Schistosoma and Leishmania: An Untold Story of Coinfection
Abstract
1. Often Forgotten, Never Gone: Neglected Tropical Diseases
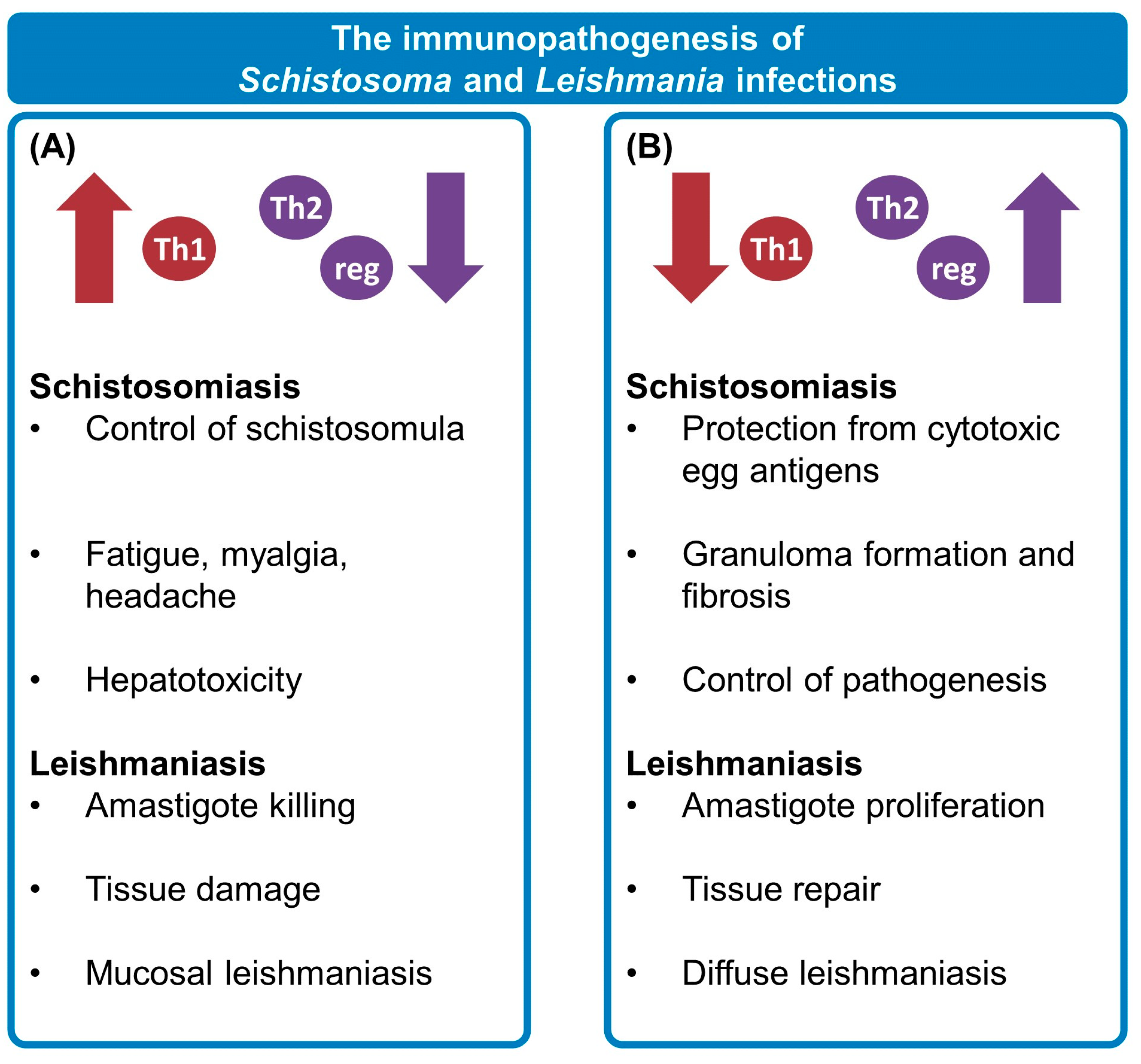
2. Two against the Host: The Influence of Schistosomiasis and Leishmaniasis on One Another
3. Making Heads and Tails: Experimental Coinfection Models
4. Concluding Remarks and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- WHO. Neglected tropical diseases: Impact of COVID-19 and WHO’s response—2021 update. Wkly. Epidemiol. Rec. 2021, 96, 461–468. [Google Scholar]
- WHO. Accelerating Work to Overcome the Global Impact of Neglected Tropical Diseases—A Roadmap for Implementation; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Ung, L.; Stothard, J.R.; Phalkey, R.; Azman, A.S.; Chodosh, J.; Hanage, W.P.; Standley, C.J. Towards global control of parasitic diseases in the COVID-19 era: One Health and the future of multisectoral global health governance. Adv. Parasitol. 2021, 114, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Brooker, S.J.; Ziumbe, K.; Negussu, N.; Crowley, S.; Hammami, M. Neglected tropical disease control in a world with COVID-19: An opportunity and a necessity for innovation. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 205–207. [Google Scholar] [CrossRef]
- Kura, K.; Ayabina, D.; Toor, J.; Hollingsworth, T.D.; Anderson, R.M. Disruptions to schistosomiasis programmes due to COVID-19: An analysis of potential impact and mitigation strategies. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 236–244. [Google Scholar] [CrossRef]
- Miguel, D.C.; Brioschi, M.B.; Rosa, L.B.; Minori, K.; Grazzia, N. The impact of COVID-19 on neglected parasitic diseases: What to expect? Trends Parasitol. 2021, 37, 694–697. [Google Scholar] [CrossRef]
- Dantas, N.M.; Andrade, L.A.; da Paz, W.S.; Borges, W.N.; Barbosa, V.G.B.; da Hora, D.P.G.; da Silva, C.E.; Carmo, R.F.D.; de Souza, C.D.F.; dos Santos, A.D.; et al. Impact of the COVID-19 pandemic on the actions of the Schistosomiasis Control Program in an endemic area in Northeastern Brazil. Acta Trop. 2023, 240, 106859. [Google Scholar] [CrossRef] [PubMed]
- WHO. Schistosomiasis and soil-transmitted helminthiases: Progress report, 2020. Wkly. Epidemiol. Rec. 2021, 96, 585–595. [Google Scholar]
- Weerakoon, K.G.A.D.; Gobert, G.N.; Cai, P.; McManus, D.P. Advances in the Diagnosis of Human Schistosomiasis. Clin. Microbiol. Rev. 2015, 28, 939–967. [Google Scholar] [CrossRef]
- Hams, E.; Aviello, G.; Fallon, P.G. The Schistosoma Granuloma: Friend or Foe? Front. Immunol. 2013, 4, 89. [Google Scholar] [CrossRef]
- Hesse, M.; Piccirillo, C.A.; Belkaid, Y.; Prufer, J.; Mentink-Kane, M.; Leusink, M.; Cheever, A.W.; Shevach, E.M.; Wynn, T.A. The Pathogenesis of Schistosomiasis Is Controlled by Cooperating IL-10-Producing Innate Effector and Regulatory T Cells. J. Immunol. 2004, 172, 3157–3166. [Google Scholar] [CrossRef]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef] [PubMed]
- Gryseels, B. Schistosomiasis. Infect. Dis. Clin. N. Am. 2012, 26, 383–397. [Google Scholar] [CrossRef]
- Scorza, B.M.; Carvalho, E.M.; Wilson, M.E. Cutaneous Manifestations of Human and Murine Leishmaniasis. Int. J. Mol. Sci. 2017, 18, 1296. [Google Scholar] [CrossRef]
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 6, 750. [Google Scholar] [CrossRef]
- Marzochi, M.C.D.A.; Marzochi, K.B.F. Tegumentary and visceral leishmaniases in Brazil: Emerging anthropozoonosis and possibilities for their control. Cad. Saude Publica 1994, 10, S359–S375. [Google Scholar] [CrossRef]
- Barral, A.; Badaro, R.; Carvalho, E.M.; Barral-Netto, M.; De Jesus, A.R.; Johnson, W.D.; Momen, H.; Almeida, R.; McMahon-Pratt, D.; Pedral-Sampaio, D.; et al. Leishmaniasis in Bahia, Brazil: Evidence that Leishmania amazonensis Produces a Wide Spectrum of Clinical Disease. Am. J. Trop. Med. Hyg. 1991, 44, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Follador, I.; Araujo, C.; Bacellar, O.; Araujo, C.B.; Carvalho, L.P.; Almeida, R.P.; Carvalho, E.M. Epidemiologic and Immunologic Findings for the Subclinical Form of Leishmania braziliensis Infection. Clin. Infect. Dis. 2002, 34, e54–e58. [Google Scholar] [CrossRef] [PubMed]
- Salam, N.; Al-Shaqha, W.M.; Azzi, A. Leishmaniasis in the Middle East: Incidence and Epidemiology. PLoS Negl. Trop. Dis. 2014, 8, e3208. [Google Scholar] [CrossRef]
- de Almeida, L.V.; Reis-Cunha, J.L.; Coqueiro-Dos-Santos, A.; Rodrigues-Luís, G.F.; Baptista, R.D.P.; Silva, S.D.O.; de Melo, M.N.; Bartholomeu, D.C. Comparative genomics of Leishmania isolates from Brazil confirms the presence of Leishmania major in the Americas. Int. J. Parasitol. 2021, 51, 1047–1057. [Google Scholar] [CrossRef]
- Ruiz-Postigo, J.A.; Jain, S.; Mikhailov, A.; Maia-Elkhoury, A.N.; Valadas, S.; Warusavithana, S.; Osman, M. Global leishmaniasis surveillance: 2019–2020, a baseline for the 2030 roadmap. Wkly. Epidemiol. Rec. 2021, 96, 401–419. [Google Scholar]
- Ruiz-Postigo, J.A.; Jain, S.; Madjou, S.; Maia-Elkhoury, A.N.; Valadas, S.; Warusavithana, S.; Osman, M.; Yajima, A.; Lin, Z.; Beshah, A.; et al. Global leishmaniasis surveillance: 2021, assessing the impact of the COVID-19 pandemic. Wkly. Epidemiol. Rec 2022, 97, 575–590. [Google Scholar]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M. Who Leishmaniasis Control the WHO Leishmaniasis Control Team Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.; Novais, P.S.F.O. Cutaneous leishmaniasis: Immune responses in protection and pathogenesis. Nat. Rev. Immunol. 2016, 16, 581–592. [Google Scholar] [CrossRef]
- Stanley, A.C.; Engwerda, C.R. Balancing immunity and pathology in visceral leishmaniasis. Immunol. Cell Biol. 2006, 85, 138–147. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, F.F.; Costa-Silva, M.F.; Pereira, A.A.S.; Rêgo, F.D.; Pereira, V.H.S.; de Souza, J.P.; Fernandes, L.O.B.; Martins-Filho, O.A.; Gontijo, C.M.F.; Peruhype-Magalhães, V.; et al. Chemokines in Leishmaniasis: Map of cell movements highlights the landscape of infection and pathogenesis. Cytokine 2021, 147, 155339. [Google Scholar] [CrossRef]
- Cox, F.E.G. Concomitant infections, parasites and immune responses. Parasitology 2001, 122, S23–S38. [Google Scholar] [CrossRef]
- Petney, T.N.; Andrews, R.H. Multiparasite communities in animals and humans: Frequency, structure and pathogenic significance. Int. J. Parasitol. 1998, 28, 377–393. [Google Scholar] [CrossRef]
- Mideo, N. Parasite adaptations to within-host competition. Trends Parasitol. 2009, 25, 261–268. [Google Scholar] [CrossRef] [PubMed]
- O’neal, S.E.; Guimarães, L.H.; Machado, P.R.; Alcântara, L.; Morgan, D.J.; Passos, S.; Glesby, M.J.; Carvalho, E.M. Influence of Helminth Infections on the Clinical Course of and Immune Response to Leishmania braziliensis Cutaneous Leishmaniasis. J. Infect. Dis. 2007, 195, 142–148. [Google Scholar] [CrossRef]
- Miranda, G.S.; Resende, S.D.; Cardoso, D.T.; Camelo, G.M.A.; Silva, J.K.A.O.; de Castro, V.N.; Geiger, S.M.; Carneiro, M.; Negrão-Corrêa, D. Previous History of American Tegumentary Leishmaniasis Alters Susceptibility and Immune Response Against Schistosoma mansoni Infection in Humans. Front. Immunol. 2021, 12, 630934. [Google Scholar] [CrossRef]
- Gregorio, A.W.; Vasconcellos, M.R.; Enokihara, M.M.; Guerra, J.M.; Nonogaki, S.; Tomimori, J. Cutaneous schistosomiasis and leishmaniasis coinfection: A case report. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1781–1783. [Google Scholar] [CrossRef] [PubMed]
- Azeredo-Coutinho, R.B.G.; Pimentel, M.I.; Zanini, G.M.; Madeira, M.F.; Cataldo, J.I.; Schubach, A.O.; Quintella, L.P.; de Mello, C.X.; Mendonça, S.C. Intestinal helminth coinfection is associated with mucosal lesions and poor response to therapy in American tegumentary leishmaniasis. Acta Trop. 2016, 154, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Paran, Y. Massive Splenomegaly: A Case Report of Visceral Leishmania and Schistosomiasis Co-infection and a Review of Infectious Causes of Massive Splenomegaly. Clin. Case Rep. Rev. 2018, 4, 1–3. [Google Scholar] [CrossRef]
- Resende, S.D.; Magalhães, F.C.; Rodrigues-Oliveira, J.L.; Castro, V.N.; Souza, C.S.A.; Oliveira, E.J.; Carneiro, M.; Geiger, S.M.; Negrão-Corrêa, D.A. Modulation of Allergic Reactivity in Humans Is Dependent on Schistosoma mansoni Parasite Burden, Low Levels of IL-33 or TNF-α and High Levels of IL-10 in Serum. Front. Immunol. 2019, 9, 3158. [Google Scholar] [CrossRef] [PubMed]
- Sokhna, C.; Le Hesran, J.-Y.; Mbaye, P.A.; Akiana, J.; Camara, P.; Diop, M.; Ly, A.; Druilhe, P. Increase of malaria attacks among children presenting concomitant infection by Schistosoma mansoni in Senegal. Malar. J. 2004, 3, 43. [Google Scholar] [CrossRef]
- Capron, M.; Béghin, L.; Leclercq, C.; Labreuche, J.; Dendooven, A.; Standaert, A.; Delbeke, M.; Porcherie, A.; Nachury, M.; Boruchowicz, A.; et al. Safety of P28GST, a Protein Derived from a Schistosome Helminth Parasite, in Patients with Crohn’s Disease: A Pilot Study (ACROHNEM). J. Clin. Med. 2019, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Newlove, T.; Machado, P.R.; Alcântara, L.; Guimarães, L.H.; Carvalho, E.M.; Morgan, D.J.; Glesby, M.J. Antihelminthic Therapy and Antimony in Cutaneous Leishmaniasis: A Randomized, Double-Blind, Placebo-Controlled Trial in Patients Co-Infected with Helminths and Leishmania braziliensis. Am. J. Trop. Med. Hyg. 2011, 84, 551–555. [Google Scholar] [CrossRef]
- El Ridi, R.; Tallima, H.; Mahana, N.; Dalton, J.P. Innate immunogenicity and in vitro protective potential of Schistosoma mansoni lung schistosomula excretory–secretory candidate vaccine antigens. Microbes Infect. 2010, 12, 700–709. [Google Scholar] [CrossRef]
- Reynolds, S.R.; Harn, D.A. Comparison of irradiated-cercaria schistosome vaccine models that use 15- and 50-kilorad doses: The 15-kilorad dose gives greater protection, smaller liver sizes, and higher gamma interferon levels after challenge. Infect. Immun. 1992, 60, 90–94. [Google Scholar] [CrossRef]
- Hervé, M.; Angeli, V.; Pinzar, E.; Wintjens, R.; Faveeuw, C.; Narumiya, S.; Capron, A.; Urade, Y.; Capron, M.; Riveau, G.; et al. Pivotal roles of the parasite PGD2 synthase and of the host D prostanoid receptor 1 in schistosome immune evasion. Eur. J. Immunol. 2003, 33, 2764–2772. [Google Scholar] [CrossRef]
- Hams, E.; Bermingham, R.; Wurlod, F.A.; Hogan, A.E.; O’Shea, D.; Preston, R.J.; McKenzie, A.N.J.; Fallon, P.G.; Rodewald, H.-R. The helminth T2 RNase ω1 promotes metabolic homeostasis in an IL-33- and group 2 innate lymphoid cell-dependent mechanism. FASEB J. 2016, 30, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Everts, B.; Perona-Wright, G.; Smits, H.H.; Hokke, C.H.; van der Ham, A.J.; Fitzsimmons, C.M.; Doenhoff, M.J.; Van Der Bosch, J.; Mohrs, K.; Haas, H.; et al. Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses. J. Exp. Med. 2009, 206, 1673–1680. [Google Scholar] [CrossRef]
- Ittiprasert, W.; Mann, V.H.; Karinshak, S.E.; Coghlan, A.; Rinaldi, G.; Sankaranarayanan, G.; Chaidee, A.; Tanno, T.; Kumkhaek, C.; Prangtaworn, P.; et al. Programmed genome editing of the omega-1 ribonuclease of the blood fluke, Schistosoma mansoni. eLife 2019, 8, 1–27. [Google Scholar] [CrossRef]
- Okano, M.; Satoskar, A.R.; Nishizaki, K.; Harn, D.A. Lacto-N-fucopentaose III Found on Schitosoma mansoni Egg Antigens Functions as Adjuvant for Proteins by Inducing Th2-Type Response. J. Immunol. 2001, 167, 442–450. [Google Scholar] [CrossRef]
- Okano, M.; Satoskar, A.R.; Nishizaki, K.; Abe, M.; Harn, D.A.A. Induction of Th2 responses and IgE is largely due to carbohydrates functioning as adjuvants on Schistosoma mansoni egg antigens. J. Immunol. 1999, 163, 6712–6717. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.D.; Jenkins, G.R.; Hogg, K.G.; Aynsley, S.A.; Paveley, R.A.; Cook, P.C.; Coles, M.C.; Mountford, A.P. CD4+CD25+ Regulatory Cells Contribute to the Regulation of Colonic Th2 Granulomatous Pathology Caused by Schistosome Infection. PLoS Negl. Trop. Dis. 2011, 5, e1269. [Google Scholar] [CrossRef]
- Haeberlein, S.; Obieglo, K.; Ozir-Fazalalikhan, A.; Chayé, M.A.M.; Veninga, H.; Van Der Vlugt, L.E.P.M.; Voskamp, A.; Boon, L.; Haan, J.M.M.D.; Westerhof, L.B.; et al. Schistosome egg antigens, including the glycoprotein IPSE/alpha-1, trigger the development of regulatory B cells. PLoS Pathog. 2017, 13, e1006539. [Google Scholar] [CrossRef]
- Layland, L.E.; Rad, R.; Wagner, H.; da Costa, C.U.P. Immunopathology in schistosomiasis is controlled by antigen-specific regulatory T cells primed in the presence of TLR2. Eur. J. Immunol. 2007, 37, 2174–2184. [Google Scholar] [CrossRef]
- Taylor, J.J.; Mohrs, M.; Pearce, E.J. Regulatory T Cell Responses Develop in Parallel to Th Responses and Control the Magnitude and Phenotype of the Th Effector Populatio. J. Immunol. 2006, 176, 5839–5847. [Google Scholar] [CrossRef]
- Bacellar, O.; Lessa, H.; Schriefer, A.; Machado, P.; de Jesus, A.R.; Dutra, W.O.; Gollob, K.J.; Carvalho, E.M. Up-Regulation of Th1-Type Responses in Mucosal Leishmaniasis Patients. Infect. Immun. 2002, 70, 6734–6740. [Google Scholar] [CrossRef]
- Faria, D.R.; Gollob, K.J.; Barbosa, J.; Schriefer, A.; Machado, P.R.L.; Lessa, H.; Carvalho, L.P.; Romano-Silva, M.A.; de Jesus, A.R.; Carvalho, E.M.; et al. Decreased In Situ Expression of Interleukin-10 Receptor Is Correlated with the Exacerbated Inflammatory and Cytotoxic Responses Observed in Mucosal Leishmaniasis. Infect. Immun. 2005, 73, 7853–7859. [Google Scholar] [CrossRef] [PubMed]
- Gaze, S.T.; Dutra, W.O.; Lessa, M.; Lessa, H.; Guimaraes, L.H.; de Jesus, A.R.; Carvalho, L.P.; Machado, P.; Carvalho, E.M.; Gollob, K.J. Mucosal Leishmaniasis Patients Display an Activated Inflammatory T-cell Phenotype Associated with a Nonbalanced Monocyte Population. Scand. J. Immunol. 2005, 63, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Cañeda-Guzmán, I.C.; Salaiza-Suazo, N.; Fernández-Figueroa, E.A.; Carrada-Figueroa, G.; Aguirre-García, M.; Becker, I. NK Cell Activity Differs between Patients with Localized and Diffuse Cutaneous Leishmaniasis Infected with Leishmania mexicana: A Comparative Study of TLRs and Cytokines. PLoS ONE 2014, 9, e112410. [Google Scholar] [CrossRef] [PubMed]
- Diaz, N.L.; Arvelaez, F.A.; Zerpa, O.; Tapia, F.J. Inducible nitric oxide synthase and cytokine pattern in lesions of patients with American cutaneous leishmaniasis. Clin. Exp. Dermatol. 2006, 31, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Diaz, N.L.; Zerpa, O.; Tapia, F.J. Chemokines and chemokine receptors expression in the lesions of patients with American cutaneous leishmaniasis. Mem. Inst. Oswaldo Cruz. 2013, 108, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Campanelli, A.P.; Brodskyn, C.I.; Boaventura, V.; Silva, C.; Roselino, A.M.; Costa, J.; Saldanha, A.C.; de Freitas, L.A.R.; de Oliveira, C.I.; Barral-Netto, M.; et al. Chemokines and chemokine receptors coordinate the inflammatory immune response in human cutaneous leishmaniasis. Hum. Immunol. 2010, 71, 1220–1227. [Google Scholar] [CrossRef]
- Bafica, A.M.B.; Cardoso, L.S.; Oliveira, S.C.; Loukas, A.; Varela, G.T.; Oliveira, R.R.; Bacellar, O.; Carvalho, E.M.; Araújo, M.I. Schistosoma mansoni antigens alter the cytokine response in vitro during cutaneous leishmaniasis. Mem. Inst. Oswaldo Cruz. 2011, 106, 856–863. [Google Scholar] [CrossRef]
- Bafica, A.M.B.; Cardoso, L.S.; Oliveira, S.C.; Loukas, A.; Góes, A.; Oliveira, R.R.; Carvalho, E.M.; Araujo, M.I. Changes in T-Cell and Monocyte Phenotypes In Vitro by Schistosoma mansoni Antigens in Cutaneous Leishmaniasis Patients. J. Parasitol. Res. 2012, 2012, 520308. [Google Scholar] [CrossRef]
- Lopes, D.M.; Fernandes, J.S.; Cardoso, T.M.d.S.; Bafica, A.M.B.; Oliveira, S.C.; Carvalho, E.M.; Araujo, M.I.; Cardoso, L.S. Dendritic Cell Profile Induced by Schistosoma mansoni Antigen in Cutaneous Leishmaniasis Patients. BioMed Res. Int. 2014, 2014, 743069. [Google Scholar] [CrossRef]
- Lopes, D.M.; de Almeida, T.V.V.S.; Souza, R.D.P.D.; Ribeiro, L.E.V.; Page, B.; Fernandes, J.D.S.; Carvalho, E.M.; Cardoso, L.S. Susceptibility of dendritic cells from individuals with schistosomiasis to infection by Leishmania braziliensis. Mol. Immunol. 2018, 93, 173–183. [Google Scholar] [CrossRef]
- Lopes, D.M.; Oliveira, S.C.; Page, B.; Carvalho, L.P.; Carvalho, E.M.; Cardoso, L.S. Schistosoma mansoni rSm29 Antigen Induces a Regulatory Phenotype on Dendritic Cells and Lymphocytes From Patients with Cutaneous Leishmaniasis. Front. Immunol. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cota, G.F.; Gomes, L.I.; Pinto, B.F.; Santos-Oliveira, J.R.; Da-Cruz, A.M.; Pedrosa, M.S.; Tafuri, W.L.; Rabello, A. Dyarrheal Syndrome in a Patient Co-Infected with Leishmania infantum and Schistosoma mansoni. Case Rep. Med. 2012, 2012, 240512. [Google Scholar] [CrossRef]
- Catorze, G.; Alberto, J.; Afonso, A.; Vieira, R.; Cortes, S.; Campino, L. Leishmania infantum/HIV co-infection: Cutaneous lesions following treatment of visceral leishmaniasis. Ann. Dermatol. Venereol. 2006, 133, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Ritmeijer, K.; Veeken, H.; Melaku, Y.; Leal, G.; Amsalu, R.; Seaman, J. Davidson Ethiopian visceral leishmaniasis: Generic and proprietary sodium stibogluconate are equivalent; HIV co-infected patients have a poor outcome. Trans. R. Soc. Trop. Med. Hyg. 2001, 95, 668–672. [Google Scholar] [CrossRef]
- James, S.L.; Cook, K.W.; Lazdins, J.K. Activation of human monocyte-derived macrophages to kill schistosomula of Schistosoma mansoni in vitro. J. Immunol. 1990, 145, 2686–2690. [Google Scholar] [CrossRef] [PubMed]
- McSorley, H.J.; Maizels, R.M. Helminth Infections and Host Immune Regulation. Clin. Microbiol. Rev. 2012, 25, 585–608. [Google Scholar] [CrossRef] [PubMed]
- Steel, C.; McCarthy, J.S.; Ottesen, E.; Guinea, A. Long-term effect of prenatal exposure to maternal microfilaraemia on immune responsiveness to filarial parasite antigens. Lancet 1994, 343, 890–893. [Google Scholar] [CrossRef]
- LaCorcia, M.; Da Costa, C.U.P. Maternal Schistosomiasis: Immunomodulatory Effects with Lasting Impact on Allergy and Vaccine Responses. Front. Immunol. 2018, 9, 2960. [Google Scholar] [CrossRef]
- Straubinger, K.; Paul, S.; da Costa, O.P.; Ritter, M.; Buch, T.; Busch, D.H.; Layland, L.E.; da Costa, C.U.P. Maternal immune response to helminth infection during pregnancy determines offspring susceptibility to allergic airway inflammation. J. Allergy Clin. Immunol. 2014, 134, 1271–1279.e10. [Google Scholar] [CrossRef]
- Cortes-Selva, D.; Gibbs, L.; Maschek, J.A.; Nascimento, M.; Van Ry, T.; Cox, J.E.; Amiel, E.; Fairfax, K.C. Metabolic reprogramming of the myeloid lineage by Schistosoma mansoni infection persists independently of antigen exposure. PLoS Pathog. 2021, 17, e1009198. [Google Scholar] [CrossRef]
- Rheinberg, C.E.; Moné, H.; Caffrey, C.R.; Imbert-Establet, D.; Jourdane, J.; Ruppel, A. Schistosoma haematobium, S. intercalatum, S. japonicum, S. mansoni, and S. rodhaini in mice: Relationship between patterns of lung migration by schistosomula and perfusion recovery of adult worms. Parasitol. Res. 1998, 84, 338–342. [Google Scholar] [CrossRef]
- Loker, E.S. A comparative study of the life-histories of mammalian schistosomes. Parasitology 1983, 87, 343–369. [Google Scholar] [CrossRef]
- Coelho, P.M.Z.; Mayrink, W.; Dias, M.; Pereira, L.H. Susceptibility to Leishmania mexicana of mice infected with Schistosoma mansoni. Trans. R. Soc. Trop. Med. Hyg. 1980, 74, 141. [Google Scholar] [CrossRef]
- La Flamme, A.C.; Scott, P.; Pearce, E.J. Schistosomiasis delays lesion resolution during Leishmania major infection by impairing parasite killing by macrophages. Parasite Immunol. 2002, 24, 339–345. [Google Scholar] [CrossRef]
- Yole, D.; Shamala, K.; Kithome, K.; Gicheru, M. Studies on the interaction of Schistosoma mansoni and Leishmania major in experimentally infected Balb/c mice. Afr. J. Health Sci. 2007, 14, 80–85. [Google Scholar] [CrossRef]
- Yoshida, A.; Maruyama, H.; Yabu, Y.; Amano, T.; Kobayakawa, T.; Ohta, N. Immune responses against protozoal and nematodal infection in mice with underlying Schistosoma mansoni infection. Parasitol. Int. 1999, 48, 73–79. [Google Scholar] [CrossRef]
- Khayeka–Wandabwa, C.; Kutima, H.L.; Nyambati, V.C.S.; Ingonga, J.; Oyoo–Okoth, E.; Karani, L.W.; Jumba, B.; Githuku, K.S.; O Anjili, C. Combination therapy using Pentostam and Praziquantel improves lesion healing and parasite resolution in BALB/c mice co-infected with Leishmania major and Schistosoma mansoni. Parasites Vectors 2013, 6, 244. [Google Scholar] [CrossRef]
- Guimarães, L.H.; Machado, P.R.L.; Lago, E.L.; Morgan, D.J.; Schriefer, A.; Bacellar, O.; Carvalho, E. Atypical manifestations of tegumentary leishmaniasis in a transmission area of Leishmania braziliensis in the state of Bahia, Brazil. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 712–715. [Google Scholar] [CrossRef]
- Schubach, A.; Marzochi, M.C.; Cuzzi-Maya, T.; Oliveira, A.V.; Araujo, M.L.; Pacheco, R.S.; Momen, H.; Conceicao-Silva, F.; Coutinho, S.G. Cutaneous scars in American tegumentary leishmaniasis patients: A site of Leishmania (Viannia) braziliensis persistence and viability eleven years after antimonial therapy and clinical cure. Am. J. Trop. Med. Hyg. 1998, 58, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.F.; Zhang, Y.; Engwerda, C.R.; Kaye, P.M.; Sharp, H.; Bickle, Q.D. The Schistosoma mansoni Hepatic Egg Granuloma Provides a Favorable Microenvironment for Sustained Growth of Leishmania donovani. Am. J. Pathol. 2006, 169, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Bertho, A.L.; Santiago, M.A.; Coutinho, S.G. An Experimental Model of the Production of Metastases in Murine Cutaneous Leishmaniasis. J. Parasitol. 1994, 80, 93. [Google Scholar] [CrossRef]
- Gomes-Silva, A.; Valverde, J.G.; Ribeiro-Romão, R.P.; Plácido-Pereira, R.M.; Da-Cruz, A.M. Golden hamster (Mesocricetus auratus) as an experimental model for Leishmania (Viannia) braziliensis infection. Parasitology 2013, 140, 771–779. [Google Scholar] [CrossRef]
- Dea-Ayuela, M.A.; Rama-Íñiguez, S.; Alunda, J.M.; Bolás-Fernandez, F. Setting New Immunobiological Parameters in the Hamster Model of Visceral Leishmaniasis for In Vivo Testing of Antileishmanial Compounds. Veter.-Res. Commun. 2007, 31, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Lindoso, J.A.L. Immunity and immunosuppression in experimental visceral leishmaniasis. Braz. J. Med. Biol. Res. 2004, 37, 615–623. [Google Scholar] [CrossRef]
- Pirmez, C.; Marzochi, M.C.A.; Coutinho, S.G. Experimental canine mucocutaneous leishmaniasis (Leishmania braziliensis braziliensis). Mem. Inst. Oswaldo Cruz. 1988, 83, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Karoum, K.O.; Amin, M.A. Domestic and wild animals naturally infected with Schistosoma mansoni in the Gezira Irrigated Scheme, Sudan. J. Trop. Med. Hyg. 1985, 88, 83–89. [Google Scholar] [PubMed]
- Moore, D.V.; Sandground, J.H. The Relative Egg Producing Capacity of Schistosoma mansoni and Schistosoma japonicum. Am. J. Trop. Med. Hyg. 1956, 5, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Dolabella, S.S.; Coelho, P.M.Z.; Borçari, I.T.; Mello, N.A.S.T.; Andrade, Z.D.A.; Silva, E.F. Morbidity due to Schistosoma mansoni–Entamoeba histolytica coinfection in hamsters (Mesocricetus auratus). Rev. Soc. Bras. Med. Trop. 2007, 40, 170–174. [Google Scholar] [CrossRef][Green Version]
- Moore, D.V.; Meleney, H.E. Comparative Susceptibility of Common Laboratory Animals to Experimental Infection with Schistosoma haematobium. J. Parasitol. 1954, 40, 392. [Google Scholar] [CrossRef]
- Gentile, R.; Costa-Neto, S.F.; D’Andrea, P.S. A review on the role of the water-rat Nectomys squamipes on the transmission dynamics of mansonic schistosomiasis: A long term multidisciplinary study in an endemic area. Oecol. Aust. 2010, 14, 711–715. [Google Scholar] [CrossRef]
- Miranda, G.S.; Rodrigues, J.G.M.; Lira, M.G.S.; Nogueira, R.A.; Gomes, G.C.C.; Silva-Souza, N. Monitoring positivity for Schistosoma mansoni in rodents Holochilus sp. naturally infected. Cien. Anim. Bras. 2015, 16, 456–463. [Google Scholar] [CrossRef]
- Bastos, O.d.C.; Sadigursky, M.; Nascimento, M.D.D.S.B.D.; Brazil, R.P.; de Holanda, J.C. Holochilus brasiliensis nanus Thomas, 1897: As an experimental model for filariasis, leishmaniasis and schistosomiasis. Rev. Inst. Med. Trop. Sao Paulo 1984, 26, 307–315. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Andrade, M.S.; Courtenay, O.; Brito, M.E.F.; Carvalho, F.G.; Carvalho, A.W.S.; Soares, F.; Carvalho, S.M.; Costa, P.L.; Zampieri, R.; Floeter-Winter, L.M.; et al. Infectiousness of Sylvatic and Synanthropic Small Rodents Implicates a Multi-host Reservoir of Leishmania (Viannia) braziliensis. PLoS Negl. Trop. Dis. 2015, 9, e0004137. [Google Scholar] [CrossRef]
- Lima, B.S.; Dantas-Torres, F.; de Carvalho, M.R.; Marinho-Junior, J.F.; de Almeida, E.L.; Brito, M.E.F.; Gomes, F.; Brandao-Filho, S.P. Small mammals as hosts of Leishmania spp. in a highly endemic area for zoonotic leishmaniasis in north-eastern Brazil. Trans. R. Soc. Trop. Med. Hyg. 2013, 107, 592–597. [Google Scholar] [CrossRef]
- Teva, A.; Porrozzi, R.; Cupolillo, E.; Pirmez, C.; Oliveira-Neto, M.P.; Grimaldi, G. Leishmania (Viannia) braziliensis-induced chronic granulomatous cutaneous lesions affecting the nasal mucosa in the rhesus monkey (Macaca mulatta) model. Parasitology 2003, 127, 437–447. [Google Scholar] [CrossRef]
- Amaral, V.F.; Ransatto, V.A.; Conceição-Silva, F.; Molinaro, E.; Ferreira, V.; Coutinho, S.G.; McMahon-Pratt, D.; Grimaldi, J.G. Leishmania amazonensis: The Asian Rhesus Macaques (Macaca mulatta) as an Experimental Model for Study of Cutaneous Leishmaniasis. Exp. Parasitol. 1996, 82, 34–44. [Google Scholar] [CrossRef]
- Torben, W.; Hailu, A. The development of hepatic granulomas in 20 Krad irradiated Schistosoma mansoni cercaria vaccinated grivet monkeys (Cercopithecus aethiops aethiops). Exp. Parasitol. 2007, 117, 376–381. [Google Scholar] [CrossRef]
- Else, J.G.; Satzger, M.; Sturrock, R.F. Natural infections of Schistosoma mansoni and S. haematobiumin Cercopithecus monkeys in Kenya. Ann. Trop. Med. Parasitol. 1982, 76, 111–112. [Google Scholar] [CrossRef]
- Fairley, N.H. A comparative study of experimental bilharziasis in monkeys contrasted with the hitherto described lesions in man. J. Pathol. Bacteriol. 1920, 23, 289–314. [Google Scholar] [CrossRef]
| Reference | Mouse Strain | S. mansoni Strain | S. mansoni Load | Leishmania sp. and Strain | Leishmania sp. Load | Time of Coinfection | Changes in Skin Lesions |
|---|---|---|---|---|---|---|---|
| Coelho et al. 1980 [75] | Outbred mice | LE strain | 70 cercariae | L. mexicana (L v 22) | 1 × 108 promastigotes | 8 weeks post S. mansoni infection | Shorter incubation period |
| Yoshida et al. 1999 [78] | BALB/c & C57BL/6 | Puerto Rico strain | 20 cercariae | L. major (5ASKH) | 4 × 107 promastigotes | 8 weeks post S. mansoni infection | Absent |
| la Flamme et al. 2002 [76] | C57BL/6 | Puerto Rico strain | 70 cercariae | L. major (FN) | 5 × 106 promastigotes | 2 weeks post S. mansoni infection | Larger lesions with delayed healing |
| Yole et al. 2007 [77] | C57BL/6 | Kenyan isolate | 150 cercariae | L. major (NLB-144) | 3 × 106 promastigotes | Simultaneous | Larger lesions |
| Khayeka-Wandabwa et al. 2013 [79] | BALB/c | KEN-lab strain | 70 cercariae | L. major (NLB-144) | 1 × 106 promastigotes | Simultaneous | Absent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camelo, G.M.A.; Silva, J.K.A.d.O.; Geiger, S.M.; Melo, M.N.; Negrão-Corrêa, D.A. Schistosoma and Leishmania: An Untold Story of Coinfection. Trop. Med. Infect. Dis. 2023, 8, 383. https://doi.org/10.3390/tropicalmed8080383
Camelo GMA, Silva JKAdO, Geiger SM, Melo MN, Negrão-Corrêa DA. Schistosoma and Leishmania: An Untold Story of Coinfection. Tropical Medicine and Infectious Disease. 2023; 8(8):383. https://doi.org/10.3390/tropicalmed8080383
Chicago/Turabian StyleCamelo, Genil Mororó Araújo, Jeferson Kelvin Alves de Oliveira Silva, Stefan Michael Geiger, Maria Norma Melo, and Deborah Aparecida Negrão-Corrêa. 2023. "Schistosoma and Leishmania: An Untold Story of Coinfection" Tropical Medicine and Infectious Disease 8, no. 8: 383. https://doi.org/10.3390/tropicalmed8080383
APA StyleCamelo, G. M. A., Silva, J. K. A. d. O., Geiger, S. M., Melo, M. N., & Negrão-Corrêa, D. A. (2023). Schistosoma and Leishmania: An Untold Story of Coinfection. Tropical Medicine and Infectious Disease, 8(8), 383. https://doi.org/10.3390/tropicalmed8080383








