Modelling Differential Diagnosis of Febrile Diseases with Fuzzy Cognitive Map
Abstract
1. Introduction
2. Related Literature
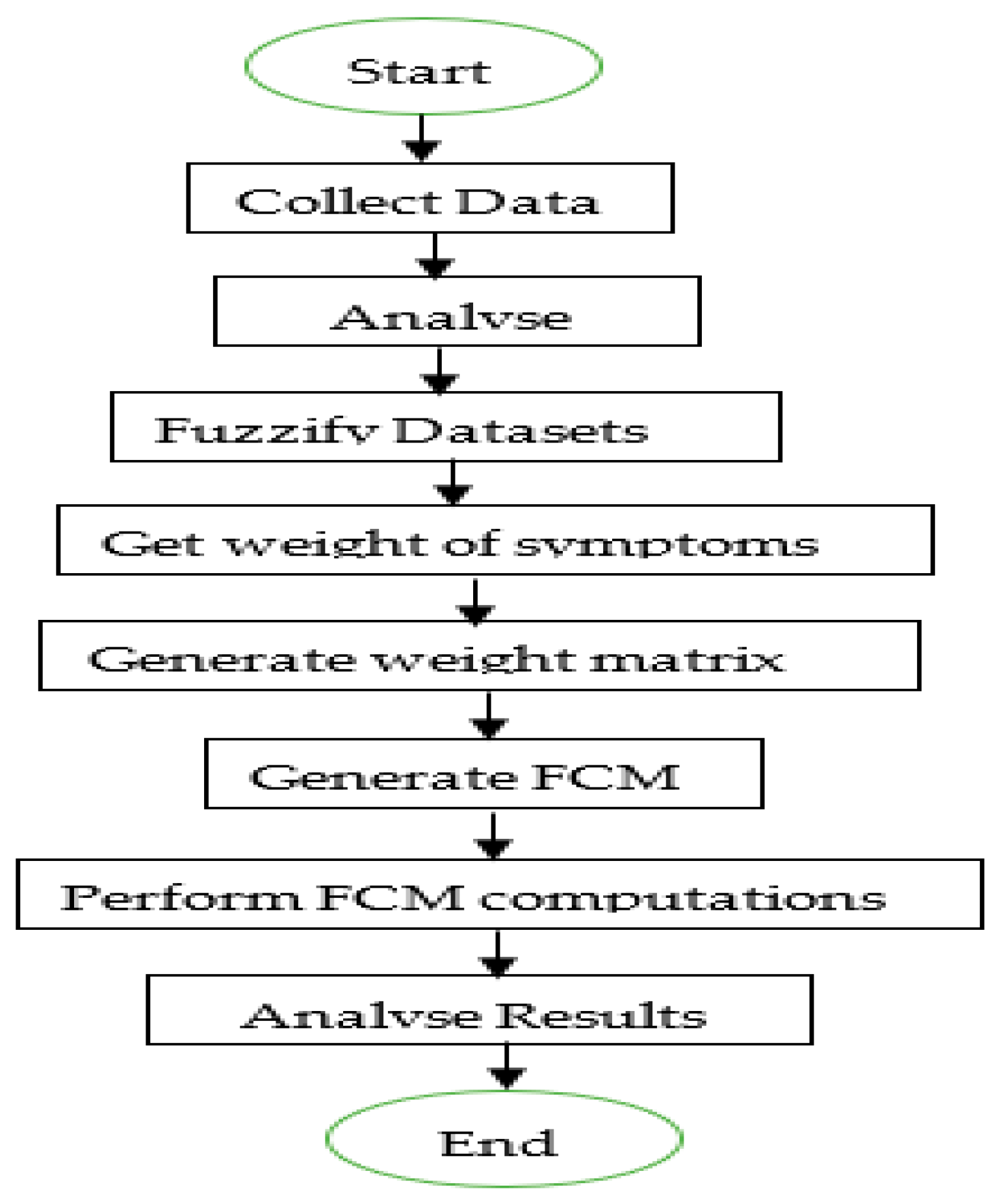
3. Methodology
3.1. Data Collection
3.2. Fuzzification of Datasets
3.3. Weight of Symptoms
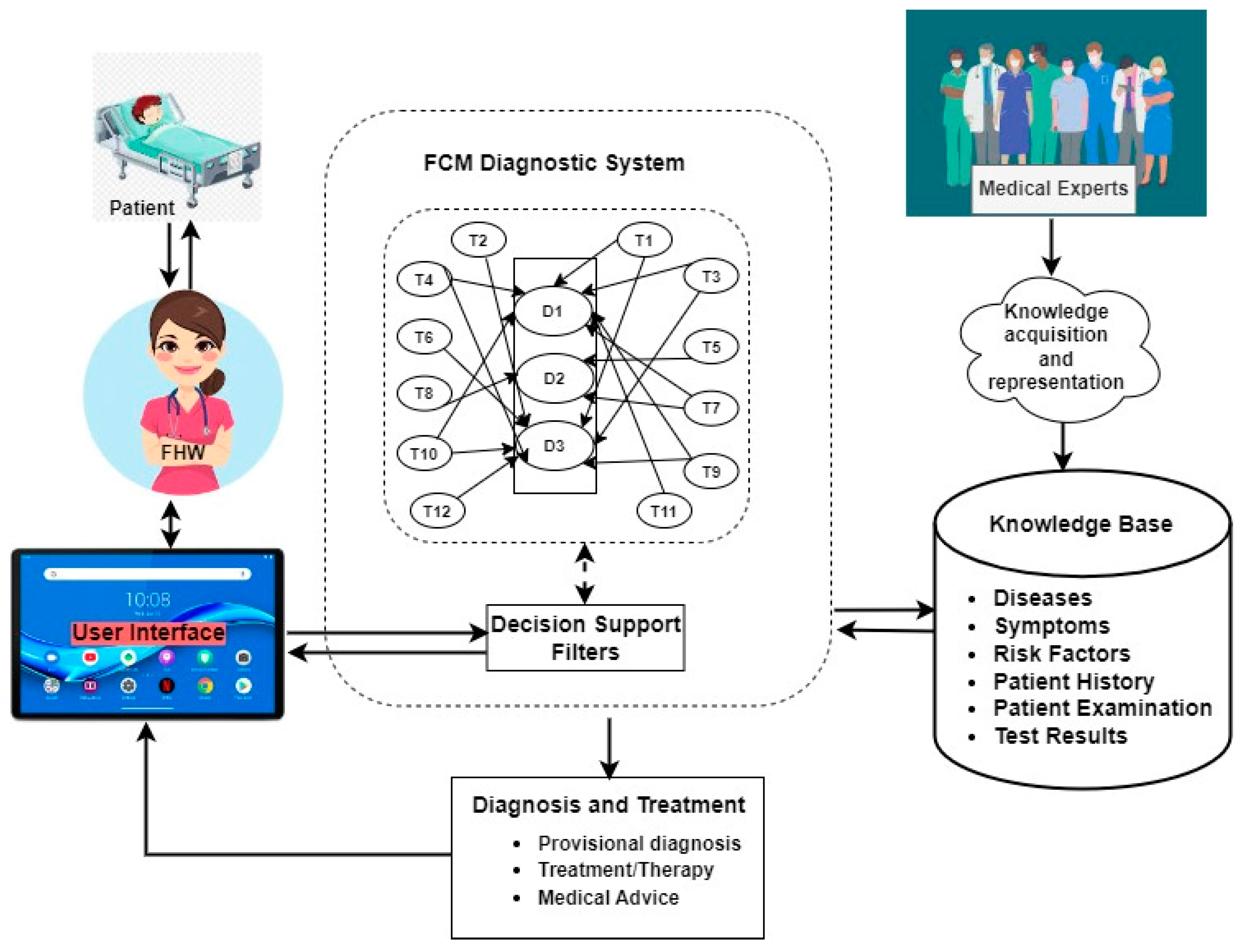
3.4. The System Architecture
3.5. FCM Computations
4. Results and Discussion
4.1. Results
4.2. Discussion
4.3. The Implication of the Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Symptom Rankings for Diseases
| SN | ENFVR Symptoms | Pearson Rank | Kendall Rank | Spearman Rank | Mean |
|---|---|---|---|---|---|
| 1 | ABDPN | 0.249 | 0.225 | 0.250 | 0.24 |
| 2 | SWRFVR | 0.227 | 0.217 | 0.236 | 0.23 |
| 3 | HDACH | 0.212 | 0.196 | 0.224 | 0.21 |
| 4 | DIZ | 0.186 | 0.206 | 0.226 | 0.21 |
| 5 | NUS | 0.185 | 0.192 | 0.211 | 0.20 |
| 6 | DRH | 0.201 | 0.178 | 0.191 | 0.19 |
| 7 | LTG | 0.167 | 0.158 | 0.174 | 0.17 |
| 8 | CNST | 0.151 | 0.167 | 0.178 | 0.17 |
| 9 | INTBLEPRF | 0.145 | 0.138 | 0.147 | 0.14 |
| 10 | PERTN | 0.125 | 0.128 | 0.136 | 0.13 |
| SN | HVAD Symptoms | Pearson Rank | Kendall Rank | Spearman Rank | Mean |
|---|---|---|---|---|---|
| 1 | GENRSH | 0.419 | 0.430 | 0.443 | 0.43 |
| 2 | SKNRSH | 0.417 | 0.418 | 0.432 | 0.42 |
| 3 | MUTUCR | 0.383 | 0.330 | 0.341 | 0.35 |
| 4 | LMPNDSWL | 0.402 | 0.320 | 0.331 | 0.35 |
| 5 | DRH | 0.299 | 0.276 | 0.287 | 0.29 |
| 6 | BDYICH | 0.232 | 0.246 | 0.256 | 0.24 |
| 7 | NGTSWT | 0.239 | 0.196 | 0.207 | 0.21 |
| 8 | SRTRT | 0.192 | 0.210 | 0.218 | 0.21 |
| 9 | FOLBRT | 0.204 | 0.188 | 0.195 | 0.20 |
| 10 | CGHDRY | 0.195 | 0.169 | 0.180 | 0.18 |
| 11 | DRYCGH | 0.160 | 0.142 | 0.150 | 0.15 |
| 12 | DRYCGH | 0.146 | 0.133 | 0.141 | 0.14 |
| 13 | LTG | 0.121 | 0.087 | 0.093 | 0.10 |
| 14 | DIZ | 0.099 | 0.073 | 0.078 | 0.08 |
| 15 | LWGDFVR | 0.079 | 0.081 | 0.088 | 0.08 |
| SN | UPUTI Symptoms | Pearson Rank | Kendall Rank | Spearman Rank | Mean |
|---|---|---|---|---|---|
| 1 | PNFLURNTN | 0.553 | 0.503 | 0.529 | 0.53 |
| 2 | URNFQC | 0.553 | 0.487 | 0.514 | 0.52 |
| 3 | SPPBPN | 0.509 | 0.479 | 0.506 | 0.50 |
| 4 | CLDYURN | 0.508 | 0.443 | 0.465 | 0.47 |
| 5 | BLDYURN | 0.244 | 0.238 | 0.247 | 0.24 |
| 6 | ABDPN | 0.216 | 0.222 | 0.243 | 0.23 |
| 7 | UPBCKPN | 0.196 | 0.211 | 0.223 | 0.21 |
| 8 | BCKPN | 0.177 | 0.189 | 0.203 | 0.19 |
| 9 | NUS | 0.144 | 0.151 | 0.163 | 0.15 |
| 10 | SUDONFVR | 0.124 | 0.125 | 0.135 | 0.13 |
| 11 | HGPSFVR | 0.127 | 0.120 | 0.131 | 0.13 |
| SN | LWUTI Symptoms | Pearson Rank | Kendall Rank | Spearman Rank | Mean |
|---|---|---|---|---|---|
| 1 | URNFQC | 0.592 | 0.551 | 0.584 | 0.58 |
| 2 | PNFLURNTN | 0.589 | 0.554 | 0.584 | 0.58 |
| 3 | SPPBPN | 0.528 | 0.529 | 0.562 | 0.54 |
| 4 | CLDYURN | 0.487 | 0.465 | 0.493 | 0.48 |
| 5 | BLDYURN | 0.268 | 0.263 | 0.277 | 0.27 |
| 6 | ABDPN | 0.212 | 0.225 | 0.249 | 0.23 |
| 7 | UPBCKPN | 0.194 | 0.195 | 0.207 | 0.20 |
| 8 | BCKPN | 0.120 | 0.140 | 0.152 | 0.14 |
| 9 | LWGDFVR | 0.106 | 0.102 | 0.113 | 0.11 |
| SN | URTI Symptoms | Pearson Rank | Kendall Rank | Spearman Rank | Mean |
|---|---|---|---|---|---|
| 1 | CTRH | 0.447 | 0.403 | 0.429 | 0.43 |
| 2 | CGHDRY | 0.407 | 0.414 | 0.444 | 0.42 |
| 3 | SRTRT | 0.314 | 0.327 | 0.346 | 0.33 |
| 4 | DIFBRT | 0.302 | 0.318 | 0.338 | 0.32 |
| 5 | DRYCGH | 0.314 | 0.300 | 0.322 | 0.31 |
| 6 | DRYCGH | 0.311 | 0.296 | 0.317 | 0.31 |
| 7 | FOLBRT | 0.222 | 0.246 | 0.260 | 0.24 |
| 8 | MUTUCR | 0.118 | 0.163 | 0.172 | 0.15 |
| 9 | FTG | 0.112 | 0.120 | 0.135 | 0.12 |
| 10 | HDACH | 0.102 | 0.105 | 0.120 | 0.11 |
| 11 | LWGDFVR | 0.057 | 0.076 | 0.083 | 0.07 |
| SN | LRTI Symptoms | Pearson Rank | Kendall Rank | Spearman Rank | Mean |
|---|---|---|---|---|---|
| 1 | CGHDRY | 0.524 | 0.480 | 0.513 | 0.51 |
| 2 | DIFBRT | 0.429 | 0.435 | 0.461 | 0.44 |
| 3 | CHSPN | 0.411 | 0.410 | 0.438 | 0.42 |
| 4 | WHZ | 0.339 | 0.340 | 0.355 | 0.34 |
| 5 | CHSIND | 0.321 | 0.329 | 0.344 | 0.33 |
| 6 | DRYCGH | 0.282 | 0.293 | 0.313 | 0.30 |
| 7 | DRYCGH | 0.278 | 0.282 | 0.301 | 0.29 |
| 8 | LMPNDSWL | 0.229 | 0.259 | 0.272 | 0.25 |
| 9 | FOLBRT | 0.235 | 0.254 | 0.268 | 0.25 |
| 10 | NGTSWT | 0.241 | 0.247 | 0.263 | 0.25 |
| 11 | SRTRT | 0.219 | 0.254 | 0.268 | 0.25 |
| 12 | CTRH | 0.209 | 0.219 | 0.235 | 0.22 |
| 13 | MUTUCR | 0.177 | 0.213 | 0.223 | 0.20 |
| 14 | LWGDFVR | 0.078 | 0.099 | 0.108 | 0.09 |
| SN | TB Symptoms | Pearson Rank | Kendall Rank | Spearman Rank | Mean |
|---|---|---|---|---|---|
| 1 | CGHDRY | 0.481 | 0.419 | 0.443 | 0.45 |
| 2 | NGTSWT | 0.503 | 0.392 | 0.409 | 0.43 |
| 3 | CHSPN | 0.390 | 0.373 | 0.391 | 0.38 |
| 4 | DIFBRT | 0.310 | 0.334 | 0.348 | 0.33 |
| 5 | LMPNDSWL | 0.345 | 0.315 | 0.327 | 0.33 |
| 6 | MUTUCR | 0.269 | 0.271 | 0.279 | 0.27 |
| 7 | FOLBRT | 0.275 | 0.261 | 0.270 | 0.27 |
| 8 | CHSIND | 0.251 | 0.266 | 0.273 | 0.26 |
| 9 | WHZ | 0.244 | 0.265 | 0.272 | 0.26 |
| 10 | SRTRT | 0.215 | 0.233 | 0.243 | 0.23 |
| 11 | DRYCGH | 0.233 | 0.221 | 0.233 | 0.23 |
| 12 | DRYCGH | 0.223 | 0.214 | 0.226 | 0.22 |
| 13 | LWGDFVR | 0.218 | 0.185 | 0.200 | 0.20 |
| SN | LASFVR Symptoms | Pearson Rank | Kendall Rank | Spearman Rank | Mean |
|---|---|---|---|---|---|
| 1 | REDEYE | 0.216 | 0.181 | 0.182 | 0.19 |
| 2 | REDEYEFCTNG | 0.247 | 0.165 | 0.166 | 0.19 |
| 3 | SENLHT | 0.201 | 0.099 | 0.100 | 0.13 |
| 4 | PNBHEYE | 0.150 | 0.106 | 0.107 | 0.12 |
| 5 | JNTSWL | 0.113 | 0.114 | 0.115 | 0.11 |
| 6 | PERTN | 0.120 | 0.107 | 0.108 | 0.11 |
| 7 | BLDYURN | 0.089 | 0.118 | 0.119 | 0.11 |
| 8 | SHK | 0.131 | 0.087 | 0.087 | 0.10 |
| 9 | SRTRT | 0.111 | 0.082 | 0.084 | 0.09 |
| 10 | UPBCKPN | 0.070 | 0.089 | 0.091 | 0.08 |
| 11 | SUDONFVR | 0.087 | 0.076 | 0.081 | 0.08 |
| 12 | BLDN | 0.086 | 0.076 | 0.076 | 0.08 |
| SN | YELFVR Symptoms | Pearson Rank | Kendall Rank | Spearman Rank | Mean |
|---|---|---|---|---|---|
| 1 | PERTN | 0.103 | 0.093 | 0.094 | 0.10 |
| 2 | JNTSWL | 0.072 | 0.081 | 0.082 | 0.08 |
| 3 | DRH | 0.051 | 0.080 | 0.082 | 0.07 |
| 4 | BDYICH | 0.068 | 0.071 | 0.072 | 0.07 |
| 5 | SHK | 0.061 | 0.073 | 0.074 | 0.07 |
| 6 | LMPNDSWL | 0.062 | 0.071 | 0.072 | 0.07 |
| 7 | SENLHT | 0.093 | 0.052 | 0.053 | 0.07 |
| 8 | GENRSH | 0.073 | 0.062 | 0.063 | 0.07 |
| 9 | INTBLEPRF | 0.055 | 0.069 | 0.070 | 0.06 |
| 10 | BLDN | 0.063 | 0.063 | 0.064 | 0.06 |
| 11 | HGPSFVR | 0.038 | 0.068 | 0.072 | 0.06 |
| SN | DENFVR Symptoms | Pearson Rank | Kendall Rank | Spearman Rank | Mean |
|---|---|---|---|---|---|
| 1 | REDEYE | 0.054 | 0.130 | 0.131 | 0.10 |
| 2 | REDEYEFCTNG | 0.066 | 0.101 | 0.101 | 0.09 |
| 3 | HGGDFVR | 0.078 | 0.083 | 0.089 | 0.08 |
| 4 | UPBCKPN | 0.053 | 0.090 | 0.092 | 0.08 |
| 5 | SENLHT | 0.048 | 0.088 | 0.089 | 0.08 |
| 6 | SKNRSH | 0.055 | 0.079 | 0.080 | 0.07 |
| 7 | BCKPN | 0.053 | 0.077 | 0.081 | 0.07 |
| 8 | PNBHEYE | 0.059 | 0.075 | 0.076 | 0.07 |
| 9 | SHK | 0.072 | 0.069 | 0.070 | 0.07 |
| 10 | PERTN | 0.052 | 0.059 | 0.059 | 0.06 |
| 11 | LMPNDSWL | 0.047 | 0.060 | 0.062 | 0.06 |
| 12 | INTBLEPRF | 0.056 | 0.055 | 0.056 | 0.06 |
| 13 | DIZ | 0.040 | 0.061 | 0.065 | 0.06 |
| 14 | ABDPN | 0.047 | 0.056 | 0.060 | 0.05 |
| 15 | GENRSH | 0.031 | 0.065 | 0.066 | 0.05 |
| 16 | CHSPN | 0.038 | 0.059 | 0.061 | 0.05 |
| 17 | HGPSFVR | 0.025 | 0.062 | 0.066 | 0.05 |
| 18 | FTG | 0.037 | 0.047 | 0.052 | 0.05 |
| 19 | DIFBRT | 0.030 | 0.051 | 0.052 | 0.04 |
| 20 | MSCBDYPN | 0.042 | 0.043 | 0.046 | 0.04 |
| 21 | BLDYURN | 0.022 | 0.054 | 0.054 | 0.04 |
| 22 | GENBDYPN | 0.039 | 0.042 | 0.046 | 0.04 |
Appendix B. Symptoms, Diseases and Their Meaning
| Label | Symptom | Meaning | Label | Symptom | Meaning | Label | Symptom | Meaning |
|---|---|---|---|---|---|---|---|---|
| T1 | ABDPN | abdominal pain | T21 | SWRFVR | stepwise rise fever | T41 | REDEYE | Red eye |
| T2 | BCKPN | back pain | T22 | SUDONFVR | sudden onset fever | T42 | REDEYEFCTNG | red eye face and tongue |
| T3 | BITAIM | bitter test in mouth | T23 | LWGDFVR | low-grade fever | T43 | SENLHT | sensitivity to light |
| T4 | BLDN | bleeding from any sight | T24 | FOLBRT | foul breathe | T44 | SHK | shock |
| T5 | BLDYURN | bloody urine | T25 | BDYICH | body itching | T45 | SKNRSH | skin rash |
| T6 | CTRH | Catarrh | T26 | GENBDYPN | generalized body pain | T46 | SRTRT | sore throat |
| T7 | CHSIND | chest indraw | T27 | GENRSH | generalized rashes | T47 | SPPBPN | suprapubic pains |
| T8 | CHSPN | chest pain | T28 | HDACH | Headache | T48 | URNFQC | urinary frequency |
| T9 | CHLNRIG | chills and rigours | T29 | INTBLEPRF | intestinal bleeding and perforation | T49 | VMT | vomiting |
| T10 | CLDYURN | cloudy urine | T30 | JNTSWL | joint swelling | T50 | WHZ | wheeze |
| T11 | CNST | constipation | T31 | LTG | lethargy | |||
| T12 | CGHDRY | cough initial dry | T32 | LMPNDSWL | lymph node swelling | D1 | MAL | malaria |
| T13 | DRH | diarrhoea | T33 | MSCBDYPN | muscle and body pain | D2 | ENFVR | enteric ever |
| T14 | DIFBRT | difficulty breathing | T34 | MUTUCR | mouth ulcer | D3 | HVAD | HIV/AIDS |
| T15 | DIZ | dizziness | T35 | NUS | nausea | D4 | UPUTI | upper urinary-tract infection |
| T16 | DRYCGH | dry cough | T36 | NGTSWT | night sweats | D5 | LWUTI | lower urinary-tract infection |
| T17 | FTG | Fatigue | T37 | PNBHEYE | pain behind eye | D6 | URTI | upper respiratory-tract infection |
| T18 | FVR | Fever | T38 | UPBCKPN | upper back pain | D7 | LRTI | lower respiratory-tract infection |
| T19 | HGPSFVR | high persistent fever | T39 | PNFLURNTN | painful urination | D8 | TB | tuberculosis |
| T20 | HGGDFVR | high grade fever | T40 | PERTN | peritonitis | D9 | LASFVR | Laser Fever |
| D10 | YELFVR | Yellow Fever | ||||||
| D11 | DENFVR | Dengue Fever |
Appendix C. Sample Results
| Pat_No | Mal_Data | Diagnosis | FCM_Mal | Diagnosis | Ent_Data | Diagnosis | FCM_Ent | Diagnosis | HivAd_Data | Diagnosis | FCM_HivAd | Diagnosis | Uputi_data | Diagnosis | FCM_Uputi | Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1261 | 0.91 | MAL | 0.7888 | MAL | 0.08 | No | 0.701 | ENTFV | 0.08 | NO | 0.795 | No | 0.08 | No | 0.6082 | No |
| 1377 | 0.58 | MAL | 0.6727 | MAL | 0.08 | No | 0.3603 | No | 0.08 | NO | 0.4223 | No | 0.08 | No | 0.4782 | No |
| 1459 | 0.08 | No | 0.2832 | No | 0.08 | No | 0.3212 | No | 0.08 | NO | 0.4835 | No | 0.08 | No | 0.5132 | No |
| 1480 | 0.58 | MAL | 0.9987 | MAL | 0.58 | ENTFV | 0.5524 | ENTFV | 0.08 | NO | 0.3832 | No | 0.08 | No | 0.516 | No |
| 151 | 0.58 | MAL | 0.6151 | MAL | 0.08 | No | 0.4334 | No | 0.08 | NO | 0.3968 | No | 0.08 | No | 0.5173 | No |
| 1594 | 0.58 | MAL | 0.6419 | MAL | 0.08 | No | 0.3212 | No | 0.08 | NO | 0.3968 | No | 0.08 | No | 0.434 | No |
| 1728 | 0.58 | MAL | 0.7083 | MAL | 0.58 | ENTFV | 0.5474 | ENTFV | 0.58 | HVAD | 0.7915 | No | 0.08 | No | 0.5277 | No |
| 1902 | 0.75 | MAL | 0.9452 | MAL | 0.75 | ENTFV | 0.6157 | ENTFV | 0.08 | NO | 0.4162 | No | 0.08 | No | 0.5528 | No |
| 2019 | 0.08 | No | 0.3822 | No | 0.08 | No | 0.4004 | No | 0.08 | NO | 0.4138 | No | 0.08 | No | 0.6419 | No |
| 2054 | 0.58 | MAL | 0.6181 | MAL | 0.91 | ENTFV | 0.8359 | ENTFV | 0.08 | NO | 0.4699 | No | 0.08 | No | 0.5927 | No |
| 2055 | 0.75 | MAL | 0.7652 | MAL | 0.41 | ENTFV | 0.8877 | ENTFV | 0.08 | NO | 0.8364 | HVAD | 0.08 | No | 0.797 | No |
| 2106 | 0.58 | MAL | 0.6134 | MAL | 0.75 | ENTFV | 0.9595 | ENTFV | 0.08 | NO | 0.5586 | No | 0.41 | UPUTI | 0.978 | UPUTI |
| 2183 | 0.41 | MAL | 0.8928 | MAL | 0.41 | ENTFV | 0.6858 | ENTFV | 0.08 | NO | 0.8977 | HVAD | 0.08 | No | 0.6686 | No |
| 2365 | 0.25 | MAL | 0.4666 | No | 0.58 | ENTFV | 0.6031 | ENTFV | 0.08 | NO | 0.3968 | No | 0.08 | No | 0.5881 | No |
| 2417 | 0.08 | No | 0.6344 | MAL | 0.08 | No | 0.638 | ENTFV | 0.08 | NO | 0.4232 | No | 0.08 | No | 0.5226 | No |
| 2622 | 0.75 | MAL | 0.7044 | MAL | 0.75 | ENTFV | 0.6647 | ENTFV | 0.08 | NO | 0.86 | HVAD | 0.08 | No | 0.564 | No |
| 2846 | 0.75 | MAL | 0.6576 | MAL | 0.41 | ENTFV | 0.6022 | ENTFV | 0.08 | NO | 0.4597 | No | 0.08 | No | 0.4835 | No |
| 2958 | 0.75 | MAL | 0.8948 | MAL | 0.75 | ENTFV | 0.8347 | ENTFV | 0.08 | NO | 0.5859 | No | 0.08 | No | 0.7519 | No |
| 2983 | 0.08 | No | 0.4082 | No | 0.08 | No | 0.467 | No | 0.08 | NO | 0.4232 | No | 0.08 | No | 0.5054 | No |
| 3056 | 0.91 | MAL | 0.7035 | MAL | 0.08 | No | 0.3212 | No | 0.08 | NO | 0.5137 | No | 0.08 | No | 0.434 | No |
| 3237 | 0.41 | MAL | 0.7368 | MAL | 0.08 | No | 0.4719 | No | 0.08 | NO | 0.4667 | No | 0.08 | No | 0.5347 | No |
| 3499 | 0.75 | MAL | 0.7079 | MAL | 0.08 | No | 0.4466 | No | 0.08 | NO | 0.4519 | No | 0.08 | No | 0.434 | No |
| 350 | 0.58 | MAL | 0.6704 | MAL | 0.08 | No | 0.5976 | ENTFV | 0.08 | NO | 0.6519 | No | 0.25 | UPUTI | 0.969 | UPUTI |
| 3562 | 0.08 | No | 0.4119 | No | 0.08 | No | 0.4664 | No | 0.08 | NO | 0.3832 | No | 0.08 | No | 0.5594 | No |
| 3582 | 0.58 | MAL | 0.7343 | MAL | 0.58 | ENTFV | 0.5578 | ENTFV | 0.08 | NO | 0.4232 | No | 0.08 | No | 0.4769 | No |
| 3624 | 0.75 | MAL | 0.6654 | MAL | 0.91 | ENTFV | 0.797 | ENTFV | 0.08 | NO | 0.4718 | No | 0.08 | No | 0.8133 | UPUTI |
| 3683 | 0.41 | MAL | 0.6176 | MAL | 0.58 | ENTFV | 0.852 | ENTFV | 0.75 | HVAD | 0.9717 | HVAD | 0.58 | UPUTI | 0.9 | UPUTI |
| 3737 | 0.08 | No | 0.5208 | No | 0.08 | No | 0.4664 | No | 0.08 | NO | 0.3832 | No | 0.08 | No | 0.5594 | No |
| 3738 | 0.08 | No | 0.5208 | No | 0.08 | No | 0.4664 | No | 0.08 | NO | 0.3832 | No | 0.08 | No | 0.5594 | No |
| 3886 | 0.58 | MAL | 0.7871 | MAL | 0.58 | ENTFV | 0.4423 | No | 0.08 | NO | 0.3832 | No | 0.08 | No | 0.4769 | No |
| 3890 | 0.58 | MAL | 0.7079 | MAL | 0.58 | ENTFV | 0.5474 | ENTFV | 0.08 | NO | 0.5251 | No | 0.08 | No | 0.5693 | No |
| 4221 | 0.75 | MAL | 0.8449 | MAL | 0.08 | No | 0.4004 | No | 0.08 | NO | 0.4232 | No | 0.08 | No | 0.5099 | No |
| 4490 | 0.08 | No | 0.6315 | MAL | 0.08 | No | 0.4434 | No | 0.58 | HVAD | 0.8481 | HVAD | 0.08 | No | 0.434 | No |
| 4644 | 0.58 | MAL | 0.7216 | MAL | 0.58 | ENTFV | 0.6535 | ENTFV | 0.08 | NO | 0.3832 | No | 0.08 | No | 0.4835 | No |
| 4742 | 0.41 | MAL | 0.6811 | MAL | 0.08 | No | 0.3212 | No | 0.08 | NO | 0.3832 | No | 0.08 | No | 0.4769 | No UPUTI |
| 479 | 0.25 | MAL | 0.7379 | MAL | 0.58 | ENTFV | 0.5875 | ENTFV | 0.08 | NO | 0.4096 | No | 0.08 | No | 0.4769 | No |
| 797 | 0.08 | No | 0.5769 | No | 0.75 | ENTFV | 0.6548 | ENTFV | 0.08 | NO | 0.7837 | No | 0.08 | No | 0.7335 | No UPUTI |
| 802 | 0.08 | No | 0.5769 | No | 0.75 | ENTFV | 0.6548 | ENTFV | 0.08 | NO | 0.7837 | No | 0.08 | No | 0.7335 | No |
| 1224 | 0.91 | MAL | 0.7292 | MAL | 0.91 | ENTFV | 0.8821 | ENTFV | 0.08 | NO | 0.6275 | No | 0.08 | No | 0.8373 | UPUTI |
| 129 | 0.08 | No | 0.2832 | No | 0.08 | No | 0.3212 | No | 0.08 | NO | 0.3832 | No | 0.08 | No | 0.434 | No |
| 1364 | 0.08 | No | 0.5451 | No | 0.75 | ENTFV | 0.5888 | ENTFV | 0.75 | HVAD | 0.556 | No | 0.08 | No | 0.499 | No |
| 1374 | 0.08 | No | 0.6869 | MAL | 0.58 | ENTFV | 0.5507 | ENTFV | 0.58 | HVAD | 0.5179 | No | 0.08 | No | 0.4782 | No |
| 1584 | 0.08 | No | 0.2832 | No | 0.08 | No | 0.3212 | No | 0.08 | NO | 0.3832 | No | 0.08 | No | 0.434 | No |
| 174 | 0.58 | MAL | 0.6079 | MAL | 0.91 | ENTFV | 0.8052 | ENTFV | 0.08 | NO | 0.3968 | No | 0.08 | No | 0.5985 | No |
| 183 | 0.08 | No | 0..8079 | MAL | 0.08 | No | 0.8052 | ENTFV | 0.08 | NO | 0.3968 | No | 0.08 | No | 0.5985 | No |
| 2023 | 0.75 | MAL | 0.8929 | MAL | 0.08 | No | 0.3926 | No | 0.08 | NO | 0.4727 | No | 0.08 | No | 0.434 | No |
| 2024 | 0.75 | MAL | 0.7779 | MAL | 0.41 | ENTFV | 0.5887 | ENTFV | 0.08 | NO | 0.3968 | No | 0.58 | UPUTI | 0.782 | No UPUTI |
| 2045 | 0.91 | MAL | 0.8546 | MAL | 0.41 | ENTFV | 0.7918 | ENTFV | 0.08 | NO | 0.4426 | No | 0.08 | No | 0.7668 | No UPUTI |
| 2088 | 0.41 | MAL | 0.5897 | No | 0.08 | No | 0.4181 | No | 0.08 | NO | 0.5935 | No | 0.08 | No | 0.4731 | No UPUTI |
| Pat_No | Lwuti_Data | Diagnosis | FCM_Lwuti | Diagnosis | Urti_Data | Diagnosis | FCM_Urti | Diagnosis | Lrti_Data | Diagnosis | FCM_Lrti | Diagnosis | TB_Data | Diagnosis | FCM_TB | Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1261 | 0.08 | No | 0.40 | No | 0.91 | URTI | 0.892 | URTI | 0.08 | No | 0.9434 | LRTI | 0.08 | No TB | 0.7501 | TB |
| 1377 | 0.08 | No | 0.79 | No | 0.58 | URTI | 0.5492 | No | 0.08 | No | 0.5669 | No | 0.08 | No TB | 0.3843 | No TB |
| 1459 | 0.75 | LWUTI | 0.864 | LWUTI | 0.08 | No | 0.2505 | No | 0.08 | No | 0.462 | No | 0.08 | No TB | 0.3571 | No TB |
| 1480 | 0.08 | No | 0.51 | No | 0.08 | No | 0.2783 | No | 0.08 | No | 0.428 | No | 0.08 | No TB | 0.3112 | No TB |
| 151 | 0.08 | No | 0.51 | No | 0.08 | No | 0.2403 | No | 0.08 | No | 0.428 | No | 0.08 | No TB | 0.3112 | No TB |
| 1594 | 0.08 | No | 0.49 | No | 0.08 | No | 0.3754 | No | 0.08 | No | 0.5159 | No | 0.08 | No TB | 0.3452 | No TB |
| 1728 | 0.08 | No | 0.49 | No | 0.08 | No | 0.469 | No | 0.08 | No | 0.6966 | No | 0.08 | No TB | 0.5787 | TB |
| 1902 | 0.08 | No | 0.65 | No | 0.08 | No | 0.2816 | No | 0.08 | No | 0.428 | No | 0.08 | No TB | 0.3112 | No TB |
| 2019 | 0.08 | No | 0.981 | LWUTI | 0.08 | No | 0.293 | No | 0.08 | No | 0.5147 | No | 0.08 | No TB | 0.3877 | No TB |
| 2054 | 0.08 | No | 0.77 | No | 0.08 | No | 0.3162 | No | 0.08 | No | 0.428 | No | 0.08 | No TB | 0.3112 | No TB |
| 2055 | 0.08 | No | 0.892 | LWUTI | 0.08 | No | 0.6618 | No | 0.08 | No | 0.7454 | No | 0.08 | No TB | 0.6142 | TB |
| 2106 | 0.58 | LWUTI | 0.921 | LWUTI | 0.08 | No | 0.4268 | No | 0.08 | No | 0.553 | No | 0.08 | No TB | 0.4432 | No TB |
| 2183 | 0.08 | No | 0.864 | LWUTI | 0.41 | URTI | 0.871 | URTI | 0.91 | LRTI | 0.9204 | LRTI | 0.91 | TB | 0.9922 | TB |
| 2365 | 0.08 | No | 0.543 | LWUTI | 0.08 | No | 0.2335 | No | 0.08 | No | 0.4433 | No | 0.08 | No TB | 0.3452 | No TB |
| 2417 | 0.08 | No | 0.671 | No | 0.08 | No | 0.297 | No | 0.08 | No | 0.428 | No | 0.08 | No TB | 0.3112 | No TB |
| 2622 | 0.08 | No | 0.783 | No | 0.75 | URTI | 0.8249 | URTI | 0.08 | No | 0.8363 | LRTI | 0.75 | TB | 0.8226 | TB |
| 2846 | 0.08 | No | 0.657 | No | 0.08 | No | 0.3162 | No | 0.08 | No | 0.4705 | No | 0.08 | No TB | 0.3673 | No TB |
| 2958 | 0.08 | No | 0.846 | LWUTI | 0.08 | No | 0.3502 | No | 0.08 | No | 0.4858 | No | 0.08 | No TB | 0.3911 | No TB |
| 2983 | 0.08 | No | 0.73 | No | 0.08 | No | 0.336 | No | 0.08 | No | 0.6117 | No | 0.08 | No TB | 0.4531 | No TB |
| 3056 | 0.08 | No | 0.567 | No | 0.08 | No | 0.2743 | No | 0.08 | No | 0.604 | No | 0.08 | No TB | 0.5653 | TB |
| 3237 | 0.08 | No | 0.721 | No | 0.08 | No | 0.3834 | No | 0.08 | No | 0.5695 | No | 0.08 | No TB | 0.433 | No TB |
| 3499 | 0.08 | No | 0.432 | No | 0.08 | No | 0.2783 | No | 0.08 | No | 0.4705 | No | 0.08 | No TB | 0.3843 | No TB |
| 350 | 0.08 | No | 0.965 | LWUTI | 0.08 | No | 0.5196 | No | 0.08 | No | 0.8165 | LRTI | 0.08 | No TB | 0.8177 | TB |
| 3562 | 0.08 | No | 0.6545 | No | 0.08 | No | 0.2216 | No | 0.08 | No | 0.428 | No | 0.08 | No TB | 0.3112 | No TB |
| 3582 | 0.08 | No | 0.4323 | No | 0.08 | No | 0.2929 | No | 0.08 | No | 0.473 | No | 0.08 | No TB | 0.4112 | No TB |
| 3624 | 0.08 | No | 0.8542 | LWUTI | 0.41 | URTI | 0.8058 | URTI | 0.41 | LRTI | 0.9551 | LRTI | 0.08 | No TB | 0.7166 | TB |
| 3683 | 0.58 | LWUTI | 0.879 | LWUTI | 0.41 | URTI | 0.7496 | No | 0.41 | LRTI | 0.8036 | LRTI | 0.58 | TB | 0.8128 | TB |
| 3737 | 0.08 | No | 0.65 | No | 0.08 | No | 0.2216 | No | 0.08 | No | 0.428 | No | 0.08 | No TB | 0.3112 | No TB |
| 3738 | 0.08 | No | 0.435 | No | 0.08 | No | 0.2216 | No | 0.08 | No | 0.428 | No | 0.08 | No TB | 0.3112 | No TB |
| 3886 | 0.08 | No | 0.567 | No | 0.08 | No | 0.2216 | No | 0.08 | No | 0.428 | No | 0.08 | No TB | 0.3112 | No TB |
| 3890 | 0.08 | No | 0.677 | No | 0.08 | No | 0.2216 | No | 0.08 | No | 0.428 | No | 0.08 | No TB | 0.3112 | No TB |
| 4221 | 0.08 | No | 0.905 | LWUTI | 0.08 | No | 0.2962 | No | 0.08 | No | 0.473 | No | 0.08 | No TB | 0.4112 | No TB |
| 4490 | 0.08 | No | 0.543 | No | 0.08 | No | 0.5862 | No | 0.08 | No | 0.7349 | No | 0.08 | No TB | 0.5414 | TB |
| 4644 | 0.08 | No | 0.432 | No | 0.08 | No | 0.2579 | No | 0.08 | No | 0.428 | No | 0.08 | No TB | 0.3112 | No TB |
| 4742 | 0.08 | No | 0.432 | No | 0.08 | No | 0.2216 | No | 0.08 | No | 0.428 | No | 0.08 | No TB | 0.3112 | No TB |
| 479 | 0.08 | No | 0.785 | No | 0.08 | No | 0.3206 | No | 0.08 | No | 0.4577 | No | 0.08 | No TB | 0.3772 | No TB |
| 797 | 0.08 | No | 0.965 | LWUTI | 0.08 | No | 0.2579 | No | 0.08 | No | 0.428 | No | 0.08 | No TB | 0.3112 | No TB |
| 802 | 0.08 | No | 0.8965 | LWUTI | 0.08 | No | 0.2579 | No | 0.08 | No | 0.428 | No | 0.08 | No TB | 0.3112 | No TB |
| 1224 | 0.08 | No | 0.971 | LWUTI | 0.08 | No | 0.3757 | No | 0.08 | No | 0.428 | No | 0.08 | No TB | 0.3112 | No TB |
| 129 | 0.08 | No | 0.77 | No | 0.08 | No | 0.2216 | No | 0.08 | No | 0.428 | No | 0.08 | No TB | 0.3112 | No TB |
| 1364 | 0.08 | No | 0.69 | No | 0.08 | No | 0.2698 | No | 0.08 | No | 0.4433 | No | 0.08 | No TB | 0.3452 | No TB |
| 1374 | 0.08 | No | 0.49 | No | 0.08 | No | 0.3094 | No | 0.08 | No | 0.4433 | No | 0.08 | No TB | 0.3452 | No TB |
| 1584 | 0.08 | No | 0.647 | No | 0.08 | No | 0.2216 | No | 0.08 | No | 0.428 | No | 0.08 | No TB | 0.3112 | No TB |
| 174 | 0.08 | No | 0.919 | LWUT | 0.08 | No | 0.2975 | No | 0.08 | No | 0.428 | No | 0.08 | No TB | 0.3112 | No TB |
| 183 | 0.08 | No | 0.935 | LWUT | 0.08 | No | 0.2975 | No | 0.08 | No | 0.428 | No | 0.08 | No TB | 0.3112 | No TB |
| 2023 | 0.08 | No | 0.431 | No | 0.08 | No | 0.3861 | No | 0.08 | No | 0.5567 | No | 0.08 | No TB | 0.4531 | No TB |
| 2024 | 0.41 | LWUTI | 0.8654 | LWUTI | 0.08 | No | 0.2522 | No | 0.08 | No | 0.5147 | No | 0.08 | No TB | 0.4098 | No TB |
| 2045 | 0.08 | No | 0.874 | LWUTI | 0.08 | No | 0.3162 | No | 0.08 | No | 0.428 | No | 0.08 | No TB | 0.3112 | No TB |
| 2088 | 0.08 | No | 0.95 | LWUT | 0.75 | URTI | 0.8871 | URTI | 0.41 | LRTI | 0.878 | LRTI | 0.08 | No TB | 0.6539 | TB |
| Pat_No | Lasfv_Data | Diagnosis | FCM_lasfv | Diagnosis | Yelfv_Data | Diagnosis | FCM_Yelfv | Diagnosis | Denfv_Data | Diagnosis | FCM_Denfv | Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1261 | 0.08 | No | 0.7451 | No | 0.08 | No | 0.6226 | No | 0.08 | No | 0.7809 | No |
| 1377 | 0.08 | No | 0.6448 | No | 0.08 | No | 0.5926 | No | 0.08 | No | 0.6509 | No |
| 1459 | 0.08 | No | 0.6675 | No | 0.08 | No | 0.5943 | No | 0.08 | No | 0.642 | No |
| 1480 | 0.08 | No | 0.6312 | No | 0.08 | No | 0.6022 | No | 0.08 | No | 0.7023 | No |
| 151 | 0.08 | No | 0.6448 | No | 0.08 | No | 0.5926 | No | 0.08 | No | 0.6696 | No |
| 1594 | 0.08 | No | 0.6312 | No | 0.08 | No | 0.5824 | No | 0.08 | No | 0.6288 | No |
| 1728 | 0.08 | No | 0.6601 | No | 0.08 | No | 0.6045 | No | 0.08 | No | 0.6713 | No |
| 1902 | 0.08 | No | 0.6312 | No | 0.08 | No | 0.6022 | No | 0.08 | No | 0.6936 | No |
| 2019 | 0.08 | No | 0.6939 | No | 0.08 | No | 0.6088 | No | 0.08 | No | 0.7212 | No |
| 2054 | 0.08 | No | 0.6448 | No | 0.08 | No | 0.6345 | No | 0.08 | No | 0.7575 | No |
| 2055 | 0.08 | No | 0.9334 | LASFVR | 0.08 | No | 0.9036 | No | 0.08 | No | 1.581 | No |
| 2106 | 0.08 | No | 0.6932 | No | 0.08 | No | 0.6413 | No | 0.08 | No | 0.829 | No |
| 2183 | 0.08 | No | 0.8012 | No | 0.08 | No | 0.626 | No | 0.08 | No | 0.8661 | No |
| 2365 | 0.08 | No | 0.6312 | No | 0.08 | No | 0.5824 | No | 0.08 | No | 0.6623 | No |
| 2417 | 0.08 | No | 0.6312 | No | 0.08 | No | 0.5824 | No | 0.08 | No | 0.6758 | No |
| 2622 | 0.08 | No | 0.6712 | No | 0.08 | No | 0.6355 | No | 0.08 | No | 0.7703 | No |
| 2846 | 0.08 | No | 0.6312 | No | 0.08 | No | 0.5943 | No | 0.08 | No | 0.7091 | No |
| 2958 | 0.08 | No | 0.6856 | No | 0.08 | No | 0.648 | No | 0.08 | No | 0.6957 | No |
| 2983 | 0.08 | No | 0.6712 | No | 0.08 | No | 0.6124 | No | 0.08 | No | 0.724 | No |
| 3056 | 0.08 | No | 0.6448 | No | 0.08 | No | 0.5926 | No | 0.08 | No | 0.682 | No |
| 3237 | 0.08 | No | 0.6652 | No | 0.08 | No | 0.6124 | No | 0.08 | No | 0.7393 | No |
| 3499 | 0.08 | No | 0.6312 | No | 0.08 | No | 0.5824 | No | 0.08 | No | 0.65 | No |
| 350 | 0.08 | No | 0.756 | No | 0.08 | No | 0.661 | No | 0.08 | No | 0.8382 | No |
| 3562 | 0.08 | No | 0.6312 | No | 0.08 | No | 0.5824 | No | 0.08 | No | 0.6453 | No |
| 3582 | 0.08 | No | 0.6576 | No | 0.08 | No | 0.6418 | No | 0.08 | No | 0.6651 | No |
| 3624 | 0.08 | No | 0.7448 | No | 0.08 | No | 0.6124 | No | 0.08 | No | 0.8425 | No |
| 3683 | 0.08 | No | 0.6894 | No | 0.08 | No | 0.6688 | No | 0.08 | No | 0.7573 | No |
| 3737 | 0.08 | No | 0.6312 | No | 0.08 | No | 0.5824 | No | 0.08 | No | 0.6453 | No |
| 3738 | 0.08 | No | 0.6312 | No | 0.08 | No | 0.5824 | No | 0.08 | No | 0.6453 | No |
| 3886 | 0.08 | No | 0.6312 | No | 0.08 | No | 0.6022 | No | 0.08 | No | 0.6849 | No |
| 3890 | 0.08 | No | 0.6576 | No | 0.08 | No | 0.6253 | No | 0.08 | No | 0.7014 | No |
| 4221 | 0.08 | No | 0.6312 | No | 0.08 | No | 0.5824 | No | 0.08 | No | 0.675 | No |
| 4490 | 0.08 | No | 0.6312 | No | 0.08 | No | 0.6276 | No | 0.08 | No | 0.6543 | No |
| 4644 | 0.08 | No | 0.6576 | No | 0.08 | No | 0.6022 | No | 0.08 | No | 0.6552 | No |
| 4742 | 0.08 | No | 0.6576 | No | 0.08 | No | 0.5824 | No | 0.08 | No | 0.6288 | No |
| 479 | 0.08 | No | 0.6312 | No | 0.08 | No | 0.6022 | No | 0.08 | No | 0.6882 | No |
| 797 | 0.08 | No | 0.6312 | No | 0.08 | No | 0.6405 | No | 0.08 | No | 0.682 | No |
| 802 | 0.08 | No | 0.6312 | No | 0.08 | No | 0.6405 | No | 0.08 | No | 0.682 | No |
| 1224 | 0.08 | No | 0.6848 | No | 0.08 | No | 0.6695 | No | 0.08 | No | 0.8029 | No |
| 129 | 0.08 | No | 0.6312 | No | 0.08 | No | 0.5824 | No | 0.08 | No | 0.6288 | No |
| 1364 | 0.08 | No | 0.6448 | No | 0.08 | No | 0.6022 | No | 0.08 | No | 0.6589 | No |
| 1374 | 0.08 | No | 0.6448 | No | 0.08 | No | 0.5926 | No | 0.08 | No | 0.6674 | No |
| 1584 | 0.08 | No | 0.6312 | No | 0.08 | No | 0.5824 | No | 0.08 | No | 0.6288 | No |
| 174 | 0.08 | No | 0.6312 | No | 0.08 | No | 0.5824 | No | 0.08 | No | 0.7273 | No |
| 183 | 0.08 | No | 0.6312 | No | 0.08 | No | 0.5824 | No | 0.08 | No | 0.7273 | No |
| 2023 | 0.08 | No | 0.6312 | No | 0.08 | No | 0.5824 | No | 0.08 | No | 0.6543 | No |
| 2024 | 0.08 | No | 0.6576 | No | 0.08 | No | 0.6226 | No | 0.08 | No | 0.738 | No |
| 2045 | 0.08 | No | 0.6312 | No | 0.08 | No | 0.6124 | No | 0.08 | No | 0.7846 | No |
| 2088 | 0.08 | No | 0.6312 | No | 0.08 | No | 0.5824 | No | 0.08 | No | 0.6538 | No |
References
- Oken, D. Multiaxial diagnosis and the psychosomatic model of disease. Psychosom. Med. 2000, 62, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Graber, M.L. The incidence of diagnostic error in medicine. BMJ Qual. Saf. 2013, 22 (Suppl. S2), ii21–ii27. [Google Scholar] [CrossRef] [PubMed]
- Attai, K.; Amannejad, Y.; Vahdat Pour, M.; Obot, O.; Uzoka, F.M. A Systematic Review of Applications of Machine Learning and Other Soft Computing Techniques for the Diagnosis of Tropical Diseases. Trop. Med. Infect. Dis. 2022, 7, 398. [Google Scholar] [CrossRef]
- Johnson-Laird, P.N.; Khemlani, S.S.; Goodwin, G.P. Logic, probability, and human reasoning. Trends Cogn. Sci. 2015, 19, 201–214. [Google Scholar] [CrossRef]
- De Silva, C.W. Intelligent Control: Fuzzy Logic Applications; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Mehta, A.; Awuah, W.A.; Aborode, A.T.; Ng, J.C.; Candelario, K.; Vieira, I.M.P.; Bulut, H.I.; Toufik, A.-R.; Hasan, M.M.; Sikora, V. Telesurgery’s potential role in improving surgical access in Africa. Ann. Med. Surg. 2022, 82, 104511. [Google Scholar] [CrossRef] [PubMed]
- WHO. The State of the Health Workforce in the WHO African Region; World Health Organization Universal Health Coverage/Life Course Cluster Brazzaville: Brazzaville, Congo, 2021. [Google Scholar]
- Prasad, N.; Murdoch, D.R.; Reyburn, H.; Crump, J.A. Etiology of Severe Febrile Illness in Low- and Middle-Income Countries: A Systematic Review. PLoS ONE 2015, 10, e0127962. [Google Scholar] [CrossRef]
- Bell, D. Acute Febrile Syndrome Strategy: A Major Challenge to Global Public Health; Foundation for Innovation New Diagnostic (FIND) Communications, 2012; pp. 1–36. Available online: https://assets.publishing.service.gov.uk/media/57a08a7340f0b652dd00072c/0031-FIND-NMFI-document-print-inhouse.pdf (accessed on 22 January 2023).
- Crump, J.A. Time for a Comprehensive Approach to the Syndrome of Fever in the Tropics. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 61–62. [Google Scholar] [CrossRef]
- Crump, J.; Newton, P.; Baird, S.; Lubell, Y. Febrile illness in adolescents and adults–ORA–Oxford University Research Archive. Febrile Illness in Adolescents and Adults–ORA–Oxford University Research Archive. 2017, pp. 1–40. Available online: https://ora.ox.ac.uk/objects/uuid:808876da-b46f-4307-b8a4-43cfc914273a (accessed on 22 January 2023).
- Crump, J.A.; Ramadhani, H.O.; Morrissey, A.B.; Saganda, W.; Mwako, M.S. Invasive Bacterial and Fungal Infections among Hospitalized HIV-Infected and HIV-Uninfected Adults and Adolescents in Northern Tanzania. Clin. Infect. Dis. 2011, 52, 341–348. [Google Scholar] [CrossRef]
- WHO. WHO Informal Consultation on Fever Management in Peripheral Health Care Settings: A Global Review of Evidence and Practice; WHO: Geneva, Switzerland, 2013; pp. 1–66. [Google Scholar]
- Nabarro, L.E.; McCann, N.; Herdman, M.T.; Dugan, C.; Ladhani, S.; Patel, D.; Morris-Jones, S.; Balasegaram, S.; Heyderman, R.S.; Brown, M. British infection association guidelines for the diagnosis and management of enteric fever in England. J. Infect. 2022, 84, 469–489. [Google Scholar] [CrossRef]
- Goletti, D.; Delogu, G.; Matteelli, A.; Migliori, G.B. The role of IGRA in the diagnosis of tuberculosis infection, differentiating from active tuberculosis, and decision making for initiating treatment or preventive therapy of tuberculosis infection. Int. J. Infect. Dis. 2022, 124, S12–S19. [Google Scholar] [CrossRef]
- Maillard, O.; Belot, J.; Adenis, T.; Rollot, O.; Adenis, A.; Guihard, B.; Gérardin, P.; Bertolotti, A. Early diagnosis of dengue: Diagnostic utility of the SD BIOLINE Dengue Duo rapid test in Reunion Island. PLoS Negl. Trop. Dis. 2023, 17, e0011253. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, D.; Chadha, S.; Sarin, S.; Sen, R.; Arafah, S.; Dittrich, S. Diagnostic tools used in the evaluation of acute febrile illness in South India: A scoping review. BMC Infect. Dis. 2019, 19, 970. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Guha, D.; Dutta, B. Medical diagnosis with the aid of using fuzzy logic and intuitionistic fuzzy logic. Appl. Intell. 2016, 45, 850–867. [Google Scholar] [CrossRef]
- Nilashi, M.; Ibrahim, O.; Ahmadi, H.; Shahmoradi, L. A knowledge-based system for Breast cancer classification using fuzzy logic method. Telemat. Inform. 2017, 34, 133–144. [Google Scholar] [CrossRef]
- Amjad, M.; Ameer, I.; Gelbukh, A. A distinct approach to diagnose Dengue Fever with the help of Soft Set Theory. arXiv 2018. [Google Scholar] [CrossRef]
- Sharma, M.K.; Dhiman, N.; Mishra, V.N.; Mishra, L.N.; Dhaka, A.; Koundal, D. Post-symptomatic detection of COVID-2019 grade based mediative fuzzy projection. Comput. Electr. Eng. 2022, 101, 108028. [Google Scholar] [CrossRef]
- Magwili, G.V.; Latina, M.A.E.; Miguel, F.I.C.; Ortega, T.K.P.; Pastoril, T.K.L.; Tanglao, E.J.D. Raspberry pi-based medical expert system for pre-diagnosis of mosquito-borne diseases. In Proceedings of the 2018 IEEE 10th Inter-national Conference on Humanoid, Nanotechnology, Information Technology, Communication and Control, Environment and Management (HNICEM), Baguio City, Philippines, 29 November–2 December 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 1–6. [Google Scholar] [CrossRef]
- Putra, I.K.G.D.; Prihatini, P.M. Fuzzy expert system for tropical infectious disease by certainty factor. TELKOMNIKA (Telecommun. Comput. Electron. Control) 2012, 10, 825–836. [Google Scholar] [CrossRef][Green Version]
- Ekong, B.; Ifiok, I.; Udoeka, I.; Anamfiok, J. Integrated fuzzy based decision support system for the management of human dis-ease. Int. J. Adv. Comput. Sci. Appl. 2020, 11, 268–274. [Google Scholar] [CrossRef]
- Bourgani, E.; Stylios, C.D.; Georgopoulos, V.C.; Manis, G. A study on Fuzzy Cognitive Map structures for Medical Decision Support Systems. In Proceedings of the 8th Conference of the European Society for Fuzzy Logic and Technology (EUSFLAT 2013), Milan, Italy, 11–13 September 2013; Atlantis Press: Amsterdam, The Netherlands, 2013; pp. 744–751. [Google Scholar] [CrossRef]
- Amirkhani, A.; Papageorgiou, E.; Mosheni, A.; Mosavi, M. A Review of Fuzzy Cognitive map in Medicine: Taxonomy Methods and Applications. Comput. Methods Programs Biomed. 2017, 142, 129–145. [Google Scholar] [CrossRef]
- Groumpos, P.P. Modelling COVID-19 using Fuzzy Cognitive Maps (FCM). EAI Endorsed Transaction. Bioeng. Bioinform. 2021, 21, e3. [Google Scholar] [CrossRef]
- Papageorgiou, E.I.; Papandrianos, N.I.; Karagianni, G.; Kyriazopoulos, G.C.; Sfyras, D. A Fuzzy Cognitive Map based tool for prediction of infectious diseases. In Proceedings of the IEEE International Conference on Fuzzy Systems, Jeju, Republic of Korea, 20–24 August 2009. [Google Scholar] [CrossRef]
- N’apoles, G.; Espinosa, M.; Grau, I.; Vanhoof, K. FCM Expert: Software Tool for Scenario Analysis and Pattern Classification Based on Fuzzy Cognitive Maps. Int. J. Artif. Intell. Tools 2018, 27, 1860010. [Google Scholar] [CrossRef]
- Mpelogianni, V.; Groumpos, P.P. Re-approaching Fuzzy Cognitive Maps to increase Knowledge of system. Ai Soc. 2018, 33, 175–188. [Google Scholar] [CrossRef]
- Apotolospoulos, I.D.; Groumpos, P.P. Fuzzy Cognitive Maps: The Role of explainable Artificial Intelligence. Appl. Sci. 2023, 13, 3412. [Google Scholar] [CrossRef]
- Obot, O.U.; Udo, I.I.; Udoh, S.S. Differential Diagnosis of Eye Diseases Based on Fuzzy Cognitive Map. IOSR J. Nurs. Health Sci. (IOSR-JNHS) 2018, 7, 42–52. [Google Scholar]
- Apostolopoulos, I.D.; Groumpos, P.P.; Apostolopoulos, D.I. Advanced Fuzzy Cognitive Maps: State Space and Rule Based methodology for Coronary Artery Disease detection. Biomed. Phys. Eng. Express 2020, 7, 045007. [Google Scholar] [CrossRef]
- Apostolopoulos, I.D.; Groumpos, P.P. Non-invasive modelling methodology for the diagnosis of coronary artery disease using fuzzy cognitive maps. Comput. Methods Biomech. Biomed. Eng. 2020, 23, 879–887. [Google Scholar] [CrossRef]
- Bourgani, E.; Stylios, C.D.; Manis, G.; Georgopoulos, V.C. Time depend-ent fuzzy cognitive maps for medical diagnosis. In Artificial Intelligence: Methods and Applications: Proceedings of the 8th Hellenic Conference on AI, SETN 2014, Ioannina, Greece, 15–17 May 2014; Springer: Berlin/Heidelberg, Germany, 2014; pp. 544–554. [Google Scholar]
- Uzoka, F.M.; Akinnwunesi, B.A.; Amoo, T.; Aladi, F.; Fashoto, S.; Olaniyan, M.; Osuji, J. A Framework for Early Differential Diagnosis of Tropical Confusable Diseases using Fuzzy Cognitive Map. Int. J. Health Med. Eng. 2016, 10, 346–353. [Google Scholar]
- Uzoka, F.M.E.; Akinnuwesi, B.A.; Amoo, T.; Debele, F.; Fashoto, G.; Nwafor-Okoli, C. An expert system for malaria diagnosis using the fuzzy cognitive map engine. In Proceedings of the 2018 IST-Africa Week Conference (IST-Africa), Gaborone, Botswana, 9–11 May 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 1–13. [Google Scholar]
- Hoyos, W.; Aguilar, J.; Toro, M. A clinical decision-support system for Dengue based on fuzzy cognitive maps. Health Care Manag. Sci. 2022, 25, 666–681. [Google Scholar] [CrossRef]
- Jayashree, L.S.; Lakshmi Devi, R.; Papandrianos, N.; Papageorgiou, E.I. Application of Fuzzy Cognitive Map for geospatial dengue outbreak risk prediction of tropical regions of Southern India. Intell. Decis. Technol. 2018, 12, 231–250. [Google Scholar] [CrossRef]
- ODK. Open Data Kit (Version 1.30.1). 2021. Available online: https://opendatakit.org/ (accessed on 22 March 2023).
- Canêo, L.F.; Neirotti, R. The Importance of the Proper Definition of Adulthood: What is and What is Not Included in a Scientific Publication. Braz. J. Cardiovasc. Surg. 2017, 32, 60. [Google Scholar] [CrossRef]
- Princy, S.; Dhenakaran, S.S. Comparison of triangular and trapezoidal fuzzy membership function. J. Comput. Sci. Eng. 2016, 2, 46–51. [Google Scholar]
- Keller, A.; Frey, M.; Schmid, H.; Steffen, R.; Walker, T.; Schlagenhaut, P. Imported Tropical Fever in Switerland, 1993–2004. J. Trop. Med. 2008, 15, 248–251. [Google Scholar]
- Goodman, K.W. Ethics, Computing and Medicine: Informatics and the Transformation of Health Care; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Khan, N.; Okoli, C.N.; Ekpin, V.; Attai, K.; Chukwudi, N.; Sabi, H.; Akwaowo, C.; Osuji, J.; Benavente, L.; Uzoka, F.M. Adoption and Utilization of Medical Decision Support Systems in the Diagnosis of Febrile Diseases: A Systematic Literature Review. Expert Syst. Appl. 2023, 220, 119638. [Google Scholar] [CrossRef]
- Meredith, R.A.; Arnntt, D.R. On Ethics and Decision Support System Development. In Proceedings of the 7th Pacific Asia Conference on Information System, Adelaide, Australia, 10–13 July 2003; pp. 1–4. [Google Scholar]
- Obot, O.; Asuquo, A.; Attai, K.; Johnson, E.; Arnold, K.; Edoho, M.; Ekpenyong, M.; Akwaowo, C.; Udoh, O.; Usen, V.; et al. Development of a mobile app diagnostic system for tropical febrile diseases. In Proceedings of the International Conference on e-Health (EH 2023), Porto, Portugal, 15–17 July 2023. [Google Scholar]
- Beauchap, T.L.; Childress, J.F. Principles of Biomedical Ethics, 3rd ed.; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]

| SN | MAL Symptoms | Pearson Rank | Kendall Rank | Spearman Rank | Mean |
|---|---|---|---|---|---|
| 1 | BITAIM | 0.528 | 0.477 | 0.545 | 0.52 |
| 2 | CHLNRIG | 0.391 | 0.368 | 0.422 | 0.39 |
| 3 | GENBDYPN | 0.381 | 0.349 | 0.404 | 0.38 |
| 4 | HDACH | 0.372 | 0.324 | 0.378 | 0.36 |
| 5 | FVR | 0.339 | 0.303 | 0.343 | 0.33 |
| 6 | HGGDFVR | 0.318 | 0.284 | 0.327 | 0.31 |
| 7 | MSCBDYPN | 0.297 | 0.273 | 0.316 | 0.30 |
| 8 | FTG | 0.251 | 0.233 | 0.272 | 0.25 |
| 9 | SUDONFVR | 0.252 | 0.218 | 0.250 | 0.24 |
| 10 | LTG | 0.245 | 0.218 | 0.252 | 0.24 |
| 11 | CTRH | 0.194 | 0.188 | 0.215 | 0.20 |
| 12 | NUS | 0.185 | 0.190 | 0.219 | 0.20 |
| 13 | VMT | 0.187 | 0.180 | 0.207 | 0.19 |
| Symptom | Degree of Severity |
|---|---|
| CTRH | 0.75 |
| CHSP | 0.58 |
| CGHDRY | 0.75 |
| DIFBRT | 0.58 |
| DRYCGH | 0.75 |
| FVR | 0.41 |
| HGPSFVR | 0.75 |
| HGGDFVR | 0.75 |
| SWRFVR | 0.75 |
| SUNDONF | 0.75 |
| GENBDYP | 0.58 |
| HDACH | 0.75 |
| LTG | 0.58 |
| STRTRT | 0.75 |
| Initial Vector | 0.75 | 0.41 | 0.75 | 0.75 | 0.58 | 0.75 | 0.58 | Ai(t + 1) | Signum Function |
|---|---|---|---|---|---|---|---|---|---|
| Weight | 0.2000 | 0.3300 | 0.3100 | 0.2400 | 0.3800 | 0.3600 | 0.2400 | - | - |
| 1st iteration | 0.1500 | 0.1353 | 0.2325 | 0.1800 | 0.2204 | 0.2700 | 0.1392 | 2.327 | 0.688 |
| 2nd iteration | 0.0300 | 0.0446 | 0.0721 | 0.0432 | 0.0838 | 0.0972 | 0.0334 | 1.404 | 0.646 |
| 3rd iteration | 0.0060 | 0.0147 | 0.0223 | 0.0104 | 0.0318 | 0.0350 | 0.0080 | 1.128 | 0.630 |
| 4th iteration | 0.0012 | 0.0049 | 0.0069 | 0.0025 | 0.0121 | 0.0126 | 0.0019 | 1.042 | 0.625 |
| 5th iteration | 0.0002 | 0.0016 | 0.0021 | 0.0006 | 0.0046 | 0.0045 | 0.0005 | 1.014 | 0.623 |
| 6th iteration | 0.0000 | 0.0005 | 0.0007 | 0.0001 | 0.0017 | 0.0016 | 0.0001 | 1.005 | 0.623 |
| 7th iteration | 0.0000 | 0.0002 | 0.0002 | 0.0000 | 0.0007 | 0.0006 | 0.0000 | 1.002 | 0.623 |
| Initial Vector | 0.75 | 0.75 | 0.58 | Ai(t + 1) | Signum Function |
|---|---|---|---|---|---|
| Weight | 0.23 | 0.21 | 0.17 | - | - |
| 1st iteration | 0.173 | 0.158 | 0.099 | 1.429 | 0.807 |
| 2nd iteration | 0.129 | 0.118 | 0.057 | 1.305 | 0.787 |
| 3rd iteration | 0.097 | 0.089 | 0.033 | 1.219 | 0.772 |
| 4th iteration | 0.073 | 0.066 | 0.019 | 1.158 | 0.761 |
| 5th iteration | 0.055 | 0.050 | 0.011 | 1.116 | 0.753 |
| 6th iteration | 0.041 | 0.037 | 0.006 | 1.085 | 0.747 |
| Pat_1261 | Actual Value | Expert Diagnosis | Computed Value | System Diagnosis |
|---|---|---|---|---|
| Malaria | 0.91 | Very High | 0.7888 | Yes |
| Enteric Fever | 0.08 | No | 0.701 | Yes |
| HIV AID | 0.08 | NO | 0.795 | No |
| UPUTI | 0.08 | No | 0.6082 | No |
| LWUTI | 0.08 | No | 0.40 | No |
| URTI | 0.91 | Very High | 0.892 | Yes |
| LRTI | 0.08 | No | 0.9431 | Yes |
| TB | 0.08 | No | 0.7501 | Yes |
| LASFVR | 0.08 | No | 0.745 | No |
| YELFVR | 0.08 | No | 0.6226 | No |
| DENFVR | 0.08 | No | 0.7809 | No |
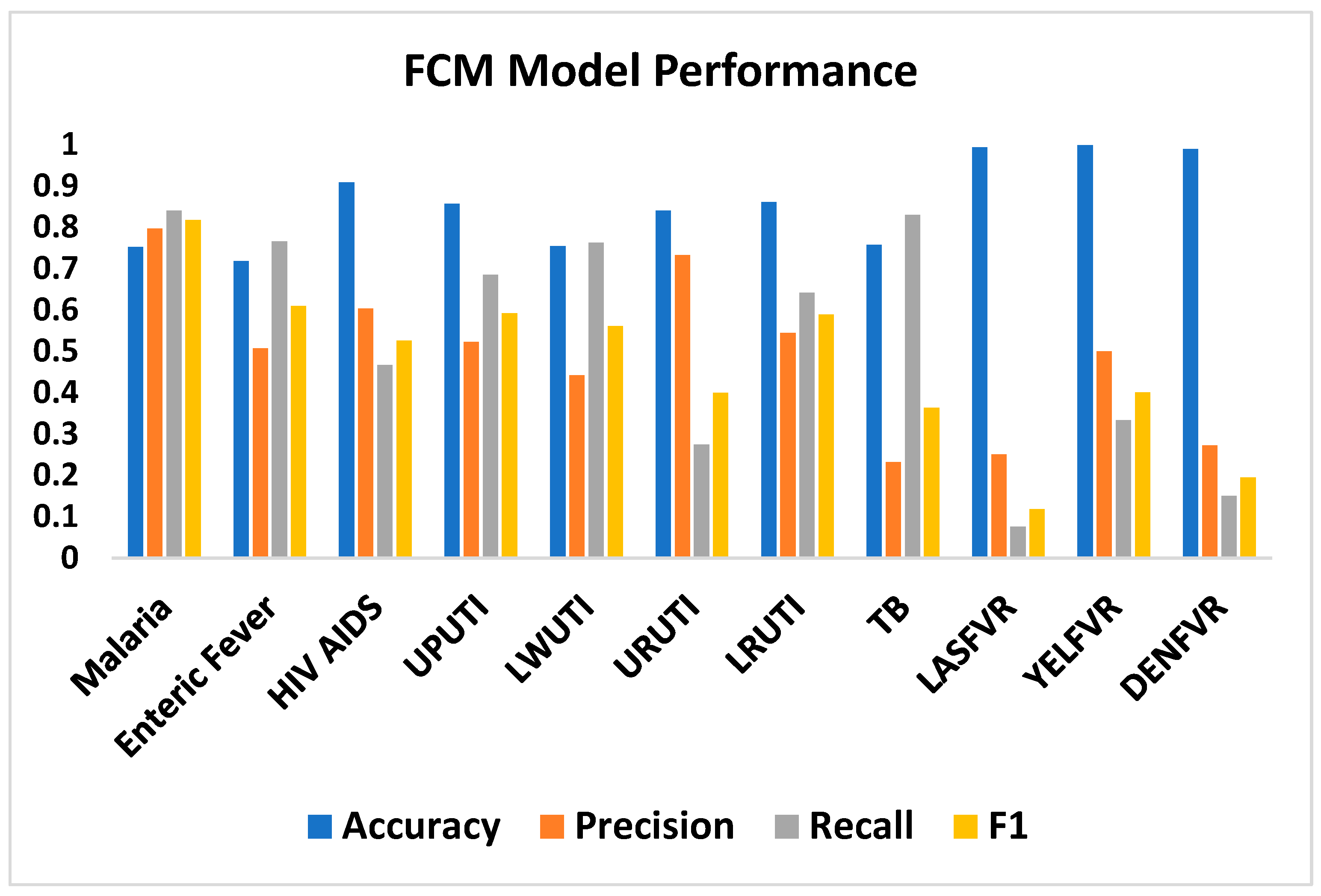
| Disease | Accuracy | Precision | Recall | F1 |
|---|---|---|---|---|
| Malaria | 0.752 | 0.796 | 0.840 | 0.817 |
| Enteric Fever | 0.718 | 0.507 | 0.765 | 0.609 |
| HIV AIDS | 0.908 | 0.603 | 0.467 | 0.526 |
| UPUTI | 0.857 | 0.522 | 0.685 | 0.592 |
| LWUTI | 0.754 | 0.442 | 0.762 | 0.561 |
| URUTI | 0.840 | 0.732 | 0.274 | 0.399 |
| LRUTI | 0.861 | 0.544 | 0.641 | 0.589 |
| TB | 0.757 | 0.232 | 0.830 | 0.363 |
| LASFVR | 0.993 | 0.250 | 0.076 | 0.118 |
| YELFVR | 0.998 | 0.5 | 0.333 | 0.400 |
| DENFVR | 0.989 | 0.272 | 0.150 | 0.194 |
| Disease | TP | FP | FN | TN | Total |
|---|---|---|---|---|---|
| Malaria | 1370 | 351 | 261 | 483 | 2465 |
| Enteric Fever | 543 | 529 | 167 | 1226 | 2465 |
| HIV/AIDS | 126 | 83 | 144 | 2112 | 2465 |
| UPUTI | 256 | 235 | 118 | 1856 | 2465 |
| LWUTI | 387 | 485 | 121 | 1472 | 2465 |
| URTI | 131 | 48 | 347 | 1939 | 2465 |
| LRTI | 245 | 205 | 137 | 1878 | 2465 |
| TB | 170 | 563 | 35 | 1697 | 2465 |
| LASFVR | 1 | 3 | 12 | 2449 | 2465 |
| YELFVR | 1 | 1 | 2 | 2461 | 2465 |
| DENFVR | 3 | 8 | 17 | 2437 | 2465 |
| Name of Disease | No. of Actual Diagnoses | No. of Predicted Diagnoses |
|---|---|---|
| Malaria | 1631 | 1721 |
| Enteric Fever | 710 | 1072 |
| HIV/AIDS | 270 | 209 |
| UPUTI | 374 | 491 |
| LWUTI | 508 | 872 |
| URTI | 478 | 179 |
| LRTI | 382 | 450 |
| TB | 205 | 733 |
| LASFVR | 13 | 04 |
| YELFVR | 02 | 01 |
| DENFVR | 11 | 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obot, O.; John, A.; Udo, I.; Attai, K.; Johnson, E.; Udoh, S.; Nwokoro, C.; Akwaowo, C.; Dan, E.; Umoh, U.; et al. Modelling Differential Diagnosis of Febrile Diseases with Fuzzy Cognitive Map. Trop. Med. Infect. Dis. 2023, 8, 352. https://doi.org/10.3390/tropicalmed8070352
Obot O, John A, Udo I, Attai K, Johnson E, Udoh S, Nwokoro C, Akwaowo C, Dan E, Umoh U, et al. Modelling Differential Diagnosis of Febrile Diseases with Fuzzy Cognitive Map. Tropical Medicine and Infectious Disease. 2023; 8(7):352. https://doi.org/10.3390/tropicalmed8070352
Chicago/Turabian StyleObot, Okure, Anietie John, Iberedem Udo, Kingsley Attai, Ekemini Johnson, Samuel Udoh, Chukwudi Nwokoro, Christie Akwaowo, Emem Dan, Uduak Umoh, and et al. 2023. "Modelling Differential Diagnosis of Febrile Diseases with Fuzzy Cognitive Map" Tropical Medicine and Infectious Disease 8, no. 7: 352. https://doi.org/10.3390/tropicalmed8070352
APA StyleObot, O., John, A., Udo, I., Attai, K., Johnson, E., Udoh, S., Nwokoro, C., Akwaowo, C., Dan, E., Umoh, U., & Uzoka, F.-M. (2023). Modelling Differential Diagnosis of Febrile Diseases with Fuzzy Cognitive Map. Tropical Medicine and Infectious Disease, 8(7), 352. https://doi.org/10.3390/tropicalmed8070352









