Evaluation of A Simple DNA Extraction Method and Its Combination with Loop-Mediated Isothermal Amplification Assays for Rapid Plasmodium knowlesi Diagnosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source of Samples
2.2. Alternative DNA Extraction from Blood Samples
2.3. SYTO-LAMP Assay
2.4. SYBR Green-LAMP
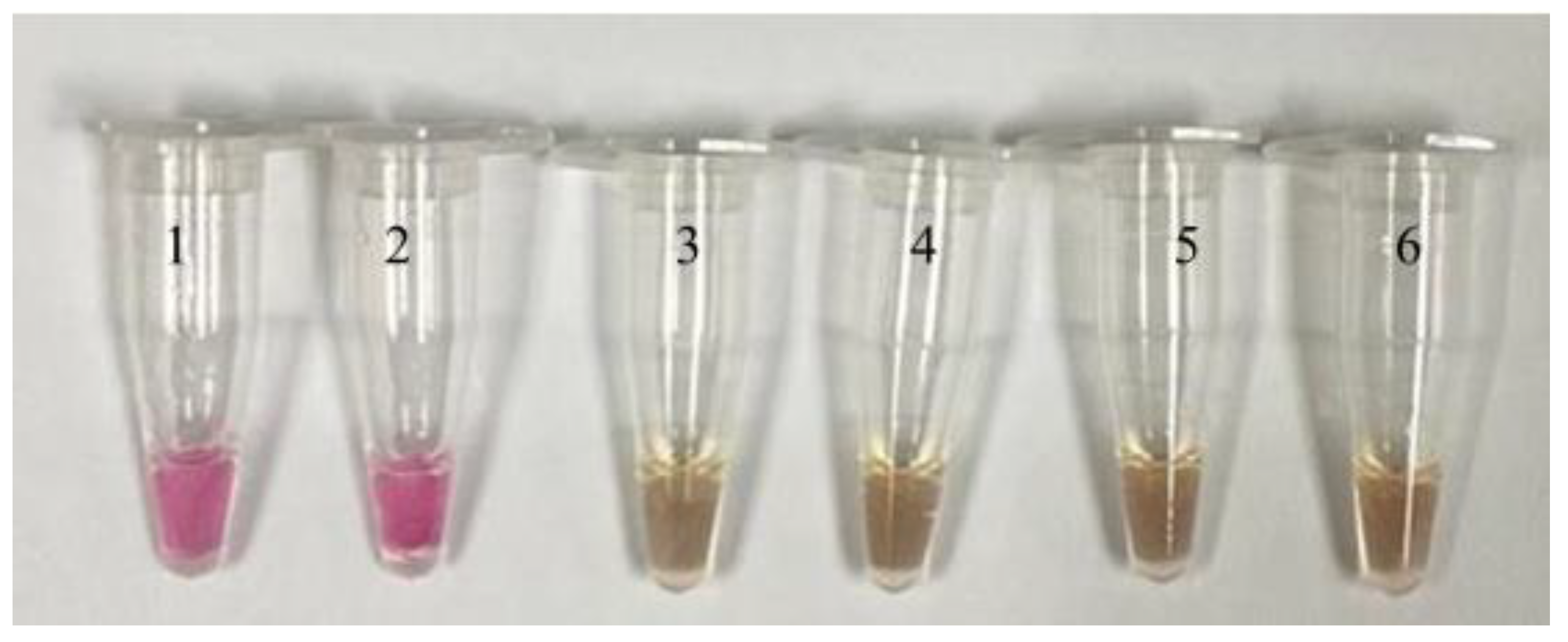
2.5. Neutral Red-LAMP
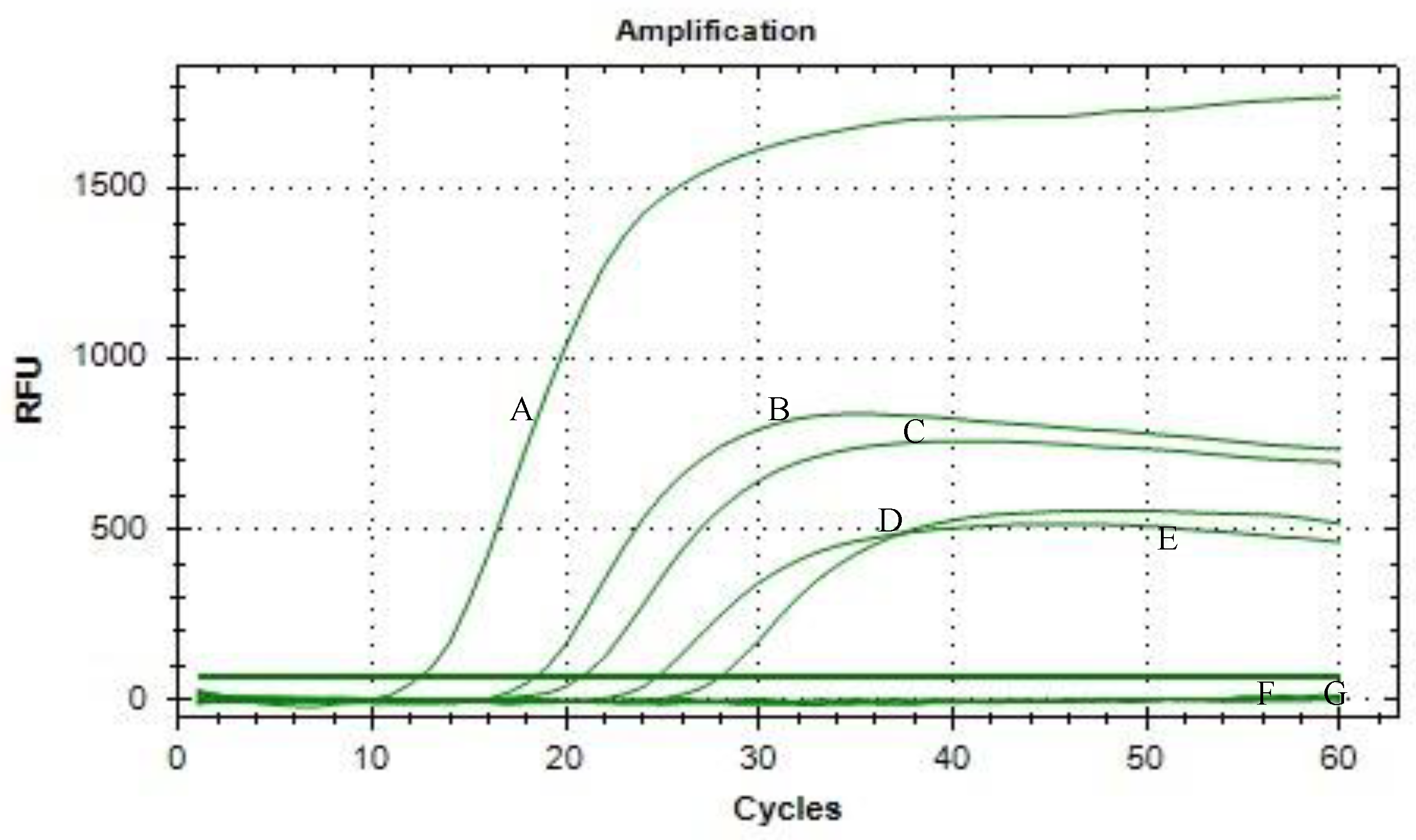
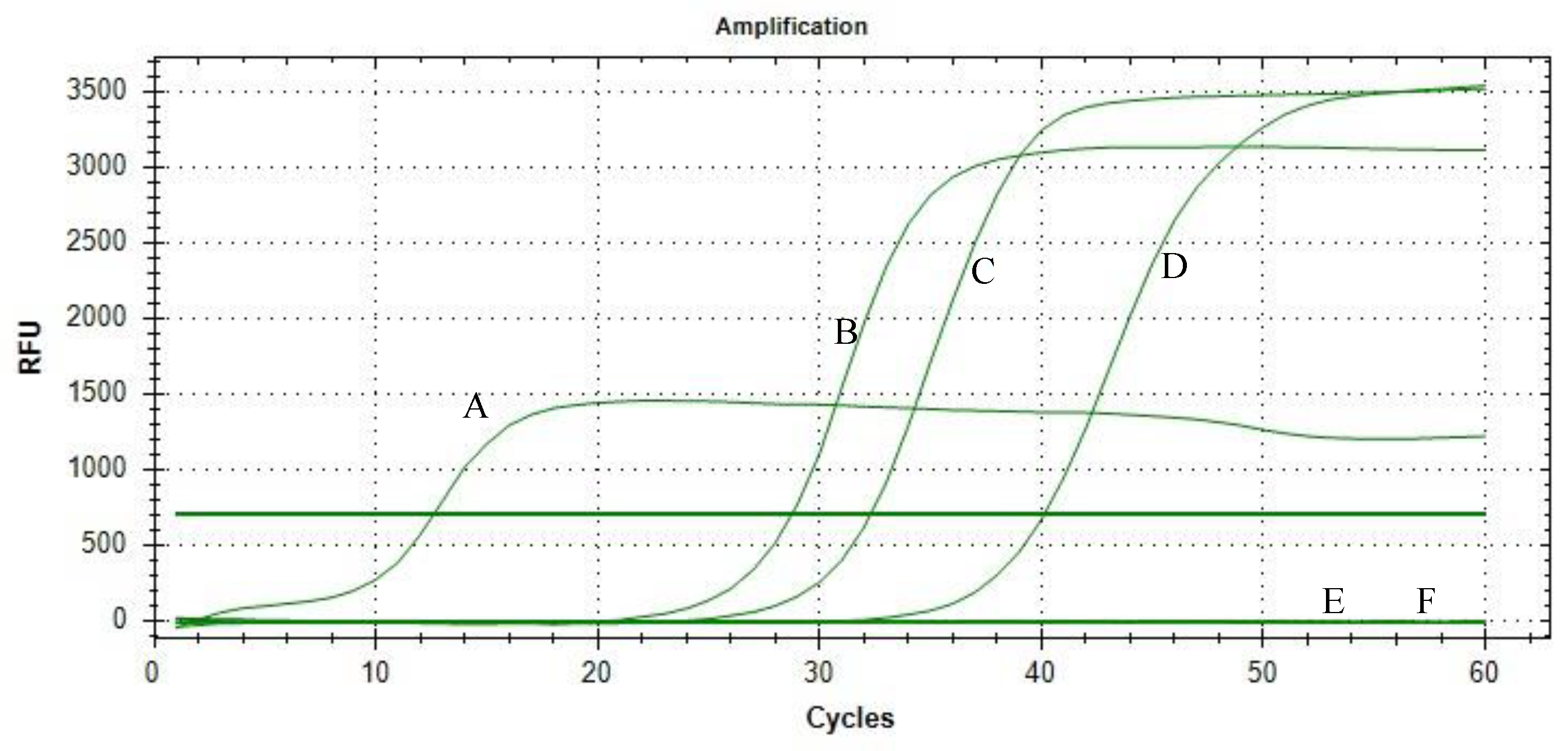
2.6. Limit of Detection of SYTO-LAMP, SYBR Green-LAMP, and Neutral Red-LAMP
2.7. Clinical Sensitivity and Specificity
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. World Malaria Report 2022; World Health Organization: Geneva, Switzerland, 2021; p. xxii.
- Singh, B.; Lee, K.S.; Matusop, A.; Radhakrishnan, A.; Shamsul, S.S.; Cox-Singh, J.; Thomas, A.; Conway, D.J. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet 2004, 363, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Jeyaprakasam, N.K.; Liew, J.W.K.; Low, V.L.; Wan-Sulaiman, W.Y.; Vythilingam, I. Plasmodium knowlesi infecting humans in Southeast Asia: What’s next? PLoS Negl. Trop. Dis. 2020, 14, e0008900. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Organization. World Malaria Report 2020. In 20 Years of Global Progress and Challenges; World Health Organization: Geneva, Switzerland, 2020; p. 4. [Google Scholar]
- Maltha, J.; Gillet, P.; Jacobs, J. Malaria rapid diagnostic tests in travel medicine. Clin. Microbiol. Infect. 2013, 19, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Tang, F.; Zhu, H.; Lu, F.; Xu, S.; Cao, Y.; Gu, Y.; He, X.; Zhou, H.; Zhu, G.; et al. Assessment of false negative rates of lactate dehydrogenase-based malaria rapid diagnostic tests for Plasmodium ovale detection. PLoS Negl. Trop. Dis. 2019, 13, e0007254. [Google Scholar] [CrossRef]
- Wu, J.; Tang, J.; Wang, W.; Chen, G.; He, X.; Xu, S.; Cao, Y.; Gu, Y.; Zhu, G.; Cao, J. Poor performance of malaria rapid diagnostic tests for the detection of Plasmodium malariae among returned international travellers in China. Malar. J. 2023, 22, 163. [Google Scholar] [CrossRef]
- Kain, K.C.; Harrington, M.A.; Tennyson, S.; Keystone, J.S. Imported malaria: Prospective analysis of problems in diagnosis and management. Clin. Infect. Dis. 1998, 27, 142–149. [Google Scholar] [CrossRef]
- Zhong, K.; Kain, K.C. Evaluation of a colorimetric PCR-based assay to diagnose Plasmodium falciparum malaria in travelers. J. Clin. Microbiol. 1996, 37, 339–341. [Google Scholar] [CrossRef]
- Gill, P.; Ghaemi, A. Nucleic acid isothermal amplification technologies: A review. Nucleos. Nucleot. Nucl. 2008, 27, 224–243. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef]
- Barazorda, K.A.; Salas, C.J.; Bishop, D.K.; Lucchi, N.; Valdivia, H.O. Comparison of real time and malachite-green based loop-mediated isothermal amplification assays for the detection of Plasmodium vivax and P. falciparum. PLoS ONE 2020, 15, e0234263. [Google Scholar] [CrossRef]
- Hu, Y.; Wan, Z.; Mu, Y.; Zhou, Y.; Liu, J.; Lan, K.; Zhang, C. A quite sensitive fluorescent loop-mediated isothermal amplification for rapid detection of respiratory syncytial virus. J. Infect. Dev. Ctries 2019, 13, 1135–1141. [Google Scholar] [CrossRef]
- Vinayaka, A.C.; Golabi, M.; Than, T.L.Q.; Wolff, A.; Bang, D.D. Point-of-care diagnosis of invasive non-typhoidal Salmonella enterica in bloodstream infections using immunomagnetic capture and loop-mediated isothermal amplification. New Biotechnol. 2022, 66, 1–7. [Google Scholar] [CrossRef]
- Lau, Y.L.; Lai, M.Y.; Teoh, B.T.; Abd-Jamil, J.; Johari, J.; Sam, S.S.; Tan, K.K.; AbuBakar, S. Colorimetric detection of dengue by single tube reverse-transcription-loop-mediated isothermal amplification. PLoS ONE 2015, 10, e0138694. [Google Scholar] [CrossRef]
- Neonakis, I.K.; Gitti, Z.; Krambovitis, E.; Spandidos, D.A. Molecular diagnostic tools in mycobacteriology. J. Microbiol. Methods 2008, 75, 1–11. [Google Scholar] [CrossRef]
- Natsu, U.; Koichi, M.; Masanobu, O.; Yoshihito, O.; Yasuhiro, S.; Hirohisa, Y.; Yoshimi, K.; Shigeru, A.; Shoji, K. Development of a loop-mediated isothermal amplification method for diagnosing Pneumocystis pneumonia. J. Med. Microbiol. 2008, 57, 50–57. [Google Scholar]
- Hong, T.C.; Mai, Q.L.; Cuong, D.V.; Parida, M.; Minekawa, H.; Notomi, T.; Hasebe, F.; Morita, K. Development and evaluation of a novel loop-mediated isothermal amplification method for rapid detection of severe acute respiratory syndrome coronavirus. J. Clin. Microbiol. 2004, 42, 1956–1961. [Google Scholar]
- Lee, S.H.; Baek, Y.H.; Kim, Y.H.; Choi, Y.K.; Song, M.S.; Ahn, J.Y. One-pot reverse transcriptional loop-mediated isothermal amplification (RT-LAMP) for detecting MERS-CoV. Front. Microbiol. 2016, 7, 2166. [Google Scholar] [CrossRef]
- Rolando, J.C.; Jue, E.; Barlow, J.T.; Ismagilov, R.F. Real-time kinetics and high-resolution melt curves in single-molecule digital LAMP to differentiate and study specific and non-specific amplification. Nucleic Acids Res. 2020, 48, e42. [Google Scholar] [CrossRef]
- Shirato, K. Detecting amplicons of loop-mediated isothermal amplification. Microbiol. Immunol. 2019, 63, 407–412. [Google Scholar] [CrossRef]
- Mori, N.; Motegi, Y.; Shimamura, Y.; Ezaki, T.; Natsumeda, T.; Yonekawa, T.; Ota, Y.; Notomi, T.; Nakayama, T. Development of a new method for diagnosis of rubella virus infection by reverse transcription-loop-mediated isothermal amplification. J. Clin. Microbiol. 2006, 44, 3268–3273. [Google Scholar] [CrossRef]
- Xiang, Z.; Li, D.; Wang, S.; Shen, T.; He, W.; Li, M.; Zeng, W.; Chen, X.; Wu, Y.; Cui, L.; et al. A simple alkali lysis method for Plasmodium falciparum DNA extraction from filter paper blood samples. Mol. Biochem. Parasitol. 2023, 254, 11557. [Google Scholar] [CrossRef] [PubMed]
- Bereczky, S.; Mårtensson, A.; Gil, J.P.; Färnert, A. Short report: Rapid DNA extraction from archive blood spots on filter paper for genotyping of Plasmodium falciparum. Am. J. Trop. Med. Hyg. 2005, 72, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Miguel, R.B.; Coura, J.R.; Samudio, F.; Suárez-Mutis, M.C. Evaluation of three different DNA extraction methods from blood samples collected in dried filter paper in Plasmodium subpatent infections from the Amazon region in Brazil. Rev. Inst. Med. Trop. Sao Paulo 2013, 55, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Modak, S.S.; Barber, C.A.; Geva, E.; Abrams, W.R.; Malamud, D.; Ongagna, Y.S.Y. Rapid point-of-care isothermal amplification assay for the detection of malaria without nucleic acid purification. Infect. Dis. 2016, 9, IDRT-S32162. [Google Scholar] [CrossRef] [PubMed]
- Snounou, G.; Viriyakosol, S.; Zhu, X.P.; Jarra, W.; Pinheiro, L.; do Rosario, V.E.; Thaithong, S.; Brown, K.N. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol. Biochem. Parasitol. 1993, 61, 315–320. [Google Scholar] [CrossRef]
- Imwong, M.; Tanomsing, N.; Pukrittayakamee, S.; Day, N.P.J.; White, N.J.; Snounou, G. Spurious amplification of a Plasmodium vivax small-subunit RNA gene by use of primers currently used to detect P. knowlesi. J. Clin. Microbiol. 2009, 47, 4173–4175. [Google Scholar] [CrossRef]
- Lau, Y.L.; Lai, M.Y.; Fong, M.Y.; Jelip, J.; Mahmud, R. Loop-mediated isothermal amplification assay for identification of five human Plasmodium species in Malaysia. Am. J. Trop. Med. Hyg. 2016, 94, 336–339. [Google Scholar] [CrossRef]
- Jaroenram, W.; Pier, P.C.; Pompa, P. Xylenol orange-based loop-mediated DNA isothermal amplification for sensitive naked-eye detection of Escherichia coli. J. Microbol. Methods 2019, 156, 9–14. [Google Scholar] [CrossRef]
- Seesui, K.; Imtawil, K.; Chanetmahun, P.; Laummaunwai, P.; Boonmars, T. An alternative method for extracting Plasmodium DNA from EDTA whole blood for malaria diagnosis. Korean J. Parasitol. 2018, 56, 25–32. [Google Scholar] [CrossRef]
- Mann, R.; Sharma, S.; Mishra, N.; Valecha, N.; Anvikar, A.R. Comparative assessment of genomic DNA extraction processes for Plasmodium: Identifying the appropriate method. J. Vector Borne. Dis. 2015, 52, 273–280. [Google Scholar]
- Piera, K.M.; Aziz, A.; William, T.; Bell, D.; González, I.J.; Barber, B.E.; Anstey, N.M.; Grigg, M.J. Detection of Plasmodium knowlesi, Plasmodium falciparum and Plasmodium vivax using loop-mediated isothermal amplification (LAMP) in a co-endemic area in Malaysia. Malar. J. 2017, 16, 29. [Google Scholar] [CrossRef]
- Port, J.R.; Nguetse, C.; Adukpo, S.; Velavan, T.P. A reliable and rapid method for molecular detection of malarial parasites using microwave irradiation and loop-mediated isothermal amplification. Malar. J. 2014, 13, 454. [Google Scholar] [CrossRef]
- Lucchi, N.W.; Demas, A.; Narayanan, J.; Sumari, D.; Kabanywanyi, A.; Kachur, S.P.; Barnwell, J.W.; Udhayakumar, V. Real-time fluorescence loop-mediated isothermal amplification for the diagnosis of malaria. PLoS ONE 2010, 5, e13733. [Google Scholar] [CrossRef]
- Amaral, C.; Antunes, W.; Moe, E.; Duarte, A.G.; Lima, L.M.P.; Santos, C.; Gomes, I.L.; Afonso, G.S.; Vieira, R.; Teles, H.S.S.; et al. A molecular test based on RT-LAMP for rapid, sensitive and inexpensive colorimetric detection of SARS-CoV-2 in clinical samples. Sci. Rep. 2021, 11, 16430. [Google Scholar] [CrossRef]
- de Oliveira, C.B.; Sanchuki, H.B.S.; Zanette, D.L.; Nardin, J.M.; Morales, H.M.P.; Fornazari, B.; Aoki, M.N.; Blanes, L. Essential properties and pitfalls of colorimetric reverse transcription loop-mediated isothermal amplification as a point-of-care test for SARS-CoV-2 diagnosis. Mol. Med. 2021, 27, 30. [Google Scholar] [CrossRef]
- Quyen, T.L.; Ngo, T.A.; Bang, D.D.; Madsen, M.; Wolff, A. Classification of multiple DNA dyes based on inhibition effects on real-time loop-mediated isothermal amplification (LAMP): Prospect for point of care setting. Front. Microbiol. 2019, 10, 2234. [Google Scholar] [CrossRef]
- Gudnason, H.; Dufva, M.; Bang, D.D.; Wolff, A. Comparison of multiple DNA dyes for real-time PCR: Effects of dye concentration and sequence composition on DNA amplification and melting temperature. Nucleic Acids Res. 2007, 35, e127. [Google Scholar] [CrossRef]
- Eischeid, A.C. SYTO dyes and EvaGreen outperform SYBR green in real-time PCR. BMC Res. 2011, 4, 263. [Google Scholar] [CrossRef]
- Lai, M.Y.; Ooi, C.H.; Lau, Y.L. Validation of SYBR green I based closed-tube loop-mediated isothermal amplification (LAMP) assay for diagnosis of knowlesi malaria. Malar. J. 2021, 20, 166. [Google Scholar] [CrossRef]
- Patel, J.C.; Oberstaller, J.; Xayavong, M.; Narayanan, J.; DeBarry, J.D.; Srinivasamoorthy, G.; Villegas, L.; Escalante, A.A.; da Silva, A.; Peterson, D.A.; et al. Real-time loop-mediated isothermal amplification (RealAmp) for the species-specific identification of Plasmodium vivax. PLoS ONE 2013, 8, e54986. [Google Scholar] [CrossRef]
| Sequence (5′ to 3′) | |
|---|---|
| FIP | GTTGTTGCCTTAAACTTCCTTGTGTTCTTGATTGTAAAGCTTCTTAGAGG |
| BIP | TGATGTCCTTAGATGAACTAGGCTTTGCAAGCAGCTAAAATCGT |
| FLP | TAGACACACATCGTT |
| BLP | GCACGCGTGCTACACT |
| F3 | CCATCTATTTCTTTTTTGCGTATG |
| B3 | CAGTGGAGGAAAAGTACGAA |
| SYTO-LAMP | SYBR Green-LAMP | Neutral Red-LAMP | |
|---|---|---|---|
| Limit of detection (parasitemia) | 0.00001% | 0.0001% | 0.01% |
| Clinical sensitivity | 100% (95% CI: 94.6–100%) | 100% (95% CI: 94.6–100%) | 91% (95% CI: 81.5–96.6) |
| Clinical specificity | 100% (95% CI: 88.4–100%) | 100% (95% CI: 88.4–100%) | 100% (95% CI: 88.4–100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, M.-Y.; Abdul Hamid, M.H.; Jelip, J.; Mudin, R.N.; Lau, Y.-L. Evaluation of A Simple DNA Extraction Method and Its Combination with Loop-Mediated Isothermal Amplification Assays for Rapid Plasmodium knowlesi Diagnosis. Trop. Med. Infect. Dis. 2023, 8, 389. https://doi.org/10.3390/tropicalmed8080389
Lai M-Y, Abdul Hamid MH, Jelip J, Mudin RN, Lau Y-L. Evaluation of A Simple DNA Extraction Method and Its Combination with Loop-Mediated Isothermal Amplification Assays for Rapid Plasmodium knowlesi Diagnosis. Tropical Medicine and Infectious Disease. 2023; 8(8):389. https://doi.org/10.3390/tropicalmed8080389
Chicago/Turabian StyleLai, Meng-Yee, Mohd Hafizi Abdul Hamid, Jenarun Jelip, Rose Nani Mudin, and Yee-Ling Lau. 2023. "Evaluation of A Simple DNA Extraction Method and Its Combination with Loop-Mediated Isothermal Amplification Assays for Rapid Plasmodium knowlesi Diagnosis" Tropical Medicine and Infectious Disease 8, no. 8: 389. https://doi.org/10.3390/tropicalmed8080389
APA StyleLai, M.-Y., Abdul Hamid, M. H., Jelip, J., Mudin, R. N., & Lau, Y.-L. (2023). Evaluation of A Simple DNA Extraction Method and Its Combination with Loop-Mediated Isothermal Amplification Assays for Rapid Plasmodium knowlesi Diagnosis. Tropical Medicine and Infectious Disease, 8(8), 389. https://doi.org/10.3390/tropicalmed8080389






