Virological Non-Suppression among Newly Diagnosed HIV-Positive Individuals on Dolutegravir-Based Antiretroviral Treatment in Eastern Ethiopia: Follow-Up Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting and Period
2.2. Study Design and Population
2.3. Sample Size and Sampling Procedure
2.4. Data and Sample Collection
2.5. Plasma Viral Load Determination
2.6. Data Analysis
3. Results
3.1. Socio-Demographics and Related Characteristics
3.2. Baseline Clinical, Laboratory, and ART Profiles
3.3. Treatment Outcomes and Virological Suppression Status at Six Months
3.4. Baseline Factors Associated with Virological Non-Suppression at Six Months
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNAIDS. Global HIV/AIDS Statistics—Fact Sheet|UNAIDS (2021). 2022. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 25 January 2023).
- UNAIDS. HIV and AIDS Estimates. Country Fact Sheet-Ethiopia|UNAIDS (2021). 2022. Available online: https://www.unaids.org/en/regionscountries/countries/ethiopia (accessed on 25 January 2023).
- WHO. End HIV/AIDS by 2030: Framework for Action in the WHO African Region, 2016–2020; WHO: Geneva, Switzerland, 2017.
- UNAIDS. 90-90-90: An Ambitious Treatment Target to Help End the AIDS Epidemic; UNAIDS: Geneva, Switzerland, 2014; pp. 9–23. [Google Scholar]
- WHO. Global Action Plan on HIV Drug Resistance 2017–2021; WHO: Geneva, Switzerland, 2017.
- Agegnehu, C.D.; Techane, M.A.; Mersha, A.T.; Atalell, K.A. Burden and Associated Factors of Virological Failure among People Living with HIV in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. AIDS Behav. 2022, 26, 3327–3336. [Google Scholar] [CrossRef] [PubMed]
- Bitwale, N.Z.; Mnzava, D.P.; Kimaro, F.D.; Jacob, T.; Mpondo, B.C.T.; Jumanne, S. Prevalence and factors associated with virological treatment failure among children and adolescents on antiretroviral therapy attending HIV/AIDS care and treatment clinics in dodoma municipality, central tanzania. J. Pediatr. Infect. Dis. Soc. 2021, 10, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Endebu, T.; Deksisa, A.; Moges, T.; Kisi, T.; Ensermu, T. Incidence of Virological failure and associated factors among adult HIV-positive patients on first line antiretroviral therapy regimen. Cen Ethiop. 2019, 5, 8. [Google Scholar]
- Osman, F.T.; Yizengaw, M.A. Virological failure and associated risk factors among HIV/AIDS pediatric patients at the ART clinic of Jimma University Medical Center, Southwest Ethiopia. Open AIDS J. 2020, 14, 61–67. [Google Scholar] [CrossRef]
- Kiros, T.; Taye, A.; Workineh, L.; Eyayu, T.; Damtie, S.; Hailemichael, W.; Tiruneh, T. Immuno-virological status and its associated factors among HIV-positive patients receiving highly active antiretroviral therapy at delgi primary hospital, northwest Ethiopia, 2020/2021: A cross-sectional study. Heliyon 2022, 8, e10169. [Google Scholar] [CrossRef] [PubMed]
- Chekole, B.; Belachew, A.; Geddif, A.; Amsalu, E.; Tigabu, A. Survival status and predictors of mortality among HIV-positive children initiated antiretroviral therapy in Bahir Dar town public health facilities Amhara region, Ethiopia, 2020. SAGE Open Med. 2022, 10, 20503121211069477. [Google Scholar] [CrossRef] [PubMed]
- Negash, H.; Welay, M.; Legese, H.; Adhanom, G.; Mardu, F.; Tesfay, K.; Gebrewahd, A.; Berhe, B. Increased virological failure and determinants among HIV patients on highly active retroviral therapy in Adigrat General Hospital, Northern Ethiopia, 2019: Hospital-based cross-sectional study. Infect. Drug Resist. 2020, 13, 1863–1872. [Google Scholar] [CrossRef]
- Nega, J.; Taye, S.; Million, Y.; Rodrigo, C.; Eshetie, S. Antiretroviral treatment failure and associated factors among HIV patients on first-line antiretroviral treatment in Sekota, northeast Ethiopia. AIDS Res. Ther. 2020, 17, 39. [Google Scholar] [CrossRef]
- Meshesha, H.M.; Nigussie, Z.M.; Asrat, A.; Mulatu, K. Determinants of virological failure among adults on first-line highly active antiretroviral therapy at public health facilities in Kombolcha town, Northeast, Ethiopia: A case-control study. BMJ Open 2020, 10, e036223. [Google Scholar] [CrossRef]
- Nabukeera, S.; Kagaayi, J.; Makumbi, F.E.; Mugerwa, H.; Matovu, J.K.B. Factors associated with virological non-suppression among HIV-positive children receiving antiretroviral therapy at the Joint Clinical Research Centre in Lubowa, Kampala Uganda. PLoS ONE 2021, 16, e0246140. [Google Scholar] [CrossRef]
- Ansah, D.; Kumah, E.; Bawontuo, V.; Agyei-Baffour, P.; Afriyie, E.K. Determinants of viral load non-suppression among people living with HIV on antiretroviral therapy in Kumasi, Ghana. Ghana Med. J. 2021, 55, 111–117. [Google Scholar] [CrossRef]
- Bisetegn, G.; Arefaynie, M.; Mohammed, A.; Fentaw, Z.; Muche, A.; Dewau, R.; Seid, Y. Predictors of Virological Failure after Adherence-Enhancement Counseling among First-Line Adults Living with HIV/AIDS in Kombolcha Town, Northeast Ethiopia. HIV/AIDS-Res. Palliat. Care 2021, 13, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Bogale, B.; Asefa, A.; Yosef, T.; Destaw, A.; Midaksa, G.; Asaye, Z.; Alemu Gebremichael, M.; Yimer, E. Determinants of Virological Failure among Patients on First Line Highly Active Antiretroviral Therapy (HAART) at Mizan Tepi University Teaching Hospital, Southwest Ethiopia: A Case Control Study. Front. Public Health 2022, 10, 916454. [Google Scholar] [CrossRef] [PubMed]
- Abubakari, A.; Issah, H.; Mutaka, M.; Asumah, M.N. Determinants of Virological Failure in HIV Patients on Highly Active Antiretroviral Therapy (HAART): A Retrospective Cross-Sectional Study in the Upper East Region of Ghana. Venereology 2023, 2, 16–29. [Google Scholar] [CrossRef]
- Perez-Molina, J.A.; Crespillo-Andújar, C.; Zamora, J.; Fernández-Félix, B.M.; Gaetano-Gil, A.; López-Bernaldo de Quirós, J.C.; Serrano-Villar, S.; Moreno, S.; Álvarez-Díaz, N.; Berenguer, J. Contribution of Low CD4 Cell Counts and High Human Immunodeficiency Virus (HIV) Viral Load to the Efficacy of Preferred First-Line Antiretroviral Regimens for Treating HIV Infection: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2023, 76, 2027–2037. [Google Scholar] [CrossRef]
- FMOH—Federal Ministry of Health Ethiopia. National Consolidated Guidelines for Comprehensive HIV Prevention, Care and Treatment, August 2018; FMOH: Addis Ababa, Ethiopia, 2018.
- Shoko, C.; Chikobvu, D. A superiority of viral load over CD4 cell count when predicting mortality in HIV patients on therapy. BMC Infect. Dis. 2019, 19, 169. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, W.; Lu, R.-R.; Ouyang, L.; Xing, H.; Shao, Y.-M.; Wu, G.-H.; Ruan, Y.-H. Baseline Viral Load Predicts Antiretroviral Therapy Outcomes among HIV-Infected Patients: An Observational Cohort Study. 2021; PREPRINT (Version 1). [Google Scholar] [CrossRef]
- Ngongo, N.M.; Ntambwe, E.K.; Nani-Tuma, H.S.; Mambimbi, M.M.; Ndona, M.M.; Mashi, M.L.; Izizag, B.B.; Lukiana, T.; Ossam, J.O.; Sonzi, D.M. Human Immunodeficiency Virus Viral Load Monitoring and Rate of Virologic Suppression Among Patients Receiving Antiretroviral Therapy in Democratic Republic of the Congo, 2013–2020; Oxford University Press: Oxford, UK, 2023. [Google Scholar]
- Vitoria, M.; Hill, A.; Ford, N.; Doherty, M.; Clayden, P.; Venter, F.; Ripin, D.; Flexner, C.; Domanico, P.L. The transition to dolutegravir and other new antiretrovirals in low-income and middle-income countries: What are the issues? Aids 2018, 32, 1551–1561. [Google Scholar] [CrossRef]
- Mnzava, D.; Okuma, J.; Ndege, R.; Kimera, N.; Ntamatungiro, A.; Nyuri, A.; Byakuzana, T.; Abilahi, F.; Mayeka, P.; Temba, E. Decentralization of viral load testing to improve HIV care and treatment cascade in rural Tanzania: Observational study from the Kilombero and Ulanga Antiretroviral Cohort. BMC Infect. Dis. 2023, 23, 222. [Google Scholar] [CrossRef]
- WHO. Surveillance of HIV Drug Resistance in Populations Initiating Antiretroviral Therapy (Pre-Treatment HIV Drug Resistance): Concept Note; WHO: Geneva, Switzerland, 2014.
- Haile, G.S.; Berha, A.B. Predictors of treatment failure, time to switch and reasons for switching to second line antiretroviral therapy in HIV infected children receiving first line anti-retroviral therapy at a Tertiary Care Hospital in Ethiopia. BMC Pediatr. 2019, 19, 37. [Google Scholar] [CrossRef]
- Derseh, B.T.; Shewayerga, B.; Dagnew Mekuria, A.; Admasu Basha, E. Virological treatment failure among adult HIV/AIDS patients from selected hospitals of North Shoa Zone, Amhara Region, Ethiopia. Infect. Drug Resist. 2020, 13, 4417–4425. [Google Scholar] [CrossRef]
- Ayele, G.; Tessema, B.; Amsalu, A.; Ferede, G.; Yismaw, G. Prevalence and associated factors of treatment failure among HIV/AIDS patients on HAART attending University of Gondar Referral Hospital Northwest Ethiopia. BMC Immunol. 2018, 19, 37. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, B.T.; Kinloch, N.N.; Baraki, B.; Lapointe, H.R.; Cobarrubias, K.D.; Brockman, M.A.; Brumme, C.J.; Foster, B.A.; Jerene, D.; Makonnen, E. High Levels of Dual-Class Drug Resistance in HIV-Infected Children Failing First-Line Antiretroviral Therapy in Southern Ethiopia. Viruses 2018, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Samizi, F.G.; Panga, O.D.; Mulugu, S.S.; Gitige, C.G.; Mmbaga, E.J. Rate and predictors of HIV virological failure among adults on first-line antiretroviral treatment in Dar Es Salaam, Tanzania. J. Infect. Dev. Ctries. 2021, 15, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.S.; Nabwera, H.M.; Mwaringa, S.M.; Obonyo, C.A.; Sanders, E.J.; Rinke de Wit, T.F.; Cane, P.A.; Berkley, J.A. HIV-1 virologic failure and acquired drug resistance among first-line antiretroviral experienced adults at a rural HIV clinic in coastal Kenya: A cross-sectional study. AIDS Res. Ther. 2014, 11, 9. [Google Scholar] [CrossRef]
- Meriki, H.D.; Tufon, K.A.; Afegenwi, M.H.; Nyindem, B.A.; Atanga, P.N.; Anong, D.N.; Cho-Ngwa, F.; Nkuo-Akenji, T. Immuno-haematologic and virologic responses and predictors of virologic failure in HIV-1 infected adults on first-line antiretroviral therapy in Cameroon. Infect. Dis. Poverty 2014, 3, 5. [Google Scholar] [CrossRef][Green Version]
- Hailu, G.G.; Hagos, D.G.; Hagos, A.K.; Wasihun, A.G.; Dejene, T.A. Virological and immunological failure of HAART and associated risk factors among adults and adolescents in the Tigray region of Northern Ethiopia. PLoS ONE 2018, 13, e0196259. [Google Scholar] [CrossRef]
- Bulage, L.; Ssewanyana, I.; Nankabirwa, V.; Nsubuga, F.; Kihembo, C.; Pande, G.; Ario, A.R.; Matovu, J.K.B.; Wanyenze, R.K.; Kiyaga, C. Factors associated with virological non-suppression among HIV-positive patients on antiretroviral therapy in Uganda, August 2014–July 2015. BMC Infect. Dis. 2017, 17, 326. [Google Scholar] [CrossRef]
- Joseph Davey, D.; Abrahams, Z.; Feinberg, M.; Prins, M.; Serrao, C.; Medeossi, B.; Darkoh, E. Factors associated with recent unsuppressed viral load in HIV-1-infected patients in care on first-line antiretroviral therapy in South Africa. Int. J. STD AIDS 2018, 29, 603–610. [Google Scholar] [CrossRef]
- Mundamshimu, J.S.; Malale, K.; Kidenya, B.R.; Gunda, D.W.; Bwemelo, L.; Mwashiuya, M.; Omar, S.S.; Mlowe, N.; Kiyumbi, M.; Ngocho, J.S. Failure to Attain HIV Viral Suppression After Intensified Adherence Counselling—What Can We Learn About Its Factors? Infect. Drug Resist. 2023, 16, 1885–1894. [Google Scholar] [CrossRef]
- Barlow-Mosha, L.; Angelidou, K.; Lindsey, J.; Archary, M.; Cotton, M.; Dittmer, S.; Fairlie, L.; Kabugho, E.; Kamthunzi, P.; Kinikar, A. Nevirapine-versus lopinavir/ritonavir-based antiretroviral therapy in HIV-infected infants and young children: Long-term follow-up of the IMPAACT P1060 randomized trial. Clin. Infect. Dis. 2016, 63, 1113–1121. [Google Scholar] [CrossRef]
- WHO. Guidelines on the Public Health Response to Pretreatment HIV Drug Resistance; WHO: Geneva, Switzerland, 2017.
- Bourgi, K.; Rebeiro, P.F.; Turner, M.; Castilho, J.L.; Hulgan, T.; Raffanti, S.P.; Koethe, J.R.; Sterling, T.R. Greater weight gain in treatment-naive persons starting dolutegravir-based antiretroviral therapy. Clin. Infect. Dis. 2020, 70, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Eifa, B.A.; Ketema, W. Could a Dolutegravir-Based Antiretroviral Therapy Lead to Clinical Obesity? A Retrospective Cohort Study Conducted at Hawassa University Comprehensive Specialized Hospital in Hawassa, Sidama, Ethiopia. AIDS Res. Treat. 2022, 2022, 2965325. [Google Scholar] [CrossRef] [PubMed]
- Esber, A.L.; Chang, D.; Iroezindu, M.; Bahemana, E.; Kibuuka, H.; Owuoth, J.; Singoei, V.; Maswai, J.; Dear, N.F.; Crowell, T.A. Weight gain during the dolutegravir transition in the African Cohort Study. J. Int. AIDS Soc. 2022, 25, e25899. [Google Scholar] [CrossRef] [PubMed]
- Xavier Hall, C.D.; Morgan, E.; Bundy, C.; Foran, J.E.; Janulis, P.; Newcomb, M.E.; Mustanski, B. Substance use predicts sustained viral suppression in a community cohort of sexual and gender minority youth living with HIV. AIDS Behav. 2021, 25, 3303–3315. [Google Scholar] [CrossRef]
- Shapiro, A.E.; Govere, S.; Galagan, S.; Krows, M.; Moosa, M.-Y.; Celum, C.L.; Drain, P.K. Prevalence and Effects of Alcohol and Substance use on HIV Clinical Outcomes in a Clinic-Based Cohort in South Africa. 13 June 2023; PREPRINT (Version 1). [Google Scholar] [CrossRef]
- Kapogiannis, B.G.; Koenig, L.J.; Xu, J.; Mayer, K.H.; Loeb, J.; Greenberg, L.; Monte, D.; Banks-Shields, M.; Fortenberry, J.D. The HIV continuum of care for adolescents and young adults attending 13 urban US HIV care centers of the NICHD-ATN-CDC-HRSA SMILE Collaborative. J. Acquir. Immune Defic. Syndr. 2020, 84, 92. [Google Scholar] [CrossRef]
- Hussen, S.; Mama, M.; Mekonnen, B.; Shegaze, M.; Boti, N.; Shure, M. Predictors of time to viral load suppression of adult PLWHIV on ART in Arba Minch General Hospital: A Follow up Study. Ethiop. J. Health Sci. 2019, 29, 751–758. [Google Scholar] [CrossRef]
- Pyngottu, A.; Scherrer, A.U.; Kouyos, R.; Huber, M.; Hirsch, H.; Perreau, M.; Yerly, S.; Calmy, A.; Cavassini, M.; Stöckle, M. Predictors of Virological Failure and Time to Viral Suppression of First-Line Integrase Inhibitor–Based Antiretroviral Treatment. Clin. Infect. Dis. 2021, 73, e2134–e2141. [Google Scholar] [CrossRef]
- Huang, S.-W.; Shen, M.-C.; Wang, W.-H.; Li, W.-Y.; Wang, J.-H.; Tseng, C.-Y.; Liu, P.-Y.; Wang, L.-S.; Lee, Y.-L.; Chen, Y.-M.A. High prevalence of HIV-1 transmitted drug resistance and factors associated with time to virological failure and viral suppression in Taiwan. J. Antimicrob. Chemother. 2022, 77, 185–195. [Google Scholar] [CrossRef]
- Yehadji, D.; Gray, G.; Vicente, C.A.; Isaakidis, P.; Diallo, A.; Kamano, S.A.; Diallo, T.S. Development of Machine Learning Algorithms to Predict Viral Load Suppression among HIV Patients in Conakry (Guinea). 16 May 2023; PREPRINT (Version 1). [Google Scholar] [CrossRef]
- Kouamou, V.; Machekano, R.; Mapangisana, T.; Maposhere, C.; Mutetwa, R.; Manasa, J.; Shamu, T.; McCarty, K.; Munyati, S.; Mutsvangwa, J. Clinic-based SAMBA-II vs centralized laboratory viral load assays among HIV-1 infected children, adolescents and young adults in rural Zimbabwe: A randomized controlled trial. PLoS ONE 2023, 18, e0281279. [Google Scholar] [CrossRef]
- Singsumran, K.; Sungkanuparph, S. Long-term virological and immunological outcomes between HIV-positive individuals with and without pretreatment HIV drug resistance. Int. J. STD AIDS 2023, 34, 322–327. [Google Scholar] [CrossRef]
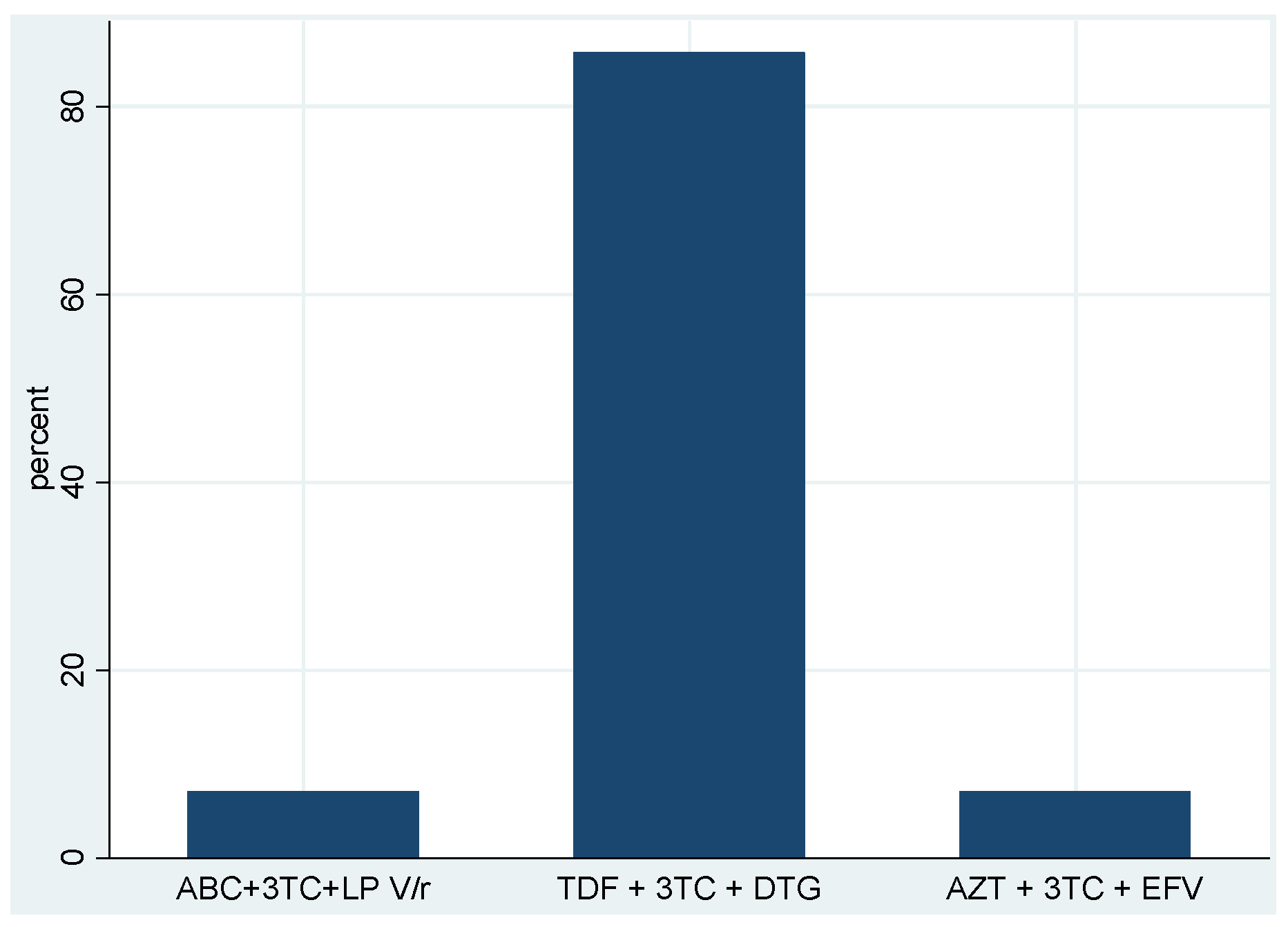
| Variables | Category | Number | Percent (%) |
|---|---|---|---|
| Sex | Male | 69 | 29.4 |
| Female | 166 | 70.6 | |
| Age groups in years | <18 | 15 | 6.4 |
| 18–29 | 71 | 30.2 | |
| 30–39 | 76 | 32.3 | |
| 40–49 | 48 | 20.4 | |
| ≥50 | 25 | 10.6 | |
| Occupational status | Govt employee | 33 | 14.0 |
| Farmer | 11 | 4.7 | |
| Merchant | 34 | 14.5 | |
| Daily laborer | 56 | 23.8 | |
| Jobless | 35 | 14.9 | |
| Housewife | 20 | 8.5 | |
| Other | 31 | 13.2 | |
| Not applicable * | 15 | 6.4 | |
| Marital status | Married | 95 | 40.4 |
| Single | 39 | 16.6 | |
| Divorced/separated | 56 | 23.8 | |
| Widowed | 30 | 12.8 | |
| Not applicable * | 15 | 6.4 | |
| Educational status | No education | 83 | 35.3 |
| Primary education | 68 | 28.9 | |
| Secondary education | 44 | 18.7 | |
| College or University | 25 | 10.6 | |
| Not applicable * | 15 | 6.4 | |
| Other family member with HIV | Yes | 100 | 42.5 |
| No | 135 | 57.5 | |
| History of substance use | Yes | 83 | 35.3 |
| No | 152 | 64.7 | |
| Khat chewing habit | Yes | 80 | 34.0 |
| No | 155 | 65.9 | |
| Alcohol consumption habit | Yes | 31 | 13.2 |
| No | 204 | 86.8 | |
| Smoking habit | Yes | 16 | 6.8 |
| No | 219 | 93.2 |
| Variables | Category | Number | Percent (%) |
|---|---|---|---|
| Type of health facility | Hospital | 176 | 74.9 |
| Health center | 59 | 25.1 | |
| Baseline comorbidity | Yes | 92 | 39.2 |
| No | 143 | 60.8 | |
| Functionality status | Working | 187 | 79.6 |
| Ambulatory | 29 | 12.3 | |
| Bed-ridden | 19 | 8.1 | |
| AIDS-defining illness | Yes | 64 | 27.2 |
| No | 171 | 72.8 | |
| Current TB history | Yes | 40 | 17.0 |
| No | 195 | 82.9 | |
| Baseline INH eligibility * | Yes | 170 | 87.2 |
| No | 25 | 12.8 | |
| Baseline CPT | Yes | 148 | 62.9 |
| No | 87 | 37.0 | |
| Gateways to ART care | VCT/ART clinic | 45 | 19.1 |
| OPD/MCH | 142 | 60.4 | |
| Family | 10 | 4.3 | |
| Other | 38 | 16.2 | |
| Baseline WHO clinical stages | I | 131 | 55.7 |
| II | 33 | 14.0 | |
| III | 51 | 21.7 | |
| IV | 20 | 8.5 | |
| Initiated ART classes | 2 NRTI + INSTI | 226 | 96.2 |
| 2 NRTI + PI | 3 | 1.3 | |
| 2 NRTI + NNRTI | 6 | 2.5 | |
| Baseline first-line ART regimens | TDF + 3TC + DTG | 223 | 94.9 |
| AZT + 3TC + EFV | 4 | 1.7 | |
| ABC + 3TC + DTG | 3 | 1.3 | |
| ABC + 3TC + LP V/r | 3 | 1.3 | |
| TDF + 3TC + EFV | 2 | 0.9 | |
| Number of ART pills/day | One pill/day | 231 | 98.3 |
| ≥2 pills/day | 4 | 1.7 | |
| Same-day ART initiation | Yes | 171 | 72.8 |
| No | 64 | 27.2 | |
| Time from diagnosis to ART initiation | Within 7 days | 18 | 7.7 |
| 8–15 days | 17 | 7.2 | |
| >15 days | 29 | 12.3 | |
| Baseline CD4 cell count availability | Yes | 91 | 38.7 |
| No | 144 | 61.3 | |
| Baseline hemoglobin availability | Yes | 131 | 55.7 |
| No | 104 | 44.3 | |
| Baseline viral load results (copies/mL) | Target not detected | 39 | 16.6 |
| <150 | 17 | 7.2 | |
| >150 | 179 | 76.2 | |
| Baseline viral load results category (copies/mL) | ≤1000 | 72 | 30.6 |
| 1001–10,000 | 51 | 21.7 | |
| 10,001–100,000 | 57 | 24.3 | |
| >100,000 | 55 | 23.4 | |
| Baseline HIV RNA VL median (range) copies/mL (n = 179) | 38,098 (156–4,102,070) | ||
| Variables | Category | Number | Percent (%) |
|---|---|---|---|
| Treatment outcomes | Alive | 161 | 68.5 |
| Loss to follow-up | 39 | 16.6 | |
| Transferred out | 21 | 8.9 | |
| Died | 14 | 5.9 | |
| ART adherence | Good | 154 | 95.7 |
| Fair/poor | 7 | 4.3 | |
| Comorbidities | Yes | 16 | 9.9 |
| No | 145 | 90.1 | |
| Functionality status | Working | 154 | 95.6 |
| Ambulatory | 4 | 2.5 | |
| Bed-ridden | 3 | 1.9 | |
| AIDS-defining illness/events | Yes | 10 | 6.2 |
| No | 151 | 93.8 | |
| WHO clinical stages | T1 | 151 | 93.8 |
| T2 | 5 | 3.1 | |
| T3 | 5 | 3.1 | |
| First-line ART regimen initiated | TDF + 3TC + DTG | 156 | 96.9 |
| ABC + 3TC + LP V/r | 2 | 1.2 | |
| AZT + 3TC + EFV | 1 | 0.6 | |
| AZT + 3TC + LPV/r | 1 | 0.6 | |
| TDF + 3TC + EFV | 1 | 0.6 | |
| History of treatment interruption | Yes | 2 | 1.2 |
| No | 159 | 98.8 | |
| ARV substitution | Yes | 1 | 0.6 |
| No | 160 | 99.4 | |
| Viral load results at 6 months (copies/mL) | Target not detected | 131 | 81.4 |
| ≤150 | 15 | 9.3 | |
| 151–999 | 1 | 0.6 | |
| ≥1000 | 14 | 8.7 | |
| Virological suppression status | Suppressed | 147 | 91.3 |
| Non-suppressed | 14 | 8.7 |
| Characteristics | Number (%) | Virological Status at 6 Months | Bivariate | Multivariate | ||
|---|---|---|---|---|---|---|
| Non-Suppressed N (%) | Suppressed N (%) | COR (95% CI) | AOR (95% CI) | p-Value | ||
| Sex | ||||||
| Male | 47 (29.2) | 7 (14.9) | 40 (85.1) | 2.67 (0.88, 8.11) | 1.01 (0.21, 4.8) | 0.995 |
| Female | 114 (70.8) | 7 (6.1) | 107 (93.9) | 1.00 | 1.00 | |
| Age groups (in years) | ||||||
| ≤30 | 64 (39.7) | 10 (15.6) | 54 (84.4) | 4.30 (1.3, 14.39) | 8.90 (1.85, 42.8) | 0.006 |
| >30 | 97 (60.3) | 4 (4.1) | 93 (95.9) | 1.00 | 1.00 | |
| Health facility type | ||||||
| Hospital | 118 (73.3) | 8 (6.8) | 110 (93.2) | 1.00 | 1.00 | |
| Health center | 43 (26.7) | 6 (13.9) | 37 (86.1) | 2.23 (0.72, 6.84) | 4.47 (0.69, 28.8) | 0.115 |
| Any comorbidity at baseline | ||||||
| Yes | 64 (39.7) | 8 (12.5) | 56 (87.5) | 2.17 (0.7, 6.57) | 2.58 (0.4, 15.3) | 0.297 |
| No | 97 (60.3) | 6 (6.2) | 91 (93.8) | 1.00 | 1.00 | |
| Functionality status | ||||||
| Working | 137 (85.1) | 12 (8.8) | 125 (91.2) | 1.00 | ||
| Ambulatory/bed-ridden | 24 (14.9) | 2 (8.3) | 22 (91.7) | 0.94 (0.19, 4.52) | ||
| Other family members with HIV | ||||||
| Yes | 71 (44.1) | 7 (9.9) | 64 (90.1) | 1.00 | ||
| No | 90 (55.9) | 7 (7.8) | 83 (92.2) | 0.77 (0.26, 2.31) | ||
| TB history | ||||||
| Yes | 24 (14.9) | 2 (8.3) | 22 (91.7) | 0.95 (0.19, 4.52) | ||
| No | 137 (85.1) | 12 (8.8) | 125 (91.2) | 1.00 | ||
| INH eligibility | ||||||
| Yes | 125 (91.2) | 10 (8.0) | 115 (92.0) | 1.00 | ||
| No | 12 (8.8) | 2 (16.7) | 10 (83.3) | 2.3 (0.44, 11.97) | ||
| CPT | ||||||
| Yes | 99 (61.5) | 7 (7.1) | 92 (92.9) | 1.00 | ||
| No | 62 (38.5) | 7 (11.3) | 55 (88.7) | 1.67 (0.56, 5.02) | 2.34 (0.55, 9.9) | 0.250 |
| WHO clinical stage | ||||||
| Stage I | 90 (55.9) | 4 (4.4) | 86 (95.6) | 1.00 | 1.00 | |
| Stage II–IV | 71 (44.1) | 10 (14.1) | 61 (85.9) | 3.5 (1.06, 11.76) | 2.89 (0.51, 16.26) | 0.229 |
| Same-day ART Initiation | ||||||
| Yes | 118 (73.3) | 9 (7.6) | 109 (92.4) | 1.00 | ||
| No | 43 (26.7) | 5 (11.6) | 38 (88.4) | 1.59 (0.50, 5.05) | ||
| Baseline VL category (copies/mL) | ||||||
| ≤4-log10 | 84 (52.2) | 3 (3.6) | 81 (96.4) | 1.00 | ||
| >4-log10 | 77 (47.8) | 11 (14.3) | 66 (85.7) | 4.5 (1.21, 16.80) | 12.64 (1.65, 96.5) | 0.014 |
| History of substance use | ||||||
| Yes | 56 (34.8) | 9 (16.1) | 47 (83.9) | 3.8 (1.22, 12.06) | 7.50 (1.41, 39.97) | 0.018 |
| No | 105 (65.2) | 5 (4.8) | 100 (95.2) | 1.00 | 1.00 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gemechu, A.; Mihret, A.; Atire, F.A.; Aseffa, A.; Howe, R.; Seyoum, B.; Mulu, A. Virological Non-Suppression among Newly Diagnosed HIV-Positive Individuals on Dolutegravir-Based Antiretroviral Treatment in Eastern Ethiopia: Follow-Up Study. Trop. Med. Infect. Dis. 2023, 8, 391. https://doi.org/10.3390/tropicalmed8080391
Gemechu A, Mihret A, Atire FA, Aseffa A, Howe R, Seyoum B, Mulu A. Virological Non-Suppression among Newly Diagnosed HIV-Positive Individuals on Dolutegravir-Based Antiretroviral Treatment in Eastern Ethiopia: Follow-Up Study. Tropical Medicine and Infectious Disease. 2023; 8(8):391. https://doi.org/10.3390/tropicalmed8080391
Chicago/Turabian StyleGemechu, Abdella, Adane Mihret, Fekadu Alemu Atire, Abraham Aseffa, Rawleigh Howe, Berhanu Seyoum, and Andargachew Mulu. 2023. "Virological Non-Suppression among Newly Diagnosed HIV-Positive Individuals on Dolutegravir-Based Antiretroviral Treatment in Eastern Ethiopia: Follow-Up Study" Tropical Medicine and Infectious Disease 8, no. 8: 391. https://doi.org/10.3390/tropicalmed8080391
APA StyleGemechu, A., Mihret, A., Atire, F. A., Aseffa, A., Howe, R., Seyoum, B., & Mulu, A. (2023). Virological Non-Suppression among Newly Diagnosed HIV-Positive Individuals on Dolutegravir-Based Antiretroviral Treatment in Eastern Ethiopia: Follow-Up Study. Tropical Medicine and Infectious Disease, 8(8), 391. https://doi.org/10.3390/tropicalmed8080391






