Cell Aggregation Capability of Clinical Isolates from Candida auris and Candida haemulonii Species Complex
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms and Growth Conditions
2.2. Aggregation Kinetic Assay
2.3. Aggregation after Prolonged Periods and Assessment of Viability
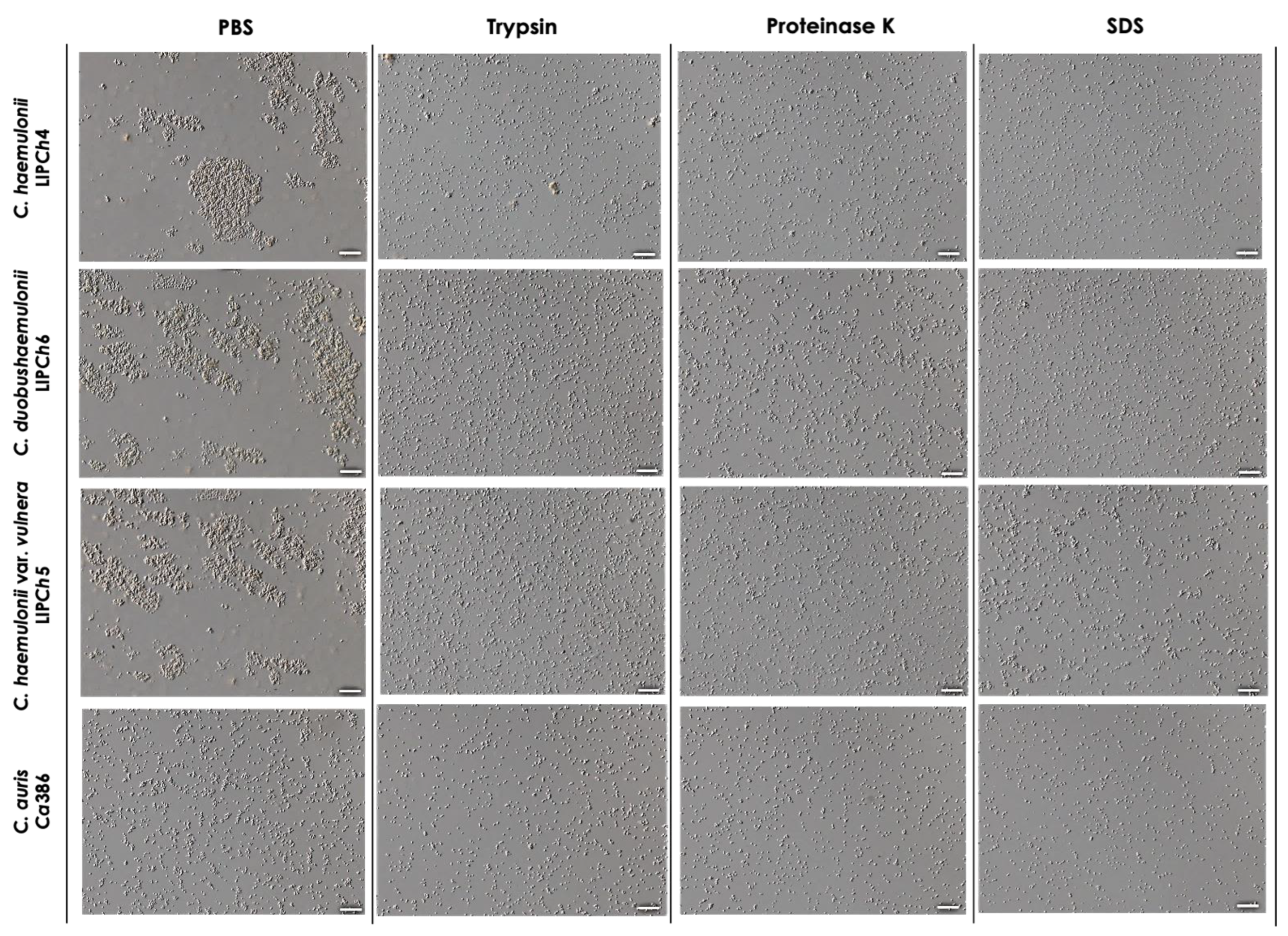
2.4. Light Microscope Imaging
2.5. Effects of Chemicals on Aggregation
2.6. Effects of Temperature on Aggregation
2.7. Statistics
3. Results and Discussion
3.1. Aggregation Is a Time-Dependent Event in C. haemulonii Clade
3.2. Aggregation after Prolonged Periods and Viability
3.3. Morphological Analysis of Cellular Aggregation
3.4. Effects of Chemical Factors on Aggregation
3.5. Effects of Temperature on Aggregation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gade, L.; Muñoz, J.F.; Sheth, M.; Wagner, D.; Berkow, E.L.; Forsberg, K.; Jackson, B.R.; Ramos-Castro, R.; Escandón, P.; Dolande, M.; et al. Understanding the emergence of multidrug-resistant Candida: Using Whole-Genome Sequencing to describe the population structure of Candida haemulonii species complex. Front. Genet. 2020, 11, 554. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gaviria, M.; Martínez-Álvarez, J.A.; Chávez-Santiago, J.O.; Mora-Montes, H.M. Candida haemulonii complex and Candida auris: Biology, virulence factors, immune response, and multidrug resistance. Infect. Drug Resist. 2023, 16, 1455–1470. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Sood, P. On the emergence, spread and resistance of Candida auris: Host, pathogen and environmental tipping points. J. Med. Microbiol. 2021, 70, 001318. [Google Scholar] [CrossRef] [PubMed]
- Borman, A.M.; Szekely, A.; Johnson, E.M. Comparative pathogenicity of United Kingdom isolates of the emerging pathogen Candida auris and other key pathogenic Candida Species. mSphere 2016, 1, e00189-16. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Kaur, M.; Chakrabarti, A.; Shankarnarayan, S.A.; Rudramurthy, S.M. Biofilm formation by Candida auris isolated from colonising sites and candidemia cases. Mycoses 2019, 62, 706–709. [Google Scholar] [CrossRef]
- Carvajal, S.K.; Alvarado, M.; Rodríguez, Y.M.; Parra-Giraldo, C.M.; Varón, C.; Morales-López, S.E.; Rodríguez, J.Y.; Gómez, B.L.; Escandón, P. Pathogenicity assessment of Colombian strains of Candida auris in the Galleria mellonella invertebrate model. J. Fungi 2021, 7, 401. [Google Scholar] [CrossRef]
- Brown, J.L.; Delaney, C.; Short, B.; Butcher, M.C.; McKloud, E.; Williams, C.; Kean, R.; Ramage, G. Candida auris phenotypic heterogeneity determines pathogenicity in vitro. mSphere 2020, 5, e00371-20. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Holowka, T.; Orner, E.P.; Fries, B.C. Gene duplication associated with increased fluconazole tolerance in Candida auris cells of advanced generational age. Sci. Rep. 2019, 9, 5052. [Google Scholar] [CrossRef] [Green Version]
- Bing, J.; Guan, Z.; Zheng, T.; Zhang, Z.; Fan, S.; Ennis, C.L.; Nobile, C.J.; Huang, G. Clinical isolates of Candida auris with enhanced adherence and biofilm formation due to genomic amplification of ALS4. PLoS Pathog. 2023, 19, e1011239. [Google Scholar] [CrossRef]
- Ramos, L.S.; Oliveira, S.S.C.; Silva, L.N.; Granato, M.Q.; Gonçalves, D.S.; Frases, S.; Seabra, S.H.; Macedo, A.J.; Kneipp, L.F.; Branquinha, M.H.; et al. Surface, adhesiveness and virulence aspects of Candida haemulonii species complex. Med. Mycol. 2020, 58, 973–986. [Google Scholar] [CrossRef]
- Ramos, L.S.; Oliveira, S.S.C.; Souto, X.M.; Branquinha, M.H.; Santos, A.L.S. Planktonic growth and biofilm formation profiles in Candida haemulonii species complex. Med. Mycol. 2017, 55, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Ramos, L.S.; Figueiredo-Carvalho, M.H.; Barbedo, L.S.; Ziccardi, M.; Chaves, A.L.; Zancope-Oliveira, R.M.; Pinto, M.R.; Sgarbi, D.B.; Dornelas-Ribeiro, M.; Branquinha, M.H.; et al. Candida haemulonii complex: Species identification and antifungal susceptibility profiles of clinical isolates from Brazil. J. Antimicrob. Chemother. 2015, 70, 111–115. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, J.E.; Ramirez, L.M.; Dias, L.D.S.; Rivas, L.A.; Ramos, L.S.; Santos, A.L.S.; Taborda, C.P.; Parra-Giraldo, C.M. Pathogenicity levels of Colombian strains of Candida auris and Brazilian strains of Candida haemulonii species complex in both murine and Galleria mellonella experimental models. J. Fungi 2020, 6, 104. [Google Scholar] [CrossRef]
- Das, T.; Krom, B.P.; van der Mei, H.C.; Busscher, H.J.; Sharma, P.K. DNA-mediated bacterial aggregation is dictated by acid–base interactions. Soft Matter 2011, 7, 2927–2935. [Google Scholar] [CrossRef]
- Liu, H.H.; Yang, Y.R.; Shen, X.C.; Zhang, Z.L.; Shen, P.; Xie, Z.X. Role of DNA in bacterial aggregation. Curr. Microbiol. 2008, 57, 139–144. [Google Scholar] [CrossRef]
- Arzmi, M.H.; Dashper, S.; Catmull, D.; Cirillo, N.; Reynolds, E.C.; McCullough, M. Coaggregation of Candida albicans, Actinomyces naeslundii and Streptococcus mutans is Candida albicans strain dependent. FEMS Yeast Res. 2015, 15, fov038. [Google Scholar] [CrossRef] [Green Version]
- Tomičić, R.; Tomičić, Z.; Raspor, P. Influence of culture conditions on co-aggregation of probiotic yeast Saccharomyces boulardii with Candida spp. and their auto-aggregation. Folia Microbiol. 2022, 67, 507–515. [Google Scholar] [CrossRef]
- Burdman, S.; Jurkevitch, E.; Schwartsburd, B.; Hampel, M.; Okon, Y. Aggregation in Azospirillum brasilense: Effects of chemical and physical factors and involvement of extracellular components. Microbiology 1998, 144 Pt 7, 1989–1999. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.; Samaranayake, L.P.; Yip, H.K. Coaggregation profiles of the microflora from root surface caries lesions. Arch. Oral Biol. 2005, 50, 23–32. [Google Scholar] [CrossRef]
- Mokhtar, M.; Rismayuddin, N.A.R.; Mat Yassim, A.S.; Ahmad, H.; Abdul Wahab, R.; Dashper, S.; Arzmi, M.H. Streptococcus salivarius K12 inhibits Candida albicans aggregation, biofilm formation and dimorphism. Biofouling 2021, 37, 767–776. [Google Scholar] [CrossRef]
- Burdman, S.; Jurkevitch, E.; Soria-Díaz, M.E.; Serrano, A.M.; Okon, Y. Extracellular polysaccharide composition of Azospirillum brasilense and its relation with cell aggregation. FEMS Microbiol. Lett. 2000, 189, 259–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Short, B.; Brown, J.; Delaney, C.; Sherry, L.; Williams, C.; Ramage, G.; Kean, R. Candida auris exhibits resilient biofilm characteristics in vitro: Implications for environmental persistence. J. Hosp. Infect. 2019, 103, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Chloe, P.; Alistair, J.P.B.; Alexander, L. Candida auris undergoes adhesin-dependent and -independent cellular aggregation. bioRxiv Prepr. Serv. Biol. 2023, 2023.2004.2021.537817. [Google Scholar] [CrossRef]
- Forgács, L.; Borman, A.M.; Prépost, E.; Tóth, Z.; Kardos, G.; Kovács, R.; Szekely, A.; Nagy, F.; Kovacs, I.; Majoros, L. Comparison of in vivo pathogenicity of four Candida auris clades in a neutropenic bloodstream infection murine model. Emerg. Microbes Infect. 2020, 9, 1160–1169. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, L.S.; Parra-Giraldo, C.M.; Branquinha, M.H.; Santos, A.L.S. Cell Aggregation Capability of Clinical Isolates from Candida auris and Candida haemulonii Species Complex. Trop. Med. Infect. Dis. 2023, 8, 382. https://doi.org/10.3390/tropicalmed8080382
Ramos LS, Parra-Giraldo CM, Branquinha MH, Santos ALS. Cell Aggregation Capability of Clinical Isolates from Candida auris and Candida haemulonii Species Complex. Tropical Medicine and Infectious Disease. 2023; 8(8):382. https://doi.org/10.3390/tropicalmed8080382
Chicago/Turabian StyleRamos, Lívia S., Claudia M. Parra-Giraldo, Marta H. Branquinha, and André L. S. Santos. 2023. "Cell Aggregation Capability of Clinical Isolates from Candida auris and Candida haemulonii Species Complex" Tropical Medicine and Infectious Disease 8, no. 8: 382. https://doi.org/10.3390/tropicalmed8080382
APA StyleRamos, L. S., Parra-Giraldo, C. M., Branquinha, M. H., & Santos, A. L. S. (2023). Cell Aggregation Capability of Clinical Isolates from Candida auris and Candida haemulonii Species Complex. Tropical Medicine and Infectious Disease, 8(8), 382. https://doi.org/10.3390/tropicalmed8080382








