Theracurmin Modulates Cardiac Inflammation in Experimental Model of Trypanosoma cruzi Infection
Abstract
1. Introduction
2. Materials and Methods
3. Results
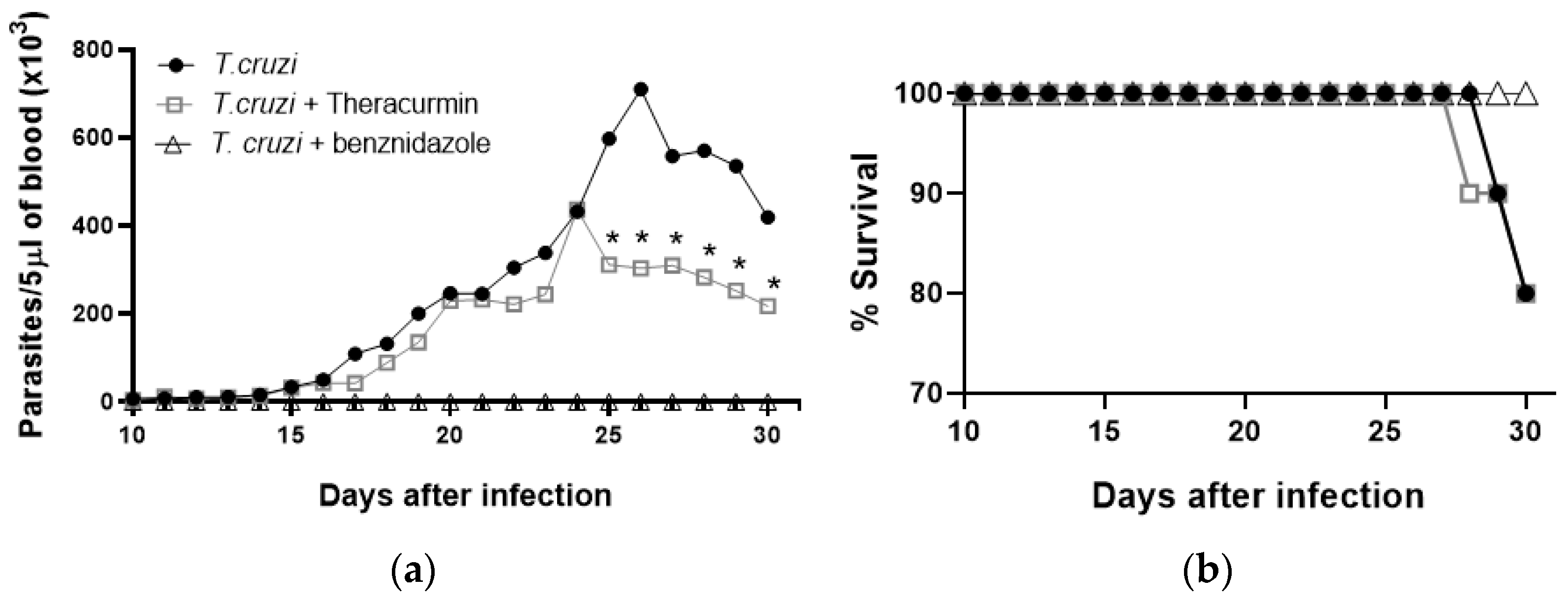
3.1. Theracurmin Has Reduced the Parasitemia Curve and Sustained Mice’s Body Mass
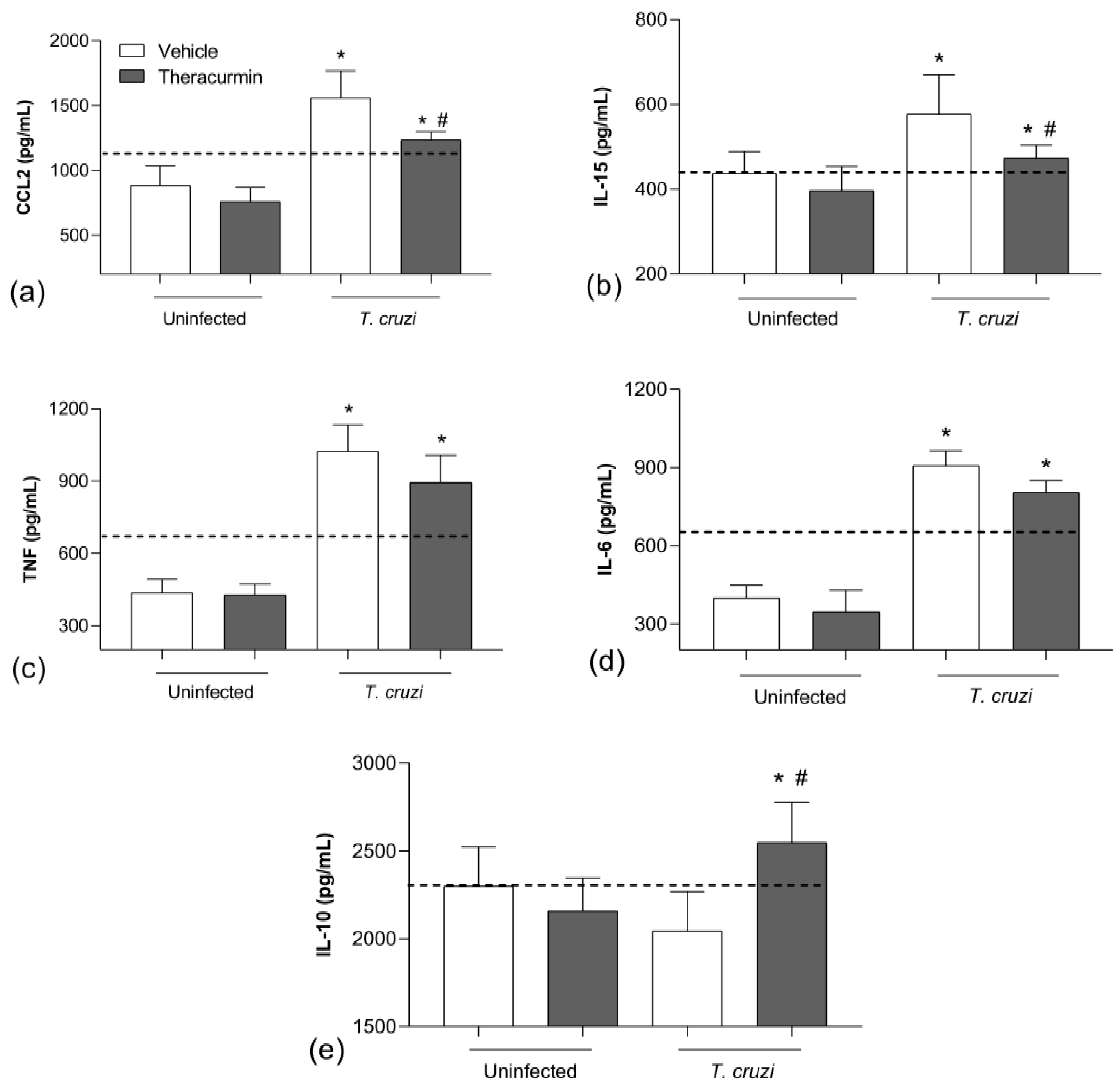
3.2. Theracurmin Has Reduced CCL2 and IL-15 Tissue and Increased IL-10 in T. cruzi Infection
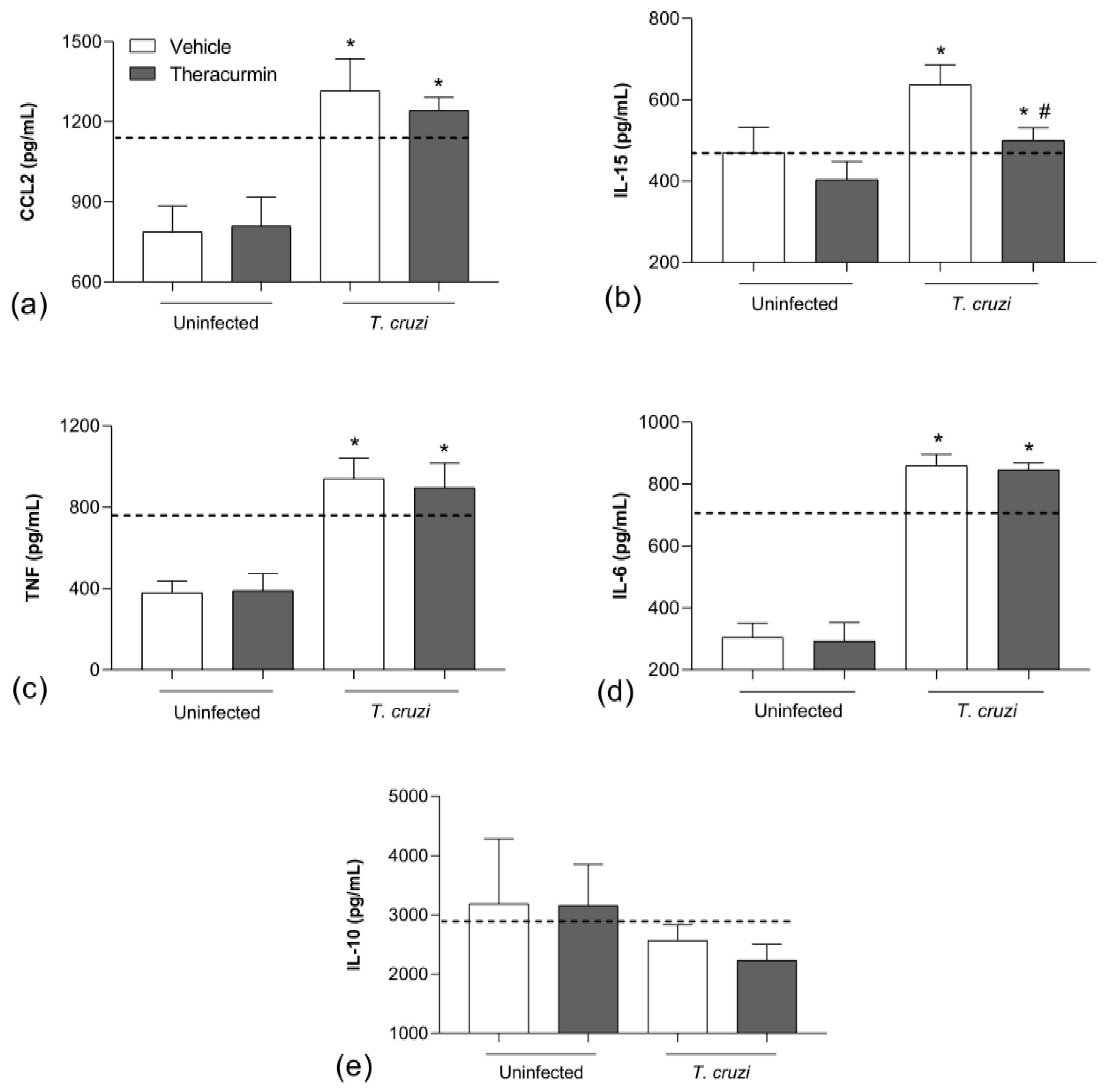
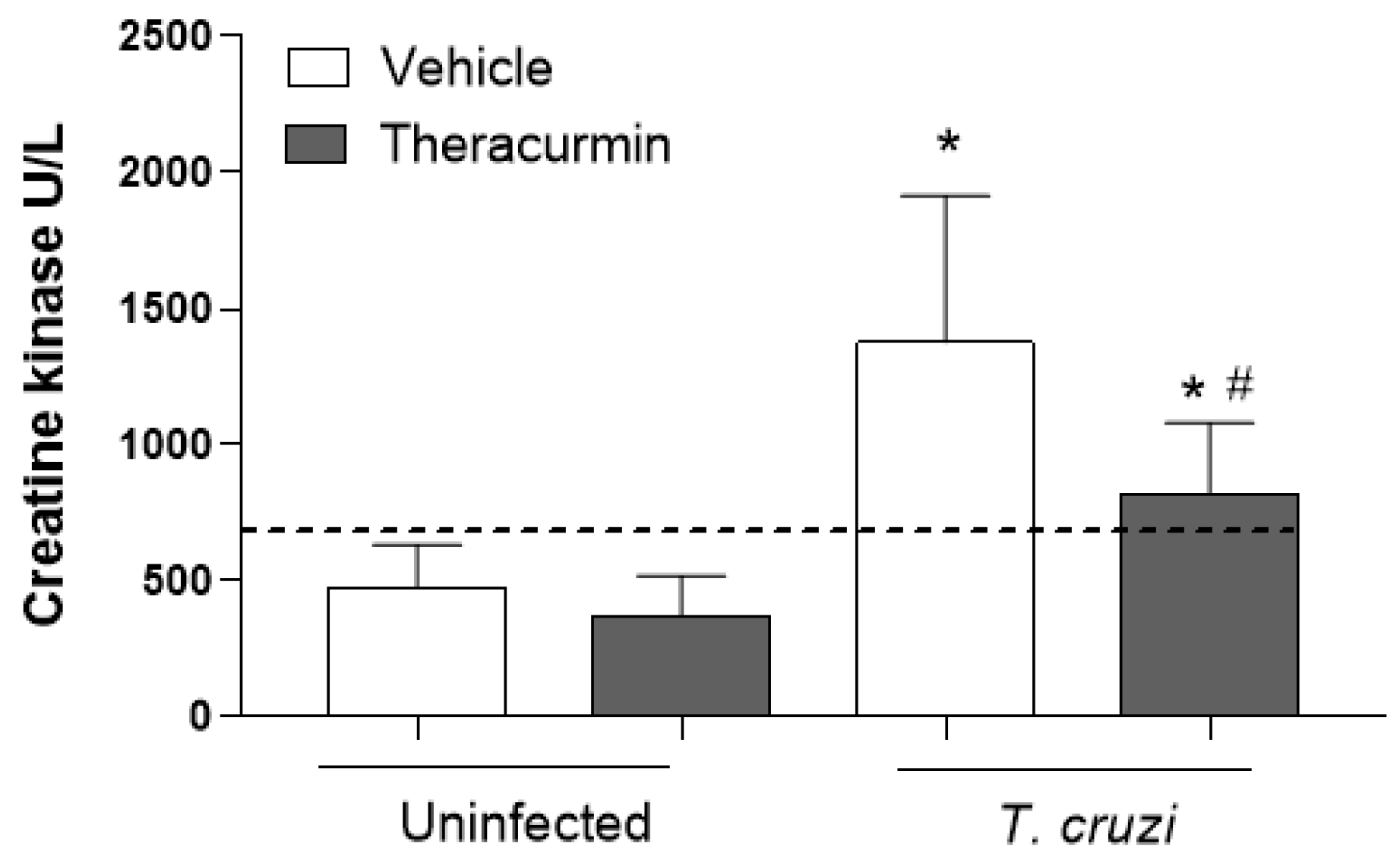
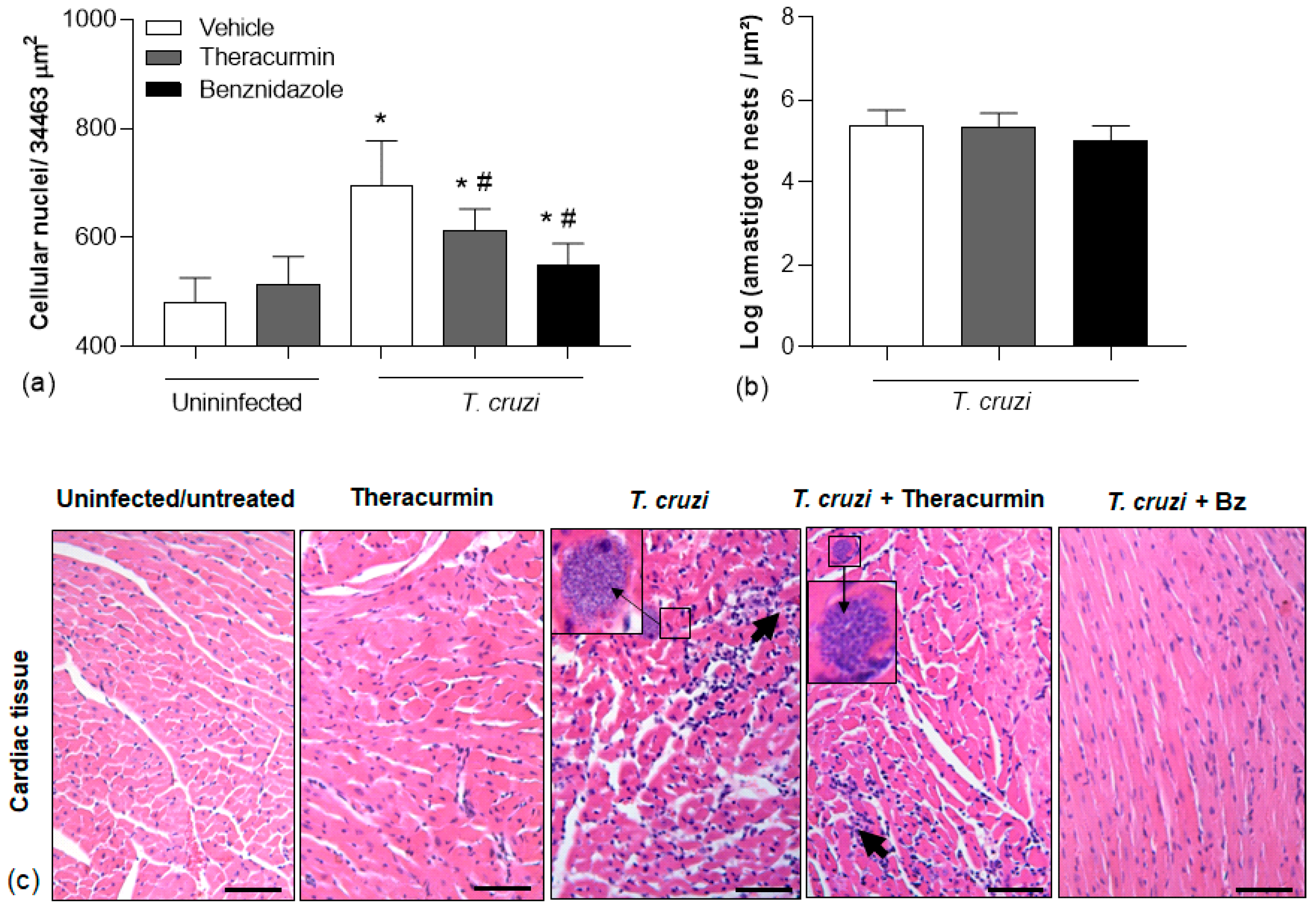
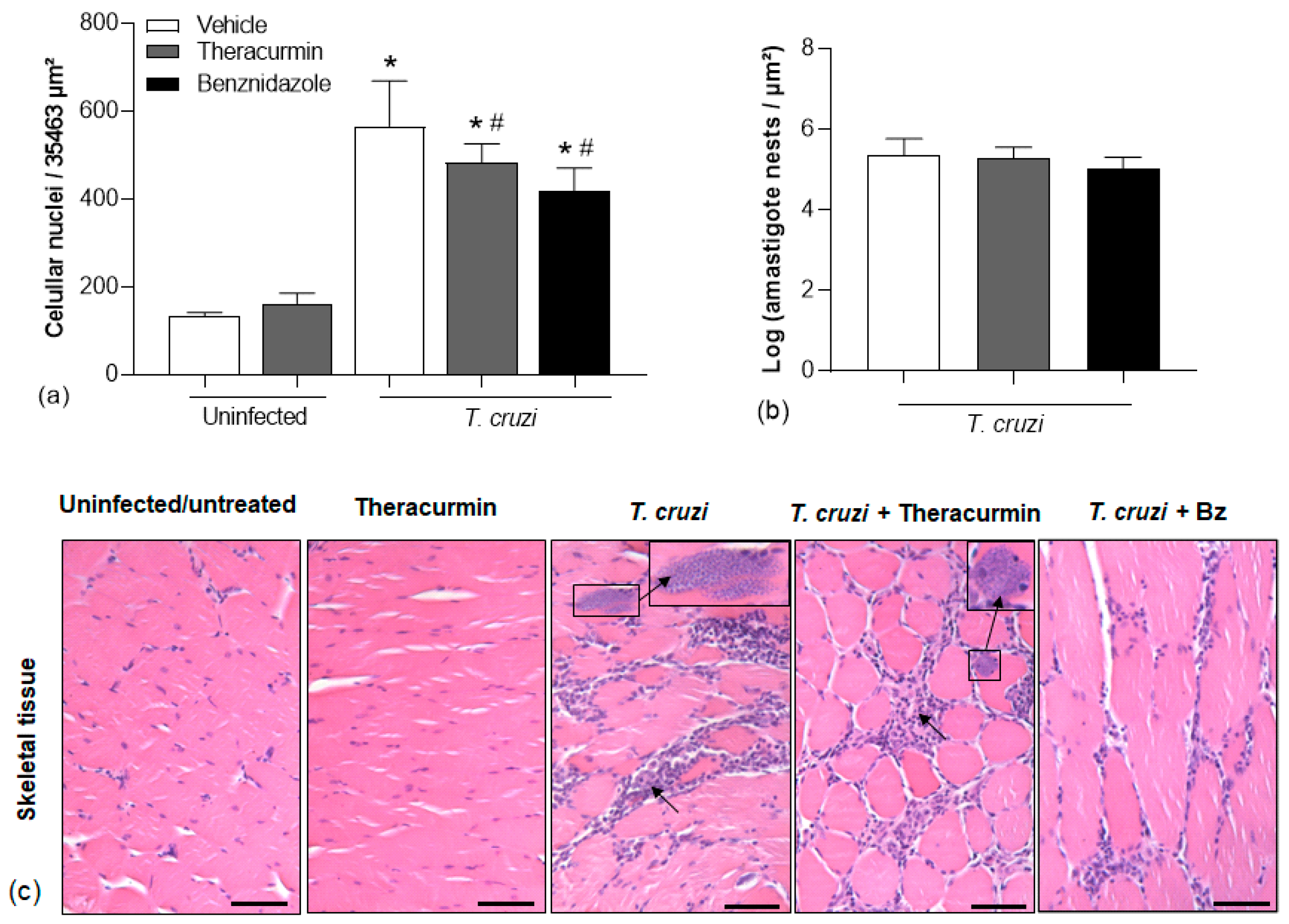
3.3. Theracurmin and Benznidazole Reduced Cardiac and Skeletal Inflammatory Infiltration in Muscles
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, S.C.; Prasad, S.; Kim, J.H.; Patchva, S.; Webb, L.J.; Priyadarsini, I.K.; Aggarwal, B.B. Multitargeting by curcumin as revealed by molecular interaction studies. Nat. Prod. Rep. 2011, 28, 1937–1955. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Kumar, A.; Aggarwal, M.S.; Shishodia, S. Curcumin Derived from Turmeric (Curcuma longa): A Spice for All Seasons. In Phytopharmaceuticals in Cancer Chemoprevention; CRC Press: Boca Raton, FL, USA, 2005; pp. 349–387. [Google Scholar]
- Hernández, M.; Wicz, S.; Santamaría, M.H.; Corral, R.S. Curcumin exerts anti-inflammatory and vasoprotective effects through amelioration of NFAT-dependent endothelin-1 production in mice with acute Chagas cardiomyopathy. Mem. Inst. Oswaldo. Cruz 2018, 113, e180171. [Google Scholar] [CrossRef]
- Nagajyothi, F.; Zhao, D.; Weiss, L.M.; Tanowitz, H.B. Curcumin treatment provides protection against Trypanosoma cruzi infection. Parasitol. Res. 2012, 110, 2491–2499. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.L.J.; de Oliveira, D.S.; Mota, L.W.R.; Carvalho, L.C.F.; Zimmermann, F.F.; de Paiva, N.C.N.; Vieira, P.M.D.A.; de Lana, M.; Afonso, L.C.C.; Talvani, A. Ectonucleotidases from trypomastigotes from different sources and various genetic backgrounds of Trypanosoma cruzi potentiate their infectivity and host inflammation. Cytokine 2020, 136, 155255. [Google Scholar] [CrossRef]
- Lobo, M.; Balouz, V.; Melli, L.; Carlevaro, G.; Cortina, M.E.; Cámara, M.D.L.M.; Cánepa, G.E.; Carmona, S.J.; Altcheh, J.; Campetella, O.; et al. Molecular and antigenic characterization of Trypanosoma cruzi Toll proteins. PLOS Neglected Trop. Dis. 2018, 13, e0007245. [Google Scholar] [CrossRef]
- Molina, I.; Salvador, F.; Sánchez-Montalvá, A.; Treviño, B.; Serre, N.; Avilés, A.S.; Almirante, B. Toxic Profile of Benznidazole in Patients with Chronic Chagas Disease: Risk Factors and Comparison of the Product from Two Different Manufacturers. Antimicrob. Agents Chemother. 2015, 59, 6125–6131. [Google Scholar] [CrossRef] [PubMed]
- Branquinho, R.T.; Mosqueira, V.C.F.; de Oliveira-Silva, J.C.V.; Simões-Silva, M.R.; Saúde-Guimarães, D.A.; de Lana, M. Sesquiterpene Lactone in Nanostructured Parenteral Dosage Form Is Efficacious in Experimental Chagas Disease. Antimicrob. Agents Chemother. 2014, 58, 2067–2075. [Google Scholar] [CrossRef] [PubMed]
- Menezes, A.P.D.J.; da Silva, M.L.; Pereira, W.L.; Costa, G.D.P.; Horta, A.L.; Mendonça, A.A.S.; Carneiro, A.C.A.; de Souza, D.M.S.; Novaes, R.D.; Teixeira, R.R.; et al. In Vitro tripanocidal effect of 1,8-dioxooctahydroxanthenes (xanthenodiones) and tetraketones and improvement of cardiac parameters in vivo. J. Glob. Antimicrob. Resist. 2020, 22, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Sasaki, H.; Sunagawa, Y.; Takahashi, K.; Imaizumi, A.; Fukuda, H.; Hashimoto, T.; Wada, H.; Katanasaka, Y.; Kakeya, H.; Fujita, M.; et al. Innovative Preparation of Curcumin for Improved Oral Bioavailability. Biol. Pharm. Bull. 2011, 34, 660–665. [Google Scholar] [CrossRef]
- Chung, H.; Yoon, S.H.; Cho, J.-Y.; Yeo, H.K.; Shin, D.; Park, J.-Y. Comparative pharmacokinetics of Theracurmin, a highly bioavailable curcumin, in healthy adult subjects. Int. J. Clin. Pharmacol. Ther. 2021, 59, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Federici, E.E.; Neva, F.A.; Abelmann, W.H. Chronic and Progressive Myocarditis and Myositis in C3H Mice Infected with Trypanosoma cruzi. Am. J. Trop. Med. Hyg. 1964, 13, 272–280. [Google Scholar] [CrossRef]
- Talvani, A.; Ribeiro, C.S.; Aliberti, J.C.; Michailowsky, V.; Santos, P.V.; Murta, S.M.; Romanha, A.J.; Almeida, I.C.; Farber, J.; Lannes-Vieira, J.; et al. Kinetics of cytokine gene expression in experimental chagasic cardiomyopathy: Tissue parasitism and endogenous IFN-γ as important determinants of chemokine mRNA expression during infection with Trypanosoma cruzi. Microbes Infect. 2000, 2, 851–866. [Google Scholar] [CrossRef] [PubMed]
- Zingales, B.; Andrade, S.; Briones, M.R.S.; Campbell, D.; Chiari, E.; Fernandes, O.; Guhl, F.; Lages-Silva, E.; Macedo, A.; Machado, C.; et al. A new consensus for Trypanosoma cruzi intraspecific nomenclature: Second revision meeting recommends TcI to TcVI. Mem. Inst. Oswaldo. Cruz 2009, 104, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D.; Zielinski, M.R.; Groschwitz, C.M.; Brown, A.S.; Gangemi, J.D.; Ghaffar, A.; Mayer, E.P.; Szymanski, M.C.; et al. Curcumin effects on inflammation and performance recovery following eccentric exercise-induced muscle damage. Am. J. Physiol. Integr. Comp. Physiol. Regul. 2007, 292, R2168–R2173. [Google Scholar] [CrossRef]
- Penitente, A.R.; Shrestha, D.; Horta, A.L.; Leite, A.L.J.; Neves, C.A.; Natali, A.J.; Costa, G.D.P.; Talvani, A. Enalapril in Combination with Benznidazole Reduces Cardiac Inflammation and Creatine Kinases in Mice Chronically Infected with Trypanosoma cruzi. Am. J. Trop. Med. Hyg. 2015, 93, 976–982. [Google Scholar] [CrossRef]
- Brener, Z. Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev. Inst. Med. Trop. Sao Paulo 1962, 4, 389–396. [Google Scholar]
- Katz, L.; Baltz, R.H. Natural product discovery: Past, present, and future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef]
- Ran, Y.; Su, W.; Gao, F.; Ding, Z.; Yang, S.; Ye, L.; Chen, X.; Tian, G.; Xi, J.; Liu, Z. Curcumin Ameliorates White Matter Injury after Ischemic Stroke by Inhibiting Microglia/Macrophage Pyroptosis through NF-κB Suppression and NLRP3 Inflammasome Inhibition. Oxidative Med. Cell Longev. 2021, 2021, 1552127. [Google Scholar] [CrossRef]
- He, H.-J.; Xiong, X.; Zhou, S.; Zhang, X.-R.; Zhao, X.; Chen, L.; Xie, C.-L. Neuroprotective effects of curcumin via autophagy induction in 6-hydroxydopamine Parkinson’s models. Neurochem. Int. 2022, 155, 105297. [Google Scholar] [CrossRef]
- Sueth-Santiago, V.; Moraes, J.D.B.B.; Alves, E.S.S.; Vannier-Santos, M.A.; Freire-De-Lima, C.G.; Castro, R.N.; Mendes-Silva, G.P.; Cistia, C.D.N.D.; Magalhães, L.G.; Andricopulo, A.D.; et al. The Effectiveness of Natural Diarylheptanoids against Trypanosoma cruzi: Cytotoxicity, Ultrastructural Alterations and Molecular Modeling Studies. PLoS ONE 2016, 11, e0162926. [Google Scholar] [CrossRef] [PubMed]
- Hesari, A.; Ghasemi, F.; Salarinia, R.; Biglari, H.; Hassan, A.T.M.; Abdoli, V.; Mirzaei, H. Effects of curcumin on NF-κB, AP-1, and Wnt/β-catenin signaling pathway in hepatitis B virus infection. J. Cell Biochem. 2018, 119, 7898–7904. [Google Scholar] [CrossRef] [PubMed]
- Horta, A.L.; Figueiredo, V.P.; Leite, A.L.J.; Costa, G.D.P.; Menezes, A.P.; Ramos, C.D.O.; Pedrosa, T.C.F.; Bezerra, F.; Vieira, P.M.D.A.; Talvani, A. The β-blocker carvedilol and the benznidazole modulate the cardiac immune response in the acute infection induced by Colombian strain of the Trypanosoma cruzi. Mem. Inst. Oswaldo Cruz 2018, 113, e180271. [Google Scholar] [CrossRef] [PubMed]
- de Souza, D.M.S.; Silva, M.C.; Farias, S.E.B.; Menezes, A.P.d.J.; Milanezi, C.M.; Lúcio, K.D.P.; Paiva, N.C.N.; de Abreu, P.M.; Costa, D.C.; Pinto, K.M.D.C.; et al. Diet Rich in Lard Promotes a Metabolic Environment Favorable to Trypanosoma cruzi Growth. Front. Cardiovasc. Med. 2021, 8, 667580. [Google Scholar] [CrossRef]
- Camandaroba, E.; Thé, T.S.; Pessina, D.H.; Andrade, S.G. Trypanosoma cruzi: Clones isolated from the Colombian strain, reproduce the parental strain characteristics, with ubiquitous histotropism. Int. J. Exp. Pathol. 2006, 87, 209–217. [Google Scholar] [CrossRef]
- Novaes, R.D.; Sartini, M.V.P.; Rodrigues, J.P.F.; Gonçalves, R.V.; Santos, E.C.; Souza, R.L.M.; Caldas, I.S. Curcumin Enhances the Anti-Trypanosoma cruzi Activity of Benznidazole-Based Chemotherapy in Acute Experimental Chagas Disease. Antimicrob. Agents Chemother. 2016, 60, 3355–3364. [Google Scholar] [CrossRef]
- Branquinho, R.T.; Roy, J.; Farah, C.; Garcia, G.M.; Aimond, F.; Le Guennec, J.-Y.; Saude-Guimarães, D.A.; Grabe-Guimaraes, A.; Mosqueira, V.C.F.; de Lana, M.; et al. Biodegradable Polymeric Nanocapsules Prevent Cardiotoxicity of Anti-Trypanosomal Lychnopholide. Sci. Rep. 2017, 7, srep44998. [Google Scholar] [CrossRef]
- Medeiros, G.A.; Silvério, J.C.; Marino, A.P.M.; Roffê, E.; Vieira, V.; Kroll-Palhares, K.; Carvalho, C.E.; Silva, A.A.; Teixeira, M.M.; Lannes-Vieira, J. Treatment of chronically Trypanosoma cruzi-infected mice with a CCR1/CCR5 antagonist (Met-RANTES) results in amelioration of cardiac tissue damage. Microbes Infect. 2009, 11, 264–273. [Google Scholar] [CrossRef]
- Gibaldi, D.; Vilar-Pereira, G.; Pereira, I.R.; Silva, A.A.; Barrios, L.C.; Ramos, I.P.; Dos Santos, H.A.M.; Gazzinelli, R.; Lannes-Vieira, J. CCL3/Macrophage Inflammatory Protein-1α Is Dually Involved in Parasite Persistence and Induction of a TNF- and IFNγ-Enriched Inflammatory Milieu in Trypanosoma cruzi-Induced Chronic Cardiomyopathy. Front. Immunol. 2020, 11, 306. [Google Scholar] [CrossRef]
- Paroli, A.F.; Gonzalez, P.V.; Díaz-Luján, C.; Onofrio, L.I.; Arocena, A.; Cano, R.C.; Silva, E.A.C.; Gea, S. NLRP3 Inflammasome and Caspase-1/11 Pathway Orchestrate Different Outcomes in the Host Protection against Trypanosoma cruzi Acute Infection. Front. Immunol. 2018, 9, 913. [Google Scholar] [CrossRef]
- Costa, R.W.; da Silveira, J.F.; Bahia, D. Interactions between Trypanosoma cruzi Secreted Proteins and Host Cell Signaling Pathways. Front. Microbiol. 2016, 7, 388. [Google Scholar] [CrossRef]
- Aoki, M.P.; Carrera-Silva, E.A.; Cuervo, H.; Fresno, M.; Gironès, N.; Gea, S. Nonimmune Cells Contribute to Crosstalk between Immune Cells and Inflammatory Mediators in the Innate Response to Trypanosoma cruzi Infection. J. Parasitol. Res. 2012, 2012, 737324. [Google Scholar] [CrossRef]
- Machado, F.S.; Dutra, W.O.; Esper, L.; Gollob, K.; Teixeira, M.M.; Factor, S.M.; Weiss, L.M.; Nagajyothi, F.; Tanowitz, H.B.; Garg, N.J. Current understanding of immunity to Trypanosoma cruzi infection and pathogenesis of Chagas disease. Semin. Immunopathol. 2012, 34, 753–770. [Google Scholar] [CrossRef]
- Colas, R.A.; Ashton, A.W.; Mukherjee, S.; Dalli, J.; Akide-Ndunge, O.B.; Huang, H.; Desruisseaux, M.S.; Guan, F.; Jelicks, L.A.; dos Santos, F.M.; et al. Trypanosoma cruzi Produces the Specialized Proresolving Mediators Resolvin D1, Resolvin D5, and Resolvin E2. Infect. Immun. 2018, 86, e00688-17. [Google Scholar] [CrossRef]
- Horta, A.L.; Williams, T.; Han, B.; Ma, Y.; Menezes, A.P.J.; Tu, V.; Talvani, A.; Weiss, L.M.; Huang, H. Resolvin D1 Administration Is Beneficial in Trypanosoma cruzi Infection. Infect. Immun. 2020, 88, e00052-20. [Google Scholar] [CrossRef]
- Shrestha, D.; Bajracharya, B.; Paula-Costa, G.; Salles, B.C.; Leite, A.L.J.; Menezes, A.P.J.; Souza, D.M.; Oliveira, L.A.; Talvani, A. Expression and production of cardiac angiogenic mediators depend on the Trypanosoma cruzi-genetic population in experimental C57BL/6 mice infection. Microvasc. Res. 2017, 110, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.L.J.; Costa, G.D.P.; Lopes, L.R.; Mota, L.W.D.R.; Vieira, P.M.D.A.; Talvani, A. The immunomodulatory effects of the Enalapril in combination with Benznidazole during acute and chronic phases of the experimental infection with Trypanosoma cruzi. Acta Trop. 2017, 174, 136–145. [Google Scholar] [CrossRef]
- de Souza, D.M.S.; Costa, G.d.P.; Leite, A.L.J.; de Oliveira, D.S.; Pinto, K.M.d.C.; Farias, S.E.B.; Simões, N.F.; de Paiva, N.C.N.; Vieira, P.M.d.A.; da Silva, C.A.M.; et al. A High-Fat Diet Exacerbates the Course of Experimental Trypanosoma cruzi Infection That Can Be Mitigated by Treatment with Simvastatin. BioMed. Res. Int. 2020, 2020, 1230461. [Google Scholar] [CrossRef] [PubMed]
- Roffê, E.; Rothfuchs, A.G.; Santiago, H.C.; Marino, A.P.M.P.; Ribeiro-Gomes, F.L.; Eckhaus, M.; Antonelli, L.R.V.; Murphy, P.M. IL-10 Limits Parasite Burden and Protects against Fatal Myocarditis in a Mouse Model of Trypanosoma cruzi Infection. J. Immunol. 2012, 188, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Stone, K.P.; Kastin, A.J.; Pan, W. NFĸB is an Unexpected Major Mediator of Interleukin-15 Signaling in Cerebral Endothelia. Cell Physiol. Biochem. 2011, 28, 115–124. [Google Scholar] [CrossRef]
- Karimian, M.S.; Pirro, M.; Majeed, M.; Sahebkar, A. Curcumin as a natural regulator of monocyte chemoattractant protein-1. Cytokine Growth Factor Rev. 2017, 33, 55–63. [Google Scholar] [CrossRef]
- Patidar, M.; Yadav, N.; Dalai, S.K. Interleukin 15: A key cytokine for immunotherapy. Cytokine Growth Factor Rev. 2016, 31, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, S.G.; Reis, M.M.; Coelho, V.; Nogueira, L.G.; Monteiro, S.M.; Mairena, E.C.; Bacal, F.; Bocchi, E.; Guilherme, L.; Zheng, X.X.; et al. Locally Produced Survival Cytokines IL-15 and IL-7 may be Associated to the Predominance of CD8+T cells at Heart Lesions of Human Chronic Chagas Disease Cardiomyopathy. Scand. J. Immunol. 2007, 66, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Menezes, T.P.; Machado, B.A.A.; Toledo, D.N.M.; dos Santos, P.V.; Ribeiro, L.; Talvani, A. Insights into CX3CL1/Fractalkine during experimental Trypanosoma cruzi infection. Parasitol. Int. 2022, 87, 102530. [Google Scholar] [CrossRef]
- Talvani, A.; Rocha, M.O.C.; Barcelos, L.S.; Gomes, Y.M.; Ribeiro, A.L.; Teixeira, M.M. Elevated Concentrations of CCL2 and Tumor Necrosis Factor–α in Chagasic Cardiomyopathy. Clin. Infect. Dis. 2004, 38, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Kanai, M.; Otsuka, Y.; Otsuka, K.; Sato, M.; Nishimura, T.; Mori, Y.; Kawaguchi, M.; Hatano, E.; Kodama, Y.; Matsumoto, S.; et al. A phase I study investigating the safety and pharmacokinetics of highly bioavailable curcumin (Theracurmin) in cancer patients. Cancer Chemother. Pharmacol. 2013, 71, 1521–1530. [Google Scholar] [CrossRef]
- Kanai, M. Therapeutic applications of curcumin for patients with pancreatic cancer. World J. Gastroenterol. 2014, 20, 9384–9391. [Google Scholar]
| Uninfected and Untreated (%) | Theracurmin (%) | T. cruzi (%) | T.cruzi + Theracurmin (%) | T. cruzi + Benznidazole (%) |
|---|---|---|---|---|
| 12.94 ± 0.96 | 9.718 ± 1.5 | 0.7850 ± 2.5 * | 1.995 ± 3.4 *,# | 3.072 ± 4.2 *,# |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Louise, V.; Machado, B.A.A.; Pontes, W.M.; Menezes, T.P.; Dias, F.C.R.; Ervilhas, L.O.G.; Pinto, K.M.d.C.; Talvani, A. Theracurmin Modulates Cardiac Inflammation in Experimental Model of Trypanosoma cruzi Infection. Trop. Med. Infect. Dis. 2023, 8, 343. https://doi.org/10.3390/tropicalmed8070343
Louise V, Machado BAA, Pontes WM, Menezes TP, Dias FCR, Ervilhas LOG, Pinto KMdC, Talvani A. Theracurmin Modulates Cardiac Inflammation in Experimental Model of Trypanosoma cruzi Infection. Tropical Medicine and Infectious Disease. 2023; 8(7):343. https://doi.org/10.3390/tropicalmed8070343
Chicago/Turabian StyleLouise, Vitória, Bianca Alves Almeida Machado, Washington Martins Pontes, Tatiana Prata Menezes, Fernanda Carolina Ribeiro Dias, Luiz Otávio Guimarães Ervilhas, Kelerson Mauro de Castro Pinto, and André Talvani. 2023. "Theracurmin Modulates Cardiac Inflammation in Experimental Model of Trypanosoma cruzi Infection" Tropical Medicine and Infectious Disease 8, no. 7: 343. https://doi.org/10.3390/tropicalmed8070343
APA StyleLouise, V., Machado, B. A. A., Pontes, W. M., Menezes, T. P., Dias, F. C. R., Ervilhas, L. O. G., Pinto, K. M. d. C., & Talvani, A. (2023). Theracurmin Modulates Cardiac Inflammation in Experimental Model of Trypanosoma cruzi Infection. Tropical Medicine and Infectious Disease, 8(7), 343. https://doi.org/10.3390/tropicalmed8070343







