Legionnaires’ Disease in Occupational Settings: A Cross-Sectional Study from Northeastern Italy (2019)
Abstract
1. Introduction
- (a)
- Italian OPs have some familiarity with the management of LD because of their role in occupational settings;
- (b)
- Italian OPs have a sufficient understanding of LD in order to provide appropriate contributions to risk assessment and risk management in occupational settings;
- (c)
- Italian OPs are involved in the design and implementation of appropriate preventive measures for LD.
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Ethical Considerations
2.4. Questionnaire
- (1)
- Demographic data: Age, gender, seniority as OP, and whether the respondents practiced as OPs in hospitals, nursing homes, or wastewater treatment plants.
- (2)
- Knowledge Status: Participants received specifically designed knowledge tests, including (a) 13 true/false statements (e.g., “LD typically has inter-human spreading”; FALSE) and (b) 2 multiple-choice questions. Every correct answer added +1 to a summary General Knowledge Score (GKS), while wrong indications or a missing/”don’t know” answer added 0 (potential range of GKS: 0 to 15). The internal consistency of the questionnaire (i.e., the degree of homogeneity among the included items) was assessed through the Cronbach alpha test. In general, a score ≥ 0.7 is considered acceptable [32]. Participants were then asked to report the perceived occurrence of a series of clinical signs and symptoms among the individual features of LD cases (range: “never”, i.e., unlikely to be noticed, to “always”, i.e., a consistent feature of the syndrome). Finally, a series of individual and environmental risk factors was shown to the participants, and they were asked whether they had any knowledge of their role in LD (“yes” vs. “no”).
- (3)
- Risk perception: According to the definition provided by Yates, risk perception can be defined as the function of the perceived probability of an event and its expected consequences [33]. Therefore, following the model developed by Betsch and Wicker [27], a sum score (Risk Perception Score, RPS) was calculated as follows. Participants were initially requested to rate, through a 5-point Likert scale, the perceived frequency (F; “extremely infrequent”, score = 1; “infrequent”, score = 2; “neutral”, score = 3; “frequent”, score = 4; “very frequent”, score = 5) and the perceived severity (C; “not at all severe”, score = 1; “low severity”, score = 2; “neutral”, score = 3; “severe”, score = 4; “very severe”, score = 5) of LD in occupational settings. RPS (potential range 1 to 25) was then calculated asRPS = F × S
- (4)
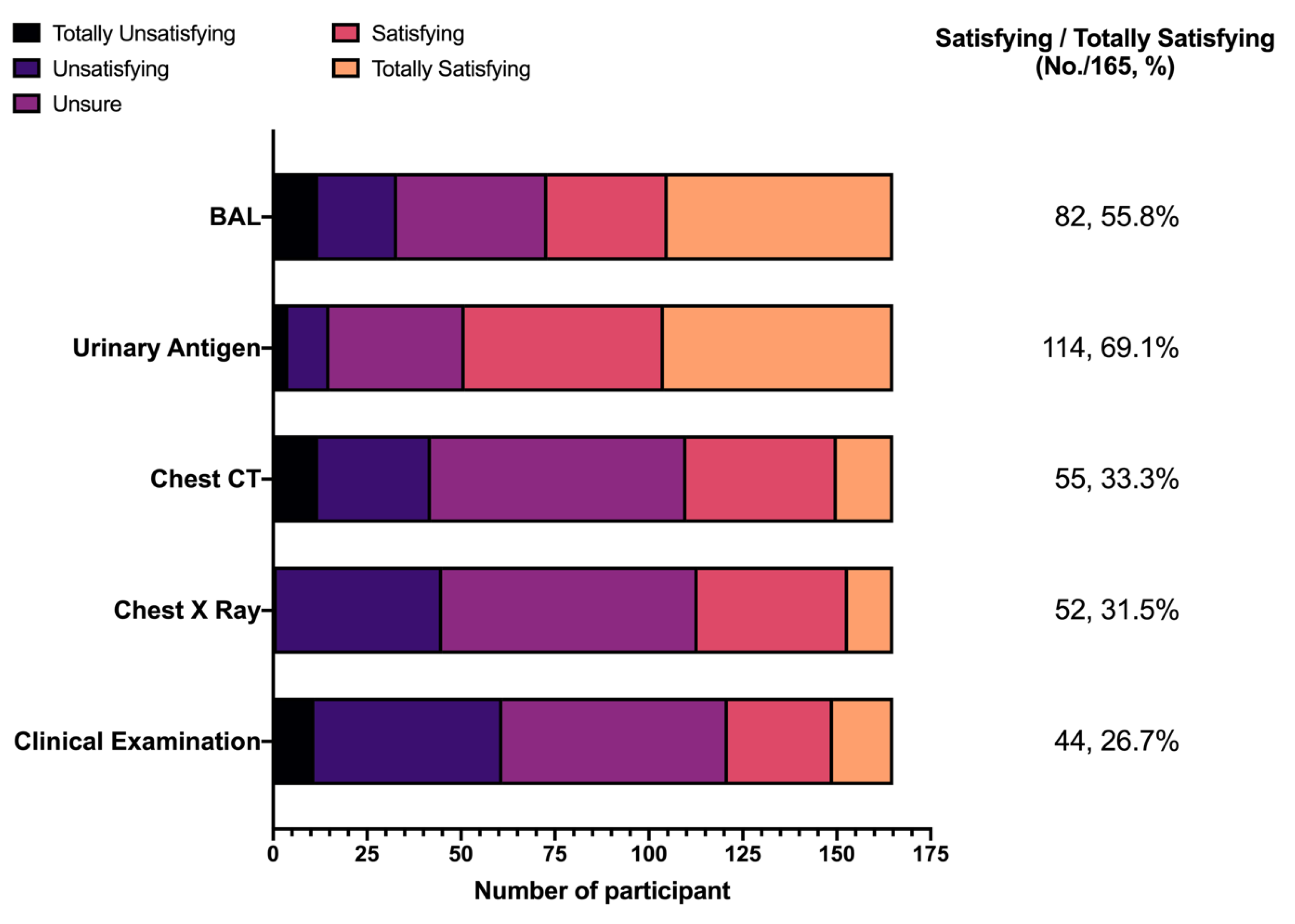
- Attitudes: To begin with, participants were asked to rate whether they were confident in properly recognizing any case of LD. Respondents were then requested to rate, through a full Likert scale of 1 to 5, a series of diagnostic options (bronchoalveolar lavage, BAL; urinary antigen assay; chest computed tomography; chest X ray; clinical examination; range: “totally unsatisfying” to “totally satisfying”).
- (5)
- Practices: Participants were requested to report whether they had previously managed any case of LD infection among assisted workers in the previous 5 years (yes vs. no) or whether any of their friends/relatives had been previously affected by LD (yes vs. no). Moreover, they were asked whether they had participated or not in the risk assessment for LD in any of the occupational settings in which they work (yes vs. no) and whether they had actively promoted any preventive measure for LD (yes vs. no).
2.5. Data Analysis
3. Results
3.1. Demographic Data
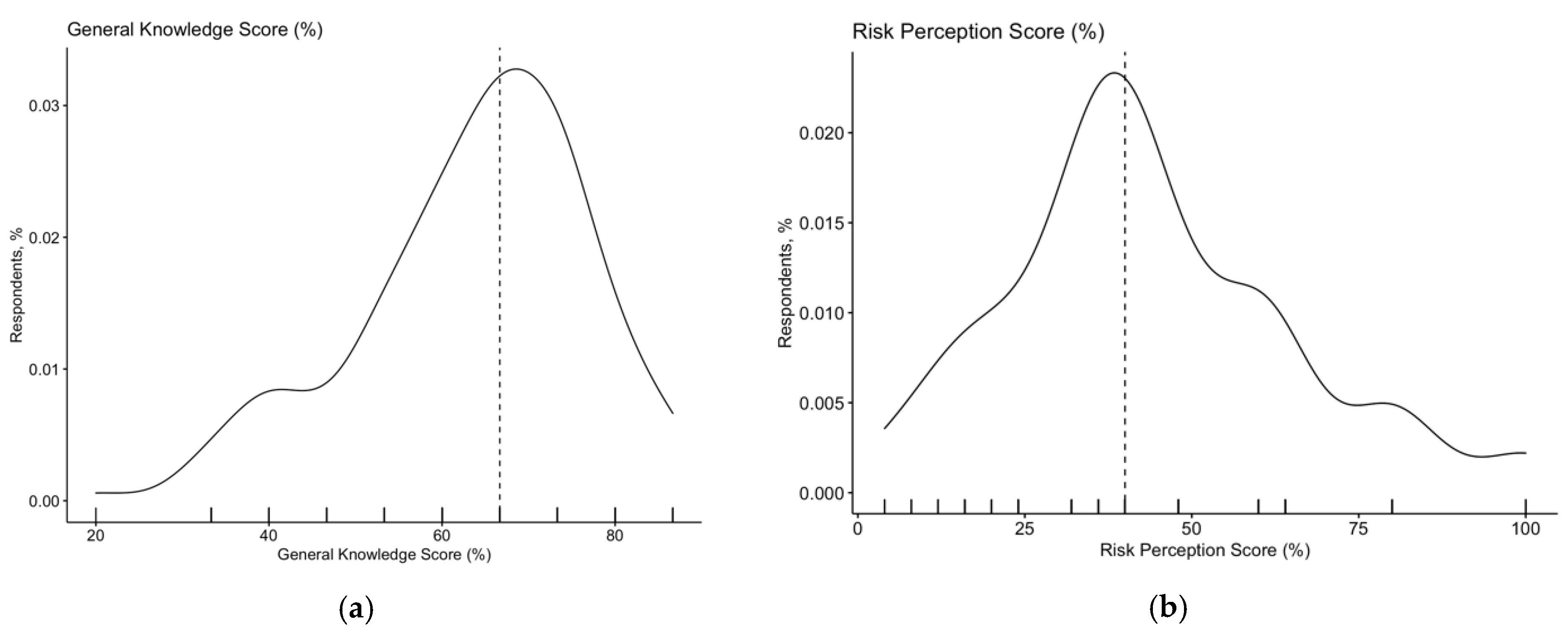
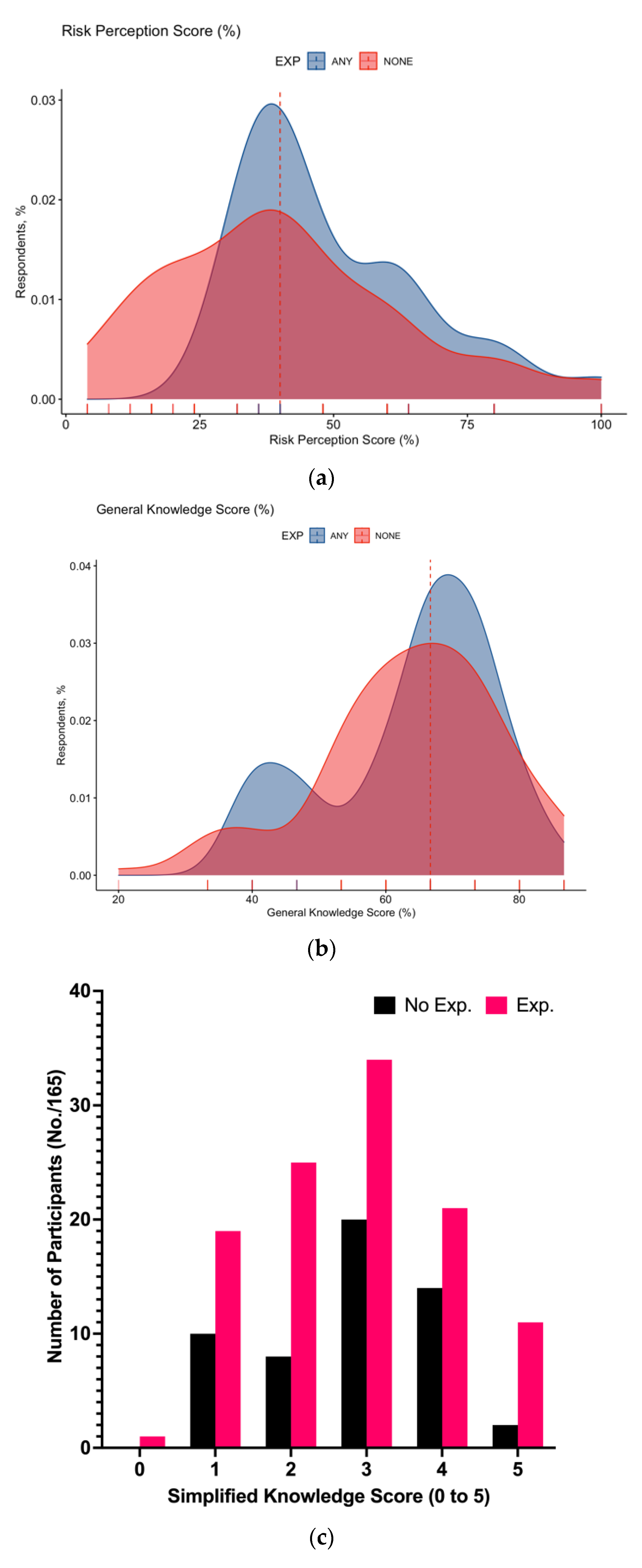
3.2. Knowledge Status
3.3. Risk Perception
3.4. Practices
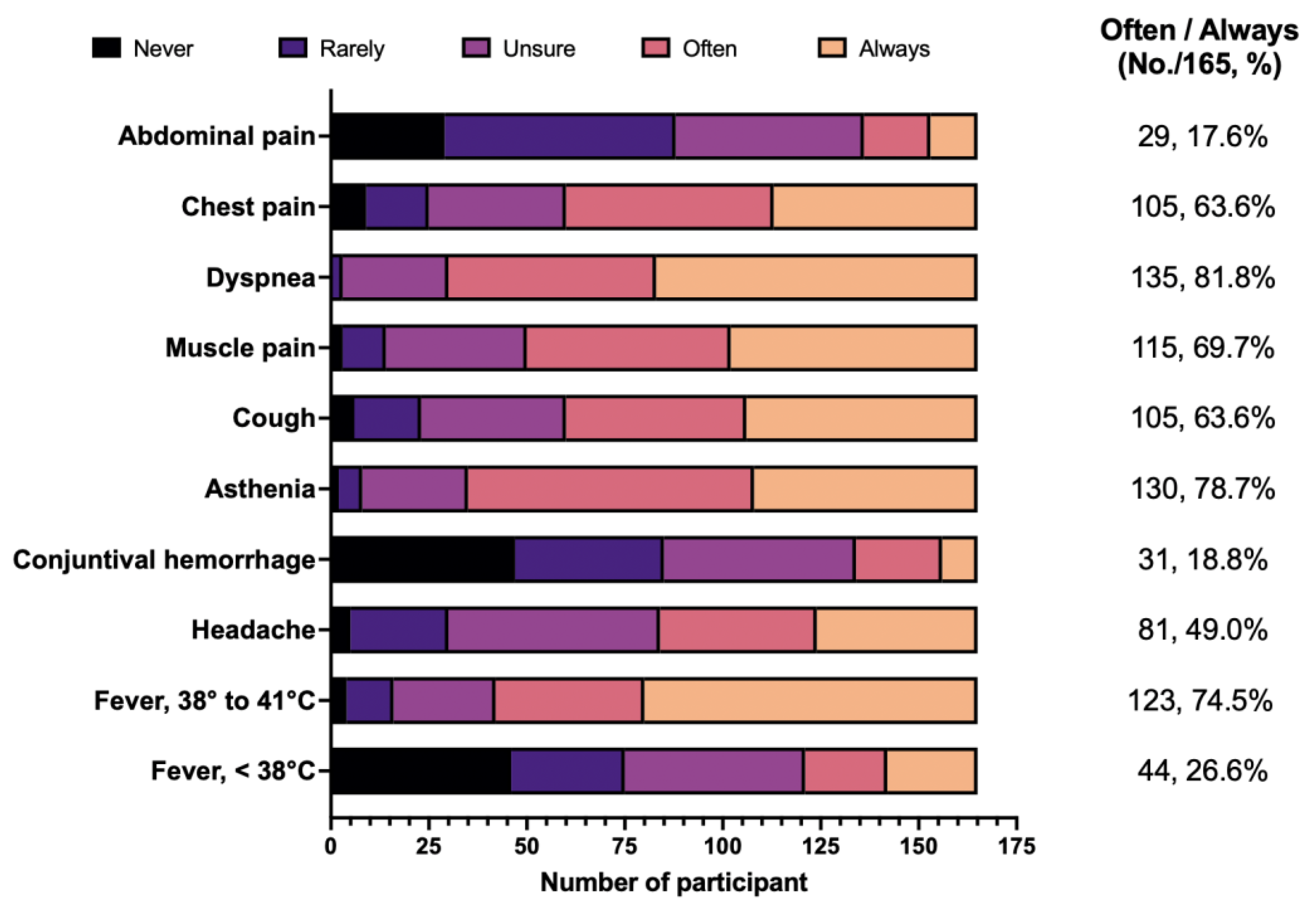
3.5. Attitudes
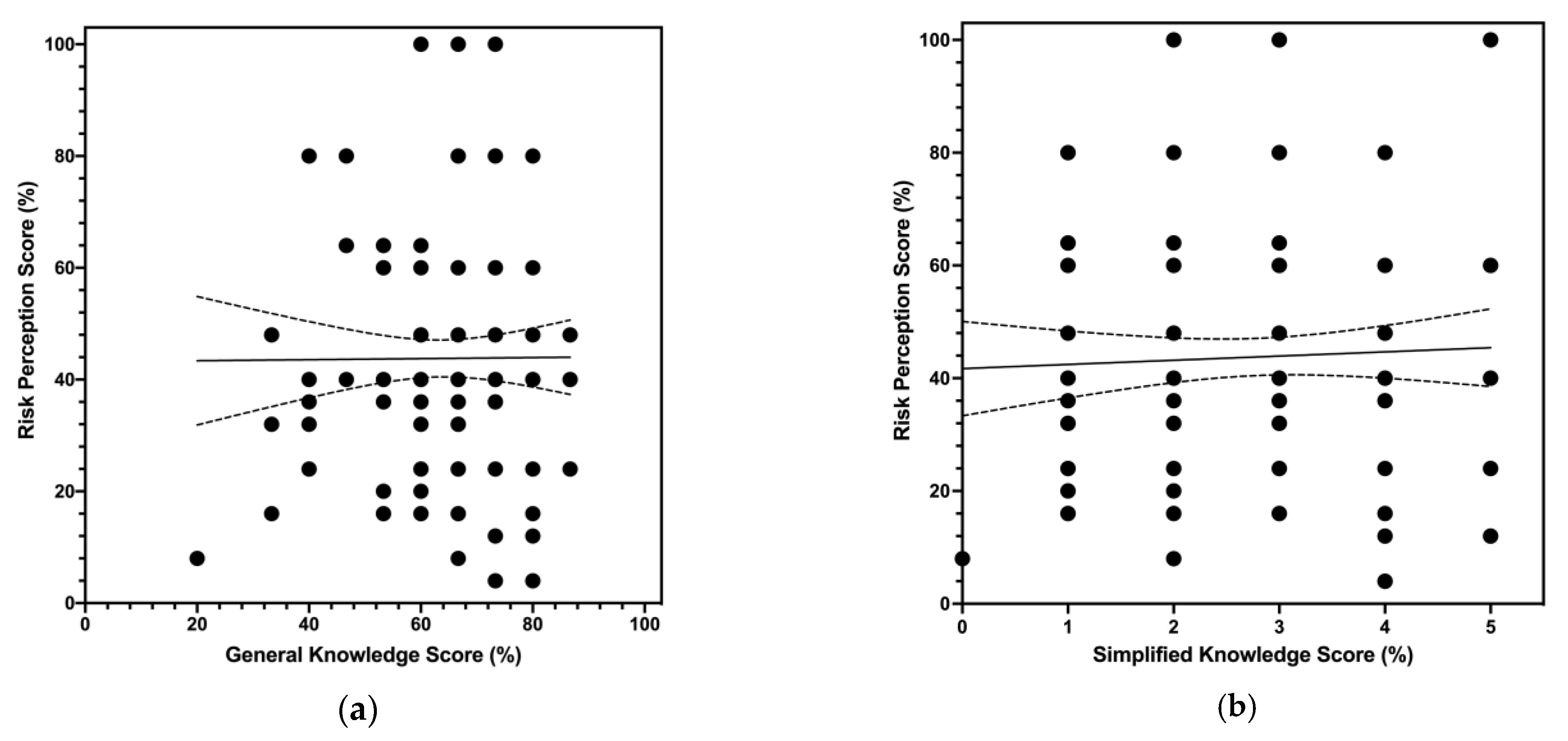
3.6. Univariate Analysis
3.7. Multivariable Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Item No. | Recommendation | Page | |
|---|---|---|---|
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract | 1 |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found | 1 | ||
| Introduction | |||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | 1–2 |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses | 2 |
| Methods | |||
| Study design | 4 | Present key elements of study design early in the paper | 3 |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection | 3 |
| Participants | 6 | (a) Give the eligibility criteria, and the sources and methods of selection of participants | 3–4 |
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable | 3–4 |
| Data sources/measurement | 8 | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group | 4 |
| Bias | 9 | Describe any efforts to address potential sources of bias | 3–4 |
| Study size | 10 | Explain how the study size was arrived at | 3 |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | 4 |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding | 4 |
| (b) Describe any methods used to examine subgroups and interactions | 4 | ||
| (c) Explain how missing data were addressed | 4 | ||
| (d) If applicable, describe analytical methods taking account of sampling strategy | 4 | ||
| (e) Describe any sensitivity analyses | - | ||
| Results | |||
| Participants | 13 * | (a) Report numbers of individuals at each stage of study—e.g., numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analyzed | 5 |
| (b) Give reasons for non-participation at each stage | 5 | ||
| (c) Consider use of a flow diagram | 5 | ||
| Descriptive data | 14 * | (a) Give characteristics of study participants (e.g., demographic, clinical, social) and information on exposures and potential confounders | 5–6 |
| (b) Indicate number of participants with missing data for each variable of interest | 5–6 | ||
| Outcome data | 15 * | Report numbers of outcome events or summary measures | 5–6 |
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (e.g., 95% confidence interval). Make clear which confounders were adjusted for and why they were included | 6–9 |
| (b) Report category boundaries when continuous variables were categorized | 9–12 | ||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | - | ||
| Other analyses | 17 | Report other analyses done—e.g., analyses of subgroups and interactions, and sensitivity analyses | - |
| Discussion | |||
| Key results | 18 | Summarize key results with reference to study objectives | 12–13 |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias | 15–16 |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | 13–15 |
| Generalizability | 21 | Discuss the generalizability (external validity) of the study results | 15 |
| Other information | |||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | - |
| Esteemed participant, the present survey has been developed and shared with the aim to assess the knowledge of occupational physicians on the topic of Legionnaires’ disease and its prevention in occupational settings. The present survey has only scientific aims. No economic or similar compensation is guaranteed to the participants. |
| While we thank you for your cooperation, we stress that web-based surveys must fulfill the requirements represented by the “Helsinki protocol” and EU Regulation 2016/679. |
| In order to fulfill the requirements of the Helsinki protocol, we’re requesting to formally share your consent. Without your consent, the survey will not continue. Even after your consent, you can leave the present survey at any moment, until the sharing of the questionnaire (button “share module” at the end of the questionnaire. Moreover, we stress that the questionnaire will be registered in anonymous form, and in no way can it be associated with the compiler, as we will not retain any specific, individual information (e.g., signature, personal address, etc.). All requested personal data are generic and functional to the demographic analyses (gender, age, etc.). |
| According to the EU Regulation 2016/279 (GDPR), we also state that: |
|
| DO YOU AGREE IN PARTICIPATING IN THE PRESENT SURVEY? (YES) (NO) |
| Section 1. Your Personal Experience with LD Infections during your clinical practice | |
| Have you previously participated in the risk assessment for LD in any of the enterprises you assist as an occupational physician? | [YES] [NO] [NO ANSWER] |
| Have you previously promoted any preventive measures for LD in any of the enterprises you assist as an occupational physician?? | [YES] [NO] [NO ANSWER] |
| Have you previously managed any case of LD among assisted workers? | [YES] [NO] [NO ANSWER] |
| To your knowledge, has any case of LD occurred among your friends/relatives? | [YES] [NO] [NO ANSWER] |
| Section 2. To your knowledge (please mark the correct answer) | |
| Q1. LD typically has inter-human spreading | [TRUE ] [FALSE] [DON’T KNOW] |
| Q2. Immunocompromised patients are at higher risk for developing LD | [TRUE ] [FALSE] [DON’T KNOW] |
| Q3. Legionella pneumophila is rare in the environment | [TRUE ] [FALSE] [DON’T KNOW] |
| Q4. Legionella pneumophila optimal growth occurs between 32 and 40 °C | [TRUE ] [FALSE] [DON’T KNOW] |
| Q5. Legionella pneumophila replicates between 20 and 50 °C | [TRUE ] [FALSE] [DON’T KNOW] |
| Q6. Legionella strains able to cause Pontiac Fever cause Legionellosis | [TRUE ] [FALSE] [DON’T KNOW] |
| Q7. Legionellosis is a vaccine-preventable disease | [TRUE ] [FALSE] [DON’T KNOW] |
| Q8. Incubation for legionellosis ranges between 2 and 10 days | [TRUE ] [FALSE] [DON’T KNOW] |
| Q9. Macrolides and Quinolones can be used in cases of suspected LD | [TRUE ] [FALSE] [DON’T KNOW] |
| Q10. LD occurs in less than 5% of all patients exposed to waters contaminated by Legionella | |
| Q11. LD must be officially reported to the Local Health Unit | [TRUE ] [FALSE] [DON’T KNOW] |
| Q12. LD is a notifiable disease to international authorities | [TRUE ] [FALSE] [DON’T KNOW] |
| Q13. LD follows ingestion of contaminated water | [TRUE ] [FALSE] [DON’T KNOW] |
| Q14. Case fatality for Legionellosis is | |
| < 1% | [ ] |
| between 1 and 5% | [ ] |
| between 5 and 10% | [ ] |
| between 10 and 15% | [ ] |
| > 15% | [ ] |
| Q15. Every year … are reported in Italy | |
| less than 100 cases | [ ] |
| 100 to 200 cases | [ ] |
| 200 to 500 cases | [ ] |
| 500 to 1000 cases | [ ] |
| over 1000 cases | [ ] |
| 3. According to your current understanding, in occupational settings, LD can be defined | |
| in terms of its occurrence, as a disease that is |
|
| in terms of its severity, as a disease that is |
|
| 4. From your current understanding, how would you rate the reliability of the following diagnostic options for LD (1 = totally unsatisfying; 5 = totally satisfying) | |
| Broncho-alveolar lavage (BAL) | (1)(2)(3)(4)(5) |
| Urinary Antigen for Legionella | (1)(2)(3)(4)(5) |
| Chest CT scans | (1)(2)(3)(4)(5) |
| Chest X ray studies | (1)(2)(3)(4)(5) |
| Clinical examination | (1)(2)(3)(4)(5) |
| 5. From your current understanding, how would you rate the occurrence of the following clinical features during LD? (1 = never; 2 = rarely; 3 = unsure; 4 = often; 5 = always) | |
| Fever, < 38 °C | (1)(2)(3)(4)(5) |
| Fever, 38 to 41 °C | (1)(2)(3)(4)(5) |
| Headache | (1)(2)(3)(4)(5) |
| Conjunctival hemorrhage | (1)(2)(3)(4)(5) |
| Asthenia | (1)(2)(3)(4)(5) |
| Cough | (1)(2)(3)(4)(5) |
| Muscle pain | (1)(2)(3)(4)(5) |
| Dyspnea | (1)(2)(3)(4)(5) |
| Chest pain | (1)(2)(3)(4)(5) |
| Abdominal pain | (1)(2)(3)(4)(5) |
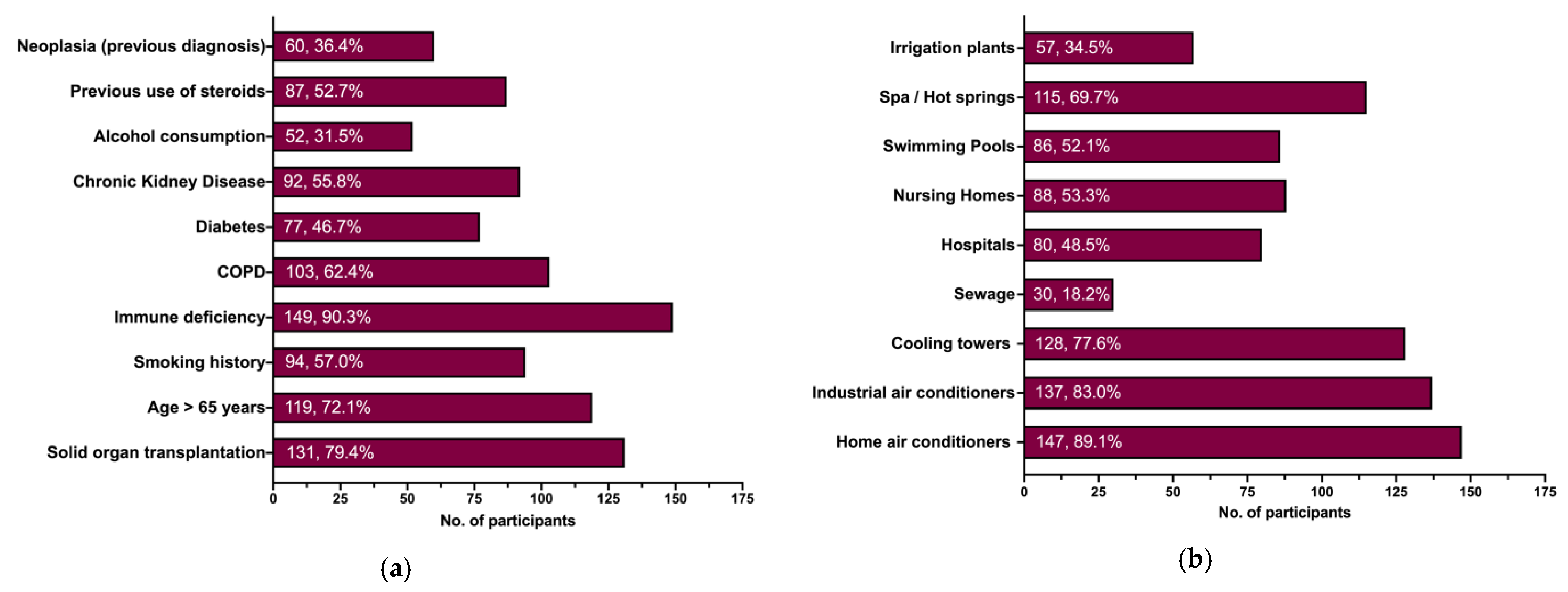
| 6. From your current understanding of LD, which one(s) of the following individual factors increase(s) the risk of developing LD? | |
| Solid organ transplantation | [ ] |
| Age > 65 years | [ ] |
| Smoking history | [ ] |
| Immune deficiency | [ ] |
| COPD | [ ] |
| Diabetes | [ ] |
| Chronic Kidney Disease | [ ] |
| Alcohol consumption | [ ] |
| Previous use of steroids | [ ] |
| Neoplasia (previous diagnosis) | [ ] |
| 7. From your current understanding of LD, which one(s) of the following environmental factors increase(s) the risk of developing LD? | |
| Home air conditioners | [ ] |
| Industrial air conditioners | [ ] |
| Cooling towers | [ ] |
| Sewage | [ ] |
| Hospitals | [ ] |
| Nursing Homes | [ ] |
| Swimming Pools | [ ] |
| Spas/Hot springs | [ ] |
| Irrigation plants | [ ] |
| 8. In the case that an effective vaccine against WNV is made available, what amount would you suggest is as acceptable expense for the general population for a single shot? | |
| Not interested | [ ] |
| Free or < 10 €/shot | [ ] |
| 10–19 €/shot | [ ] |
| 20–29 €/shot | [ ] |
| 30–39 €/shot | [ ] |
| 40–49 €/shot | [ ] |
| 50–100 €/shot | [ ] |
| >100 €/shot | [ ] |
| 9. Please provide some general information about you: | |
| Year of birth | ______________ |
| Year of medical qualification | ______________ |
| You identify yourself as | [Male] [Female] [No Answer] |
| Do you work in hospital settings? | [yes] [no] [no answer] |
| Do you work as Occupational Physician for any hospital? | [yes] [no] [no answer] |
| Do you work as Occupational Physician for any nursing home? | [yes] [no] [no answer] |
| Do you work as Occupational Physician for any wastewater treatment plant? | [yes] [no] [no answer] |

References
- Graham, F.F.; Hales, S.; White, P.S.; Baker, M.G. Review Global Seroprevalence of Legionellosis—A Systematic Review and Meta-Analysis. Sci. Rep. 2020, 10, 7337. [Google Scholar] [CrossRef]
- Cunha, B.A.; Burillo, A.; Bouza, E. Legionnaires’ Disease. Lancet 2016, 387, 376–385. [Google Scholar] [CrossRef]
- Burillo, A.; Pedro-Botet, M.L.; Bouza, E. Microbiology and Epidemiology of Legionnaire’s Disease. Infect. Dis. Clin. North. Am. 2017, 31, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.W.; Tsai, T.R.; Orenstein, W.; Parkin, W.E.; Beecham, J.H.; Sharrar, R.G.; Harris, J.; Mallison, G.F.; Martin, S.M.; McDade, J.E.; et al. Legionnaires’ Disease. NEJM 1977, 297, 1189–1197. [Google Scholar] [CrossRef]
- Löf, E.; Chereau, F.; Jureen, P.; Andersson, S.; Rizzardi, K.; Edquist, P.; Kühlmann-Berenzon, S.; Galanis, I.; Schönning, C.; Kais, M.; et al. An Outbreak Investigation of Legionella Non-Pneumophila Legionnaires’ Disease in Sweden, April to August 2018: Gardening and Use of Commercial Bagged Soil Associated with Infections. Eurosurveillance 2021, 26, 1900702. [Google Scholar] [CrossRef] [PubMed]
- Faccini, M.; Russo, A.G.; Bonini, M.; Tunesi, S.; Murtas, R.; Sandrini, M.; Senatore, S.; Lamberti, A.; Ciconali, G.; Cammarata, S.; et al. Large community-acquired Legionnaires’ disease outbreak caused by Legionella pneumophila serogroup 1, Italy, July to August 2018. Eurosurveillance 2020, 25, 1900523. [Google Scholar] [CrossRef] [PubMed]
- Scaturro, M.; Rota, M.C.; Caporali, M.G.; Girolamo, A.; Magoni, M.; Barberis, D.; Romano, C.; Cereda, D.; Gramegna, M.; Piro, A.; et al. A Community-Acquired Legionnaires’ Disease Outbreak Caused by Legionella Pneumophila Serogroup 2: An Uncommon Event, Italy, August to October 2018. Eurosurveillance 2021, 26, 2001961. [Google Scholar] [CrossRef]
- Doublet, P.; Khodr, A.; Kay, E.; Gomez-Valero, L.; Jarraud, S.; Buchrieser, C.; Ginevra, C. Molecular Epidemiology, Phylogeny and Evolution of Legionella. Infect. Genet. Evol. 2016, 43, 108–122. [Google Scholar] [CrossRef]
- Riccò, M.; Peruzzi, S.; Ranzieri, S.; Giuri, P.G. Epidemiology of Legionnaires’ Disease in Italy, 2004–2019: A Summary of Available Evidence. Microorganisms 2021, 9, 2180. [Google Scholar] [CrossRef]
- Riccò, M. Impact of Lockdown and Non-Pharmaceutical Interventions on the Epidemiology of Legionnaires’ Disease. Acta Biomed. 2022, 93, e2022090. [Google Scholar]
- Rota, M.C.; Caporali, M.G.; Giannitelli, S.; Urciuoli, R.; Scaturro, M.; Ricci, M.L. La Sorveglianza Nazionale Della Legionellosi: Risultati Relativi All’anno 2022. Boll. Epidemiol. Naz. 2023, 4, 25–32. [Google Scholar] [CrossRef]
- Magira, E.E.; Zakynthinos, S. Legionnaire’s Disease and Influenza. Infect. Dis. Clin. North. Am. 2017, 31, 137–153. [Google Scholar] [CrossRef]
- Lanternier, F.; Ader, F.; Pilmis, B.; Catherinot, E.; Jarraud, S.; Lortholary, O. Legionnaire’s Disease in Compromised Hosts. Infect. Dis. Clin. North. Am. 2017, 31, 123–135. [Google Scholar] [CrossRef]
- Hamilton, K.A.; Prussin, A.J.; Ahmed, W.; Haas, C.N. Outbreaks of Legionnaires’ Disease and Pontiac Fever 2006–2017. Curr Environ Health Rep 2018, 5, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, I.; Simoes, L.C.; Simoes, M. Legionella Pneumophila. Trends Microbiol. 2021, 29, 860–861. [Google Scholar] [CrossRef] [PubMed]
- Herwaldt, L.A.; Marra, A.R. Legionella: A Reemerging Pathogen. Curr. Opin. Infect. Dis. 2018, 31, 325–333. [Google Scholar] [CrossRef]
- Burstein, D.; Zusman, T.; Degtyar, E.; Viner, R.; Segal, G.; Pupko, T. Legionella Pneumophila. Emerg. Infect. Dis. 2009, 23, 1924–1925. [Google Scholar] [CrossRef]
- Felice, A.; Franchi, M.; De Martin, S.; Vitacolonna, N.; Iacumin, L.; Civilini, M. Environmental Surveillance and Spatio-Temporal Analysis of Legionella Spp. In a Region of Northeastern Italy (2002–2017). PLoS ONE 2019, 14, e0218687. [Google Scholar] [CrossRef]
- Rota, M.C.; Caporali, M.G.; Bella, A.; Ricci, M.L.; Napoli, C. Legionnaires’ Disease in Italy: Results of the Epidemiological Surveillance from 2000 to 2011. Eurosurveillance 2013, 18, 20497. [Google Scholar] [CrossRef] [PubMed]
- Principe, L.; Tomao, P.; Visca, P. Legionellosis in the Occupational Setting. Environ. Res. 2017, 152, 485–495. [Google Scholar] [CrossRef]
- Domingo-Pueyo, A.; Sanz-Valero, J.; Wanden-Berghe, C. Occupational Legionella in Adults over 18 Years Pf Age: A Systematic Review. Ciência Saúde Coletiva 2019, 24, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Rudbeck, M.; Viskum, S.; Mølbak, K.; Uldum, S.A. Legionella Antibodies in a Danish Hospital Staff with Known Occupational Exposure. J. Environ. Public Health 2009, 2009, 812829. [Google Scholar] [CrossRef]
- Petti, S.; Vitali, M. Occupational Risk for Legionella Infection among Dental Healthcare Workers: Meta-Analysis in Occupational Epidemiology. BMJ Open 2017, 7, e015374. [Google Scholar] [CrossRef]
- Tedjaseputra, A.; Manzoor, M.; Dendle, C.; Kanellis, J. Occupational Legionella Pneumophila Exposure in a Street Sweeper with a Renal Transplant. Nephrology 2018, 23, 493–495. [Google Scholar] [CrossRef]
- Ricci, M.L.; Fontana, S.; Bella, A.; Gaggioli, A.; Cascella, R.; Cassone, A.; Scaturro, M. A Preliminary Assessment of the Occupational Risk of Acquiring Legionnaires ’ Disease for People Working in Telephone Manholes, a New Workplace Environment for Legionella Growth. Am. J. Infect. Control. 2010, 38, 540–545. [Google Scholar] [CrossRef]
- Riccò, M.; Ferraro, P.; Peruzzi, S.; Zaniboni, A.; Ranzieri, S. SARS-CoV-2–Legionella Co-Infections: A Systematic Review and Meta-Analysis (2020–2021). Microorganisms 2022, 10, 499. [Google Scholar] [CrossRef] [PubMed]
- Betsch, C.; Wicker, S. Personal Attitudes and Misconceptions, Not Official Recommendations Guide Occupational Physicians’ Vaccination Decisions. Vaccine 2014, 32, 4478–4484. [Google Scholar] [CrossRef]
- Riccò, M.; Cattani, S.; Casagranda, F.; Gualerzi, G.; Signorelli, C. Knowledge, Attitudes, Beliefs and Practices of Occupational Physicians towards Vaccinations of Health Care Workers: A Cross Sectional Pilot Study in North-Eastern Italy. Int. J. Occup. Med. Environ. Health 2017, 30, 775–790. [Google Scholar] [CrossRef]
- Di Prinzio, R.R.; Nigri, A.G.; Zaffina, S. Total Worker Heath Strategies in Italy: New Challenges and Opportunities for Occupational Health and Safety Practice. J. Health Soc. Sci. 2021, 6, 313–318. [Google Scholar]
- Riccò, M.; Gualerzi, G.; Ranzieri, S.; Ferraro, P.; Bragazzi, N.L. Knowledge, Attitudes, Practices (KAP) of Italian Occupational Physicians towards Tick Borne Encephalitis. Trop. Med. Infect. Dis. 2020, 5, 117. [Google Scholar] [CrossRef]
- Zingg, A.; Siegrist, M. Measuring People’s Knowledge about Vaccination: Developing a One-Dimensional Scale. Vaccine 2012, 30, 3771–3777. [Google Scholar] [CrossRef]
- Cronbach, L.J. Coefficient Alpha and the Internal Structure of Tests. Psychometrika 1951, 16, 297–332. [Google Scholar] [CrossRef]
- Yates, F.J.; Stone, E.R. The Risk Construct. In Risk-Taking Behaviour; Yates, F.J., Ed.; John Wiley & Sons.: Chichester, UK, 1992; pp. 1–25. ISBN 0471922501. [Google Scholar]
- Ricci, M.L.; Grottola, A.; Serpini, G.F.; Bella, A.; Rota, M.C.; Frascaro, F.; Pegoraro, E.; Meacci, M.; Fabio, A.; Vecchi, E.; et al. Improvement of Legionnaires’ Disease Diagnosis Using Real-Time PCR Assay: A Retrospective Analysis, Italy, 2010 to 2015. Eurosurveillance 2018, 23, 1800032. [Google Scholar] [CrossRef]
- Scaturro, M.; Fontana, S.; Crippa, S.; Caporali, M.G.; Seyler, T.; Veschetti, E.; Villa, G.; Rota, M.C.; Ricci, M.L. An Unusually Long-Lasting Outbreak of Community-Acquired Legionnaires’ Disease, 2005–2008, Italy. Epidemiol. Infect. 2015, 143, 2416–2425. [Google Scholar] [CrossRef]
- Ministero della Salute. Legionellosi Controllo e Prevenzione Nelle Strutture Turistico-Ricettive. In Proceedings of the Permanent Conference for Relations between the State and Regions; Italian Health Ministry (Ministero della Salute): Rome, Italy, 2015. [Google Scholar]
- Ministero della Salute. Linee Guida per La Prevenzione e Il Controllo Della Legionellosi. In Proceedings of the Permanent Conference for Relations between the State and Regions; Italian Health Ministry (Ministero della Salute): Rome, Italy, 2015. [Google Scholar]
- Palazzolo, C.; Maffongelli, G.; D’Abrano, A.; Lepore, L.; Mariano, A.; Vulcano, A.; Ascoli Bartoli, T.; Bevilacqua, N.; Giancola, M.L.; Di Rosa, E.; et al. Legionella Pneumonia: Increased Risk after COVID-19 Lockdown? Italy, May to June 2020. Eurosurveillance 2020, 25, 2001372. [Google Scholar] [CrossRef]
- Rota, M.C.; Caporali, M.G.; Bella, A.; Scaturro, M.; Giannitelli, S.; Ricci, M.L. Il Sistema Di Sorveglianza Della Legionellosi in Italia: I Risultati Del 2019. Boll. Epidemiol. Naz. 2020, 1, 32–38. [Google Scholar]
- Rota, M.C.; Caporali, M.G.; Bella, A.; Scaturro, M.; Giannitelli, S.; Ricci, M.L. I Risultati Del Sistema Di Sorveglianza Della Legionellosi in Italia Nel 2020 Durante La Pandemia Di COVID-19. Boll. Epidemiol. Naz. 2021, 2, 9–16. [Google Scholar]
- Bushuven, S.; Juenger, J.; Moeltner, A.; Dettenkofer, M. Overconfidence in Infection Control Proficiency. Am. J. Infect. Control. 2019, 47, 545–550. [Google Scholar] [CrossRef]
- Hochlehnert, A.; Brass, K.; Moeltner, A.; Juenger, J. Does Medical Students’ Preference of Test Format (Computer-Based vs. Paper-Based) Have an Influence on Performance? BMC Med. Educ. 2011, 11, 89. [Google Scholar] [CrossRef]
- Walker, J.T. The Influence of Climate Change on Waterborne Disease and Legionella: A Review. Perspect. Public. Health 2018, 138, 282–286. [Google Scholar] [CrossRef]
- Rota, M.C.; Caporali, M.G.; Scaturro, M.; Girolamo, A.; Andrianou, X.; Ricci, M.L. Legionella Pneumophila and SARS-COV-2 Co-Infection: The Importance of Laboratory Diagnosis. Ann. Ist. Super. Sanita 2021, 57, 199–200. [Google Scholar] [CrossRef]
- Borella, P.; Bargellini, A.; Marchesi, I.; Rovesti, S.; Stancanelli, G.; Triassi, M.; Montegrosso, S.; Pennino, F.; Zotti, C.M. Prevalence of Anti-Legionella Antibodies among Italian Hospital Workers. J. Hosp. Infect. 2008, 69, 148–155. [Google Scholar] [CrossRef]
- Beauté, J. Legionnaires’ Disease in Europe, 2011 to 2015. Eurosurveillance 2017, 22, 30566. [Google Scholar] [CrossRef]
- de Jong, B.; Hallström, L.P. European Surveillance of Legionnaires’ Disease. Curr. Issues Mol. Biol. 2021, 42, 81–96. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Legionnaires’ Disease in Europe, 2013 Surveillance Report; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2017.
- European Centre for Disease Prevention and Control (ECDC). Legionnaires’ Disease—Annual Epidemiological Report for 2019; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2021.
- European Centre for Disease Prevention and Control (ECDC). Legionnaires’ Disease—Annual Epidemiological Report for 2018; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2020.
- European Centre for Disease Prevention and Control. Legionnaires’ Disease—Surveillance Atlas for Infectious Diseases; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2021.
- De Giglio, O.; Fasano, F.; Diella, G.; Lopuzzo, M.; Napoli, C.; Apollonio, F.; Brigida, S.; Calia, C.; Campanale, C.; Marzella, A.; et al. Legionella and Legionellosis in Touristic-Recreational Facilities: Influence of Climate Factors and Geostatistical Analysis in Southern Italy (2001–2017). Environ. Res. 2019, 178, 108721. [Google Scholar] [CrossRef]
- Buchholz, U.; Altmann, D.; Brodhun, B. Differential Seasonality of Legionnaires’ Disease by Exposure Category. Int. J. Environ. Res. Public. Health 2020, 17, 3049. [Google Scholar] [CrossRef]
- Larsen, T.M.; Endo, B.H.; Yee, A.T.; Do, T.; Lo, S.M. Probing Internal Assumptions of the Revised Bloom’s Taxonomy. CBE Life Sci. Educ. 2022, 21, 4. [Google Scholar] [CrossRef]
- Stringer, J.K.; Santen, S.A.; Lee, E.; Rawls, M.; Bailey, J.; Richards, A.; Perera, R.A.; Biskobing, D. Examining Bloom’s Taxonomy in Multiple Choice Questions: Students’ Approach to Questions. Med. Sci. Educ. 2021, 31, 1311–1317. [Google Scholar] [CrossRef]
- Ricco, M.; Gualerzi, G.; Ranzieri, S.; Ferraro, P. West Nile Virus Infection: Before Involving Occupational Physicians in Active Surveillance, Make Sure They Are More Aware. Infect. Dis. Now. 2021, 51, 569–572. [Google Scholar] [CrossRef]
- Chirico, F.; Magnavita, N. Commentary West Nile Virus Infection in Europe: Need for an Integration of Occupational Health Practice and Public Health Activities. Ann. Ist. Super. Sanita 2019, 55, 3–5. [Google Scholar]
- Moßhammer, D.; Michaelis, M.; Mehne, J.; Wilm, S.; Rieger, M.A. General Practitioners’ and Occupational Health Physicians’ Views on Their Cooperation: A Cross-Sectional Postal Survey. Int. Arch. Occup. Environ. Health 2016, 89, 449–459. [Google Scholar] [CrossRef]
- Ricco, M.; Gualerzi, G.; Ranzieri, S. Personal Beliefs and Misconceptions, Not Evidence Guide General Practitioners in the Managing of Travelers’ Diarrhea: Results from a Pilot Study (North-Western Italy, 2019). Med. Mal. Infect. 2020, 51, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Manzoli, L.; Sotgiu, G.; Magnavita, N.; Durando, P.; Barchitta, M.; Carducci, A.; Conversano, M.; De Pasquale, G.; Dini, G.; Firenze, A.; et al. Evidence-Based Approach for Continuous Improvement of Occupational Health. Epidemiol. Prev. 2015, 39, 81–85. [Google Scholar]
- Abbritti, G.; Apostoli, P.; Iavicoli, S.; Murgia, N.; Persechino, B.; Soleo, L.; Ambrosi, L. Needs, Education and Accreditation in Occupational Medicine in Italy. Int. Arch. Occup. Environ. Health 2005, 78, 75–78. [Google Scholar] [CrossRef]
- Bibby, K.J.; Orkis, L.T.; Harrison, L.H.; Mertz, K.J.; Stout, J.E.; Brooks, M.M. Environmental Sources of Community-Acquired Legionnaires’ Disease: A Review. Int. J. Hyg. Environ. Health 2018, 221, 764–774. [Google Scholar] [CrossRef]
- French National Academy of Medicine. The Occupational Physician, a Key Role in COVID-19 Risk Management in Enterprises. Bull. Acad. Natl. Med. 2020, 204, e132–e133. [Google Scholar]
- Spagnolo, L.; Vimercati, L.; Caputi, A.; Benevento, M.; De Maria, L.; Ferorelli, D.; Solarino, B. Role and Tasks of the Occupational Physician during the COVID-19 Pandemic. Medicina 2021, 57, 479. [Google Scholar] [CrossRef]
- Yoshida, G.J. Legionnaires ’ Disease as an Occupational Risk Related to Decontamination Work after the Fukushima Nuclear Disaster: A Case Report. J. Occup. Health 2018, 60, 525–526. [Google Scholar] [CrossRef]
- Riccò, M.; Ferraro, P.; Camisa, V.; Satta, E.; Zaniboni, A.; Ranzieri, S.; Baldassarre, A.; Zaffina, S.; Marchesi, F. When a Neglected Tropical Disease Goes Global: Knowledge, Attitudes and Practices of Italian Physicians towards Monkeypox, Preliminary Results. Trop. Med. Infect. Dis. 2022, 7, 135. [Google Scholar] [CrossRef]
- Riccò, M.; Ferraro, P.; Peruzzi, S.; Balzarini, F.; Ranzieri, S. Mandate or Not Mandate: Knowledge, Attitudes, and Practices of Italian Occupational Physicians towards SARS-CoV-2 Immunization at the Beginning of Vaccination Campaign. Vaccines 2021, 9, 889. [Google Scholar] [CrossRef]
- Betsch, C.; Schmid, P.; Heinemeier, D.; Korn, L.; Holtmann, C.; Böhm, R. Beyond Confidence: Development of a Measure Assessing the 5C Psychological Antecedents of Vaccination. PLoS ONE 2018, 13, e0208601. [Google Scholar] [CrossRef] [PubMed]
- La Vecchia, C.; Alicandro, G.; Negri, E.; Scarpino, V.; Coggiola, M.; Spatari, G. Attitudes towards COVID-19 Vaccination and Containment Measures in Italy and the Role of Occupational Physicians. Med. Del Lav. 2022, 113, e2022018. [Google Scholar] [CrossRef]
- Kirupakaran, J.; Meloche, C.; Upfal, M. Practices and Attitudes of Michigan-Based Occupational Physicians Regarding Adult Immunization. J. Occup. Environ. Med. 2018, 60, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Valente, M.; Marchesi, F. Are Symptoms Associated with SARS-CoV-2 Infections Evolving over Time? Infect. Dis. Now. 2022, 52, 110–112. [Google Scholar] [CrossRef]
- Cervellin, G.; Comelli, I.; Lippi, G. Is Google Trends a Reliable Tool for Digital Epidemiology? Insights from Different Clinical Settings. J. Epidemiol. Glob. Health 2017, 7, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Rovetta, A. Reliability of Google Trends: Analysis of the Limits and Potential of Web Infoveillance During COVID-19 Pandemic and for Future Research. Front. Res. Metr. Anal. 2021, 6, 670226. [Google Scholar] [CrossRef]
- San Martin Rodriguez, M.; Kaier, K.; Hehn, M.; Borde, J.P. Knowledge, habits and attitudes towards TBE and other tick-borne diseases in German forestry trainees. Ticks Tick Borne Dis. 2020, 11, 101307. [Google Scholar] [CrossRef]
- Riccò, M.; Vezzosi, L.; Gualerzi, G.; Bragazzi, N.L.; Balzarini, F. Pertussis immunization in healthcare workers working in pediatric settings: Knowledge, Attitudes and Practices (KAP) of Occupational Physicians. Preliminary results from a web-based survey (2017). J. Prev. Med. Hyg 2020, 61, E66–E75. [Google Scholar] [CrossRef]
- Riccò, M.; Zaniboni, A.; Satta, E.; Baldassarre, A.; Cerviere, M.P.; Marchesi, F.; Peruzzi, S. Management and Prevention of Traveler’s Diarrhea: A Cross-Sectional Study on Knowledge, Attitudes, and Practices in Italian Occupational Physicians (2019 and 2022). Trop. Med. Infect. Dis. 2022, 7, 370. [Google Scholar] [CrossRef]
- Corace, K.M.; Srigley, J.A.; Hargadon, D.P.; Yu, D.; MacDonald, T.K.; Fabrigar, L.R.; Garber, G.E. Using Behavior Change Frameworks to Improve Healthcare Worker Influenza Vaccination Rates: A Systematic Review. Vaccine 2016, 34, 3235–3242. [Google Scholar] [CrossRef]
- Palacios-Ceña, D.; Talavera, B.; Gómez-Mayordomo, V.; Garcia-Azorin, D.; Gallego-Gallego, M.; Cuadrado, M.L.; Guerrero-Peral, Á.L. Understanding the Diagnoses and Medical Care Experience of Patients with New Daily Persistent Headache: A Qualitative Study in Spain. BMJ Open 2021, 11, e048552. [Google Scholar] [CrossRef]
- Mitchell, K.C.; Ryan, P.; Howard, D.E.; Feldman, K.A. Understanding Knowledge, Attitudes, and Behaviors Toward West Nile Virus Prevention: A Survey of High-Risk Adults in Maryland. Vector-Borne Zoonotic Dis. 2018, 18, 173–180. [Google Scholar] [CrossRef]
- Riccò, M.; Cattani, S.; Casagranda, F.; Gualerzi, G.; Signorelli, C. Knowledge, Attitudes, Beliefs and Practices of Occupational Physicians towards Seasonal Influenza Vaccination: A Cross-Sectional Study from North-Eastern Italy. J. Prev. Med. Hyg. 2017, 58, E141–E154. [Google Scholar]
- Riccò, M.; Vezzosi, L.; Balzarini, F. Challenges Faced by the Italian Medical Workforce. Lancet 2020, 395, e55–e56. [Google Scholar] [CrossRef]
- Betsch, C.; Wicker, S. E-health use, vaccination knowledge and perception of own risk: Drivers of vaccination uptake in medical students. Vaccine 2012, 30, 1143–1148. [Google Scholar] [CrossRef]
- Krebs, R. The Swiss Way to Score Multiple True-False Items: Theoretical and Empirical Evidence. In Advances in Medical Education; Scherpbier, A.J.J.A., Van der Vleuten, C., Rethans, J., van der Steeg, A., Eds.; Springer Science + Business: Dordrecht, The Netherlands, 1997; pp. 158–161. [Google Scholar]
- Cassell, K.; Davis, J.L.; Berkelman, R. Legionnaires’ Disease in the Time of COVID-19. Pneumonia 2021, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Swanson, C.S.; Wang, L.; He, Q. Impact of Building Closures during the COVID-19 Pandemic on Legionella Infection Risks. Am. J. Infect. Control. 2021, 49, 1564–1566. [Google Scholar] [CrossRef] [PubMed]
- Allam, C.; Gaymard, A.; Descours, G.; Ginevra, C.; Josset, L.; Bouscambert, M.; Beraud, L.; Ibranosyan, M.; Golfier, C.; Friggeri, A.; et al. Co-Infection with Legionella and SARS-CoV-2, France, March 200. Emerg. Infect. Dis. 2021, 27, 2864–2868. [Google Scholar] [CrossRef] [PubMed]
- Verhasselt, H.L.; Buer, J.; Dedy, J.; Ziegler, R.; Steinmann, J.; Herbstreit, F.; Brenner, T.; Rath, P.M. COVID-19 Co-Infection with Legionella Pneumophila in 2 Tertiary-Care Hospitals, Germany. Emerg. Infect. Dis. 2021, 27, 1535–1537. [Google Scholar] [CrossRef]
- Chirico, F.; Sacco, A.; Bragazzi, N.L.; Magnavita, N. Can Air-Conditioning Systems Contribute to the Spread of SARS / MERS / COVID-19 Infection ? Insights from a Rapid Review of the Literature. Int. J. Environ. Res. Public. Health 2020, 17, 6052. [Google Scholar] [CrossRef] [PubMed]
- Flaxman, S.; Mishra, S.; Gandy, A.; Unwin, H.J.T.; Mellan, T.A.; Coupland, H.; Whittaker, C.; Zhu, H.; Berah, T.; Eaton, J.W.; et al. Estimating the Effects of Non-Pharmaceutical Interventions on COVID-19 in Europe. Nature 2020, 584, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Müller, O.; Razum, O.; Jahn, A. Effects of Non-Pharmaceutical Interventions against COVID-19 on the Incidence of Other Diseases. Lancet Reg. Health 2021, 6, 100139. [Google Scholar] [CrossRef] [PubMed]
| General Characteristics of the Sample | No./165, % | Average ± S.D. |
|---|---|---|
| Age | 48.3 ± 10.8 | |
| ≥50 years | 50, 30.3% | |
| Gender (No., %) | ||
| Male | 95, 57.6% | |
| Female | 64, 38.8% | |
| Undisclosed | 6, 3.6% | |
| Seniority | 21.1 ± 11.3 | |
| ≥10 years (No., %) | 144, 87.3% | |
| Any practice as Occupational Physician in | ||
| Hospitals | 44, 26.7% | |
| Nursing homes | 70, 42.4% | |
| Wastewater treatment plants | 44, 26.7% | |
| Participation in the risk assessment for LD | 69, 41.8% | |
| Promotion of any preventive measure for LD | 31, 18.8% | |
| Case of LD among assisted workers | 46, 27.9% | |
| Case of LD among friends/relatives | 15, 9.1% | |
| Previous experience with LD | 54, 32.7% | |
| General Knowledge Score | 63.7% ± 13.2 | |
| >median (66.7%) | 58, 35.2% | |
| Simplified Knowledge Score 4 to 5 | 48, 29.1% | |
| Risk Perception | ||
| Acknowledging LD as a frequent issue in occupational settings | 27, 16.4% | |
| Acknowledging LD as a severe issue in occupational settings | 126, 76.4% | |
| Risk Perception Score | 43.8% ± 21.4 | |
| >median (40.0%) | 66, 40.0% | |
| Confidence in being able to recognize an LD case | 81, 39.1% |
| Statement | Answer | No./165, % | Correlation with Higher GKS (rho) |
|---|---|---|---|
| Q1. LD typically has inter-human spreading | FALSE | 84, 79.2% | −0.043 |
| Q2. Immunocompromised patients are at higher risk for developing LD | TRUE | 97, 81.5% | 0.233 |
| Q3. Legionella pneumophila is rare in the environment | FALSE | 67, 63.2% | 0.241 |
| Q4. Legionella pneumophila optimal growth occurs between 32 and 40 °C | TRUE | 84, 79.2% | 0.357 (+) |
| Q5. Legionella pneumophila replicates between 20 and 50 °C | TRUE | 76, 71.7% | 0.231 |
| Q6. Legionella strains able to cause Pontiac Fever cause Legionellosis | TRUE | 67, 63.2% | 0.443 (+) |
| Q7. Legionellosis is a vaccine-preventable disease | FALSE | 101, 95.3% | −0.049 |
| Q8. Incubation for legionellosis ranges between 2 and 10 days | TRUE | 72, 67.9% | 0.301 (+) |
| Q9. Macrolides and Quinolones can be used in cases of suspected LD | TRUE | 76, 71.7% | 0.243 |
| Q10. LD occurs in less than 5% of all patients exposed to waters contaminated by Legionella | TRUE | 67, 63.2% | 0.291 |
| Q11. LD must be officially reported to the Local Health Unit | TRUE | 101, 95.3% | 0.166 |
| Q12. LD is a notifiable disease to international authorities | TRUE | 55, 51.9% | 0.393 (+) |
| Q13. LD follows ingestion of contaminated water | FALSE | 85, 80.2% | 0.053 |
| Q14. Case fatality for Legionellosis is | 0.114 | ||
| <1% | FALSE | 9, 8.5% | |
| between 1 and 5% | FALSE | 38, 35.8% | |
| between 5 and 10% | TRUE | 28, 26.4% | |
| between 10 and 15% | FALSE | 21, 19.8% | |
| >15% | FALSE | 10, 9.4% | |
| Q15. Every year … are reported in Italy | 0.394 (+) | ||
| less than 100 cases | FALSE | 13, 12.3% | |
| 100 to 200 cases | FALSE | 26, 24.5% | |
| 200 to 500 cases | FALSE | 21, 19.8% | |
| 500 to 1000 cases | FALSE | 13, 12.3% | |
| over 1000 cases | TRUE | 21, 19.8% |
| General Characteristics of the Sample | Participation in the Risk Assessment for LD | Promotion of any Preventive Measures for LD | ||||
|---|---|---|---|---|---|---|
| Ever (No./69, %) | Never (No./96, %) | Chi- Squared Test p Value | Ever (No./31, %) | Never (No./134, %) | Chi- Squared Test p Value | |
| Age ≥ 50 years | 24, 34.8% | 26, 27.1% | 0.374 | 10, 32.3% | 40, 29.9% | 0.963 |
| Male Gender | 36, 52.2% | 59, 61.5% | 0.303 | 26, 83.9% | 69, 51.5% | 0.002 |
| Seniority ≥ 10 years | 63, 91.3% | 81, 84.4% | 0.280 | 31, 100% | 113, 84.3% | 0.059 |
| Any practice as Occupational Physician in | ||||||
| Hospitals | 35, 50.7% | 9, 9.4% | < 0.001 | 19, 61.3% | 25, 18.7% | < 0.001 |
| Nursing homes | 51, 73.9% | 19.8% | < 0.001 | 21, 67.7% | 49, 36.6% | 0.003 |
| Wastewater treatment plants | 36, 52.2% | 8, 8.3% | < 0.001 | 17, 54.8% | 27, 20.1% | < 0.001 |
| Case of LD among assisted workers | 21, 30.4% | 25, 26.0% | 0.656 | 8, 25.8% | 38, 28.4% | 0.950 |
| Case of LD among friends/relatives | 3, 4.3% | 12, 12.5% | 0.128 | 0, - | 15, 11.2% | 0.108 |
| Previous experience with LD | 21, 30.4% | 33, 34.4% | 0.716 | 8, 25.8% | 46, 34.3% | 0.485 |
| Participation in the risk assessment for LD | - | - | - | 21, 67.7% | 48, 35.8% | 0.002 |
| Promotion of preventive measures for LD | 21, 30.4% | 10, 10.4% | 0.002 | - | - | - |
| Simplified Knowledge Score 4 to 5 | 28, 40.6% | 20, 20.8% | 0.010 | 13, 41.9% | 35, 26.1% | 0.127 |
| Risk Perception > median (40.0%) | 30, 43.5% | 36, 37.5% | 0.540 | 13, 41.9% | 53, 39.6% | 0.968 |
| Confidence in being able to recognize an LD case | 35, 50.7% | 46, 47.9% | 0.843 | 13, 41.9% | 68, 50.7% | 0.493 |
| Participation in the Risk Assessment for LD (aOR, 95%CI) | Promotion of any Preventive Measure for LD (aOR, 95%CI) | |
|---|---|---|
| Practice as Occupational Physician in | ||
| Hospitals | 2.850 (0.936; 8.676) | 6.792 (2.026; 22.764) |
| Nursing homes | 8.732 (2.991;25.487) | 0.902 (0.257; 3.164) |
| Wastewater treatment plants | 8.710 (2.844; 26.668) | 4.464 (1.363; 14.619) |
| Participation in the risk assessment for LD | - | 1.368 (0.401; 4.663) |
| Promotion of any preventive measure for LD | 1.495 (0.453; 4.929) | - |
| Simplified Knowledge Score 4 to 5 | 2.152 (0.847; 5.468) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riccò, M.; Ferraro, P.; Ranzieri, S.; Boldini, G.; Zanella, I.; Marchesi, F. Legionnaires’ Disease in Occupational Settings: A Cross-Sectional Study from Northeastern Italy (2019). Trop. Med. Infect. Dis. 2023, 8, 364. https://doi.org/10.3390/tropicalmed8070364
Riccò M, Ferraro P, Ranzieri S, Boldini G, Zanella I, Marchesi F. Legionnaires’ Disease in Occupational Settings: A Cross-Sectional Study from Northeastern Italy (2019). Tropical Medicine and Infectious Disease. 2023; 8(7):364. https://doi.org/10.3390/tropicalmed8070364
Chicago/Turabian StyleRiccò, Matteo, Pietro Ferraro, Silvia Ranzieri, Giorgia Boldini, Ilaria Zanella, and Federico Marchesi. 2023. "Legionnaires’ Disease in Occupational Settings: A Cross-Sectional Study from Northeastern Italy (2019)" Tropical Medicine and Infectious Disease 8, no. 7: 364. https://doi.org/10.3390/tropicalmed8070364
APA StyleRiccò, M., Ferraro, P., Ranzieri, S., Boldini, G., Zanella, I., & Marchesi, F. (2023). Legionnaires’ Disease in Occupational Settings: A Cross-Sectional Study from Northeastern Italy (2019). Tropical Medicine and Infectious Disease, 8(7), 364. https://doi.org/10.3390/tropicalmed8070364









