Abstract
Background: COVID-19 caused devastating effects on global healthcare systems. The elderly and people with chronic comorbidities were at a particularly high risk of mortality and morbidity. However, the evidence on the association of COVID-19 severity with noncommunicable diseases (NCDs) in the African population is scarce. Objective: The aim is to estimate COVID-19 severity among African patients with hypertension, diabetes, and cardiovascular diseases (CVDs) and its implications for case management. Methods: We will adhere to the extension for Scoping Reviews of PRISMA (PRISMA-ScR). The following electronic databases will be searched: PubMed, Scopus, Web of Science, Embase, CINAHL, and Joanna Briggs Institute. The search will be conducted after the publication of this protocol. Two reviewers will extract data from articles published after March 2020 without language restrictions. A descriptive analysis of the important findings and a narrative synthesis of the results will serve as the basis for interpretation. Expected results and conclusions: This scoping review is expected to determine the odds of patients with chronic comorbidities to progress to severe stages of COVID-19. The review will generate an evidence-based and set foundation for recommendations toward the establishment of surveillance systems and referral guidelines for the management of NCDs in the face of COVID-19 and future pandemics.
1. Introduction
Coronavirus disease 2019 (COVID-19), is an illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. The unprecedented spread of this infectious disease (pandemic) posed devastating effects on the health and well-being of people around the world, including Africa. As of 1 January 2023, according to the Africa Centre for Diseases Control and Prevention (Africa CDC) [2,3], there were more than 12.2 million confirmed cases and 256,542 deaths reported throughout Africa, representing about 2% of all cases (656.4 million) and about 4% of all deaths (6.7 million) reported globally.
The effect of the COVID-19 pandemic on global healthcare systems has been profound. In particular, the impact of the pandemic on the elderly and people with non-communicable diseases (NCDs) has been devastating [4,5,6,7,8]. Important issues include disordered regular service delivery, decreased access to existing healthcare facilities, social isolation, and supply chain disruptions [4,5,9]. Additionally, the COVID-19 pandemic severely affected access to and the utilization of healthcare facilities, management of chronic diseases, maternal and child services, vaccination programs and regular control, and treatment of endemic diseases, such as malaria, tuberculosis, HIV/AIDS, and neglected tropical diseases [6,10,11,12,13,14]. Interventions and clinical trials have also been adversely affected.
The increasing burden of NCDs along with the enduring burden of infectious diseases in Africa and other settings in low- and middle-income countries (LMICs) has been well noted [15]. Nevertheless, the treatment and control of NCDs have been routinely given less attention, as often priority is bestowed to the control and management of infectious diseases. Additionally, regular check-ups and early screenings are yet not well adapted. Treatment delays and low utilization rates of the available services have been among the daunting challenges in Africa and elsewhere in LMICs leading to a high prevalence of advanced NCD conditions and an increased burden of preventable complications. Moreover, inadequate self-management practices and nonadherence to treatment procedures of these lifelong conditions have been major hurdles to patients with NCDs. Given this existing dual burden of disease in Africa and the global impact of COVID-19, the pandemic presumably aggravated the already existing healthcare crisis in the continent [6,7,8,16,17].
Nonetheless, information on the effect of COVID-19 in Africa focused on health system challenges, and evidence on the effect of COVID-19 in African patients with hypertension, diabetes, and cardiovascular diseases (CVDs) was extrapolated from what was obtained in other parts of the globe. In order to properly allocate scarce resources and to support clinical decisions during the COVID-19 pandemic, it is important to have an evidence-based record, derived from available epidemiological and clinical data on the comorbidity between NCDs and COVID-19 among African patients. This is of particular importance as in many African countries, the demography is characterized by a large proportion of young people and a lower prevalence of lifestyle risks (e.g., obesity and smoking), which may prevent against severe SARS-CoV-2 [18,19,20,21]. Furthermore, genetic differences in COVID-19 susceptibility may exist [22]. To our knowledge, no study or report is available to date that summarizes the severity of COVID-19 in African patients with NCDs.
A preliminary search on International Prospective Register of Systematic Reviews (PROSPERO) [23], Open Science Framework (OSF) [24], and Joanna Briggs Institute (JBI) [25] showed that no scoping review on the association between COVID-19 and hypertension, diabetes, and CVDs is currently ongoing or registered. Hence, this scoping review is an attempt to fill this gap and generate evidence for improved management of COVID-19 and the aforementioned NCDs. Furthermore, it will inform African policy makers and healthcare professionals toward the establishment of surveillance systems and referral guidelines for the management of NCDs during the COVID-19 pandemic and future pandemics.
2. Aim and Review Questions
The overarching aim of this review is to focus on the potential factors related to the severity of COVID-19 for African patients with hypertension, diabetes, and CVDs and their implications for case management. The following research questions will guide to conduct this scoping review:
- What type of severity outcomes were reported in the included studies?
- What relative impact did the selected NCDs have on the severity of COVID-19?
- Are there specific patient characteristics that increase the risk of COVID-19 severity among patients with the selected NCDs, namely hypertension, diabetes, and CVDs?
- What strategies and interventions have addressed the risk factors for COVID-19 severity in comorbid patients?
- Which of the three selected NCDs had a major impact on exacerbating COVID-19 severity in Africa?
- What impact did the COVID-19 response have on the services for NCDs in Africa?
3. Materials and Methods
3.1. Protocol and Registration
The scoping review will be conducted in accordance with the guidance for pursuing systematic scoping reviews, put forth by Peters and colleagues from JBI in Australia [26]. The methodologies comply with the extension for Scoping Reviews of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA-ScR), given in Appendix A [27]. The review protocol was registered on OSF (registration link: http//:osf.io/e9r28 accessed on 12 May 2023) on 17 November 2022.
3.2. Inclusion Criteria
Studies focusing on COVID-19 patients meeting the following criteria will be included: (i) studies that estimated the quantitative relationship between COVID-19 and hypertension, diabetes, and CVDs; (ii) studies conducted on the African continent; (iii) and studies of both observational (longitudinal and cross-sectional) and interventional (randomized and nonrandomized community trials and controlled and uncontrolled before/after studies) designs.
3.3. Exclusion Criteria
Not considered will be studies that met at least one of the following exclusion criteria: (i) studies that evaluate COVID-19 patients without considering NCDs and vice versa; (ii) position papers, editorials, policy statements, case reports, case series studies, perspectives, commentaries, published abstracts, poster and oral presentations, and author reply articles; (iii) studies on the potential association between COVID-19 and other infectious diseases, malignancies, or autoimmune disorders, but not considering diabetes and hypertension; and (iv) articles that speculatively extrapolate findings from studies conducted outside Africa to explain the effects of COVID-19 in Africa.
3.4. Participants
The literature search will include results from studies reporting on COVID-19 and NCD comorbidities among African patients. The review will include studies on adults aged ≥18 years, irrespective of their gender. Data on patients participating in clinical trials, cross-sectional epidemiological studies, or cohort studies (both retrospective and prospective) will be included in the review. Data on patients admitted to any healthcare facility, including outpatient departments, emergency rooms, and intensive care units (ICUs) and reporting to have comorbidities of COVID-19 and NCDs are eligible for the review. However, the review will not consider studies that report from African diaspora patients (living outside of Africa).
3.5. Concept
The review will address the severity of COVID-19 symptoms among African patients due to either one or several of the selected NCDs. For pragmatism and homogeneousness, the review will consider the standard definitions of COVID-19 severities set forth by the World Health Organization (WHO). The common categories for the level of aggravation of COVID-19 among adult population are nonsevere (mild or early stage), moderate, severe, and critical [28]. Moreover, as many studies from our preliminary search mentioned “asymptomatic” as a category in their findings, we will include it in the search outcome, in addition to the categories used by WHO [29]. The review will uncover the weight of multicomorbidity of NCDs in further aggravating COVID-19 infection, changing the pattern or course of outcomes. Patient characteristics, which weigh in exacerbating the COVID-19 condition, will be marked. The final analysis will map and elaborate the morbidity outcome in relation to the major NCDs among African patients and identify strategies and intervention management for NCD comorbidity during pandemics on the continent.
3.6. Information Sources and Search Strategy
The systematic search strategy will mainly be aimed at published peer-reviewed articles. To identify potentially suitable articles, we will search documents from the following electronic databases: PubMed/MEDLINE, Embase/Elsevier, Scopus, Cumulative Index to Nursing & Allied Health Literature (CINAHL)/EBSCO, and Web of Science. Because the first COVID-19 case was confirmed in Africa on 14 February 2020, we will search the databases from March 2020 to 28 February 2023 (3-year period) without language restrictions.
A three-step search strategy will be used in this review. First, an initial search of PubMed will be undertaken followed by an analysis of the text words contained in the title and abstract and of the index terms used to describe the article. Second, using all the identified keywords and index terms, we will search all the other databases. Third, we will undertake a hand search of the reference list of all the identified relevant documents for potential additional articles. An example of the terms and strings of words applied on PubMed is provided in Appendix B.
3.7. Search Results
Records retrieved through the aforementioned search strategy from all databases will be imported into the bibliometric software EndNote™ X9 (Clarivate Analytics; Philadelphia, PA, USA) and screened for relevance and duplication. The criteria for relevance are based on the scope and objectives of the review. Using the inclusion criteria set above, two reviewers will conduct full assessment of the identified scientific publications, and any duplicates will be removed. Any disagreement will be resolved through discussion, and with a third reviewer, as the case might be.
3.8. Data Charting Process
The reviewers will develop a data abstraction tool to capture relevant information from the selected documents. The tool will encompass detailed information that includes (i) participant characteristics, such as demographics of patients; (ii) study characteristics, such as study setting, study types, publication dates, authors, methodology, etc.; and (iii) outcomes and key findings related to the review objective. Two reviewers will independently chart information from each selected document to ensure charting consistency and inter-reviewer reliability. In case of disagreements, the two reviewers will resolve through discussion or in consultation with a third team member.
All extracted data will be exported to Microsoft® Excel 2016 (Microsoft; Redmond, WA, USA). A draft data abstraction tool is provided in Appendix C. As the reviewers familiarize themselves with the content of the selected documents, necessary modifications to and revisions of the data abstraction tool will be made. We will include the final version of the data abstraction tool in the scoping review publication.
3.9. Data Analysis and Presentation
Outcome of the systematic search will be analyzed descriptively using frequencies and percentages. Moreover, graphical presentation including tables and charts will be used, whenever applicable. This will compare and/or reflect the effect of NCDs on the severity of COVID-19. Effect of comorbidities of selected NCDs (diabetes, hypertension, and CVDs) will be mapped accordingly and will be presented in comparison to those without any comorbidities.
Figure 1 illustrates the graphical summary of the methodological strategy applied for the protocol of the scoping review.
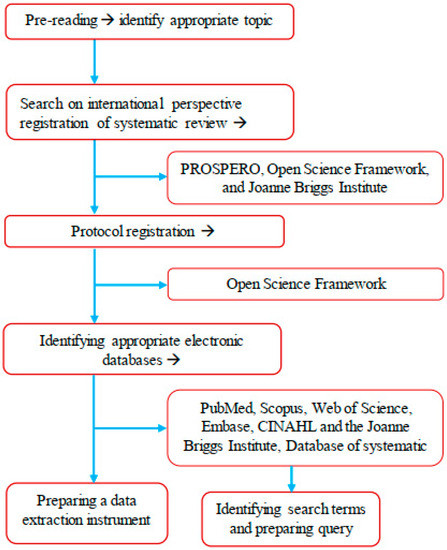
Figure 1.
Graphic summary of the methodological strategy of the scoping review.
4. Results
In this section, we will summarize the results of the search strategy and the process of document selection, inclusion, and exclusion in both text and chart formats. We will tabulate and describe detailed information about the selected studies. Emphasis will be placed on the following information: authors, year of publication, country, aim of the study, study design, study setting, participant characteristics, sample size, main findings, measures of outcomes, and effect size (if relevant).
The abstracted information in relation to the objectives of the review will be summarized and presented in detail. For example, we will present the number of studies that examined associations between COVID-19 and hypertension. We will describe the relative frequencies of the studies by geographical location and number and characteristics of the participants included in terms of age, sex, and severity of COVID-19. Moreover, the types of study designs and outcome measurements will be described. Additionally, the types and strengths of the reported relationships between COVID-19 and hypertension will be given, and the consistency of the findings will be reported. We will also recount the effect of the COVID-19 response measures (e.g., lockdowns, closure of outpatient clinics, stock-outs of medicines, diagnostics, personal protective equipment, etc.) on patients.
After describing the reported relationship between COVID-19 and the three selected NCDs, we will report the relationship between COVID-19 and concurrent NCDs. The final synthesis will present the overall severity of COVID-19 in people with all the selected NCDs.
5. Conclusions
This scoping review is expected to determine the odds of patients with chronic comorbidities to advance into severe stages of COVID-19 and to estimate the extent to which NCD services were affected during the COVID-19 response in Africa. In doing so, the review will provide new evidence and set foundations for recommendations toward the establishment of surveillance systems and referral guidelines for the management of NCDs during the COVID-19 pandemic and future pandemics. The outcome of the review will increase awareness of healthcare professionals, policy makers, and other key stakeholders. It will also enhance further collaboration among research, surveillance systems, and technological advancement to develop new advanced diagnostic tools and to set up policies that deal with the silent pandemic of the dual burden of NCDs and infectious diseases in Africa.
Author Contributions
F.N. and A.C. designed, searched and conducted the protocol. J.U., D.H.P. and N.P.-H. read and assessed the methodological quality and critically reviewed the protocol. J.U. supervised the protocol, and D.H.P. and N.P.-H. provided expert opinions. A.C. conceptualized and drafted the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank the two anonymous reviewers for their useful comments and suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) checklist.
| Section | Item | PRISMA-ScR Checklist Item | Reported on Page # |
| TITLE | |||
| Title | 1 | Identify the report as a scoping review | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary that includes (as applicable): background, objectives, eligibility criteria, sources of evidence, charting methods, results, and conclusions that relate to the review questions and objectives | 1 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. Explain why the review questions/objectives lend themselves to a scoping review approach | 1 |
| Objectives | 4 | Provide an explicit statement of the questions and objectives being addressed with reference to their key elements (e.g., population or participants, concepts, and context) or other relevant key elements used to conceptualize the review questions and/or objectives | 2 |
| METHODS | |||
| Protocol and registration | 5 | Indicate whether a review protocol exists; state if and where it can be accessed (e.g., a Web address), and if available, provide registration information, including the registration number | 3 |
| Eligibility criteria | 6 | Specify characteristics of the sources of evidence used as eligibility criteria (e.g., years considered, language, and publication status) and provide a rationale | 3 |
| Information sources * | 7 | Describe all information sources in the search (e.g., databases with dates of coverage and contact with authors to identify additional sources), as well as the date the most recent search was executed | 4 |
| Search | 8 | Present the full electronic search strategy for at least one database, including any limits used, such that it could be repeated | 4 |
| Selection of sources of evidence † | 9 | State the process for selecting sources of evidence (i.e., screening and eligibility) included in the scoping review | 4 |
| Data charting process ‡ | 10 | Describe the methods of charting data from the included sources of evidence (e.g., calibrated forms or forms that were tested by the team before their use and whether data charting was performed independently or in duplicate) and any processes for obtaining and confirming data from investigators | 4 |
| Data items | 11 | List and define all variables for which data were sought and any assumptions and simplifications made | 4 & 10 |
| Critical appraisal of individual sources of evidence § | 12 | If performed, provide a rationale for conducting a critical appraisal of included sources of evidence and describe the methods used and how this information was used in any data synthesis (if appropriate) | NA |
| Synthesis of results | 13 | Describe the methods of handling and summarizing the data that were charted | 4 |
| RESULTS | |||
| Selection of sources of evidence | 14 | Give numbers of sources of evidence screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally using a flow diagram | NA |
| Characteristics of sources of evidence | 15 | For each source of evidence, present characteristics for which data were charted and provide the citations | NA |
| Critical appraisal within sources of evidence | 16 | If performed, present data on critical appraisal of included sources of evidence (see item 12) | NA |
| Results of individual sources of evidence | 17 | For each included source of evidence, present the relevant data that were charted that relate to the review questions and objectives | NA |
| Synthesis of results | 18 | Summarize and/or present the charting results as they relate to the review questions and objectives | NA |
| DISCUSSION | |||
| Summary of evidence | 19 | Summarize the main results (including an overview of concepts, themes, and types of evidence available), link to the review questions and objectives, and consider the relevance to key groups | NA |
| Limitations | 20 | Discuss the limitations of the scoping review process | NA |
| Conclusions | 21 | Provide a general interpretation of the results with respect to the review questions and objectives, as well as potential implications and/or next steps | 5 |
| FUNDING | |||
| Funding | 22 | Describe sources of funding for the included sources of evidence, as well as sources of funding for the scoping review. Describe the role of the funders of the scoping review | NA |
| JBI = Joanna Briggs Institute; PRISMA-ScR = Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews. * Where sources of evidence (see second footnote) are compiled from, such as bibliographic databases, social media platforms, and websites. † A more inclusive/heterogeneous term used to account for the different types of evidence or data sources (e.g., quantitative and/or qualitative research, expert opinion, and policy documents) that may be eligible in a scoping review as opposed to only studies. This is not to be confused with information sources (see first footnote). ‡ The frameworks by Arksey and O’Malley (6) and Levac and colleagues (7) and the JBI guidance (4, 5) refer to the process of data extraction in a scoping review as data charting. § The process of systematically examining research evidence to assess its validity, results, and relevance before using it to inform a decision. This term is used for items 12 and 19 instead of “risk of bias” (which is more applicable to systematic reviews of interventions) to include and acknowledge the various sources of evidence that may be used in a scoping review (e.g., quantitative and/or qualitative research, expert opinion, and policy document). NA = Not Applicable. | |||
Appendix B
The following terms and strings of word combinations were applied to identify relevant studies as an example for PubMed/MEDLIN. Search conducted on 13 March 2023.
| Search | Terms | Query | Result |
| #1 | COVID-19 | “COVID-19”[tiab] OR “COVID-19”[MeSH Terms] OR “SARS-CoV-2”[tiab] OR “SARS-CoV-2”[MeSH Terms] OR “coronavirus”[MeSH Terms] OR “coronavirus”[tiab] | 336,796 |
| #2 | Severity | severity[tiab] | 125,613 |
| #3 | Cardiovascular diseases (CVDs) | “Coronary Artery Disease”[Majr] OR “Cardiovascular Diseases”[Majr] OR “Coronary artery disease”[tiab] OR “CAD”[tiab] OR “coronary heart disease”[tiab] OR “CHD”[tiab] OR “ischemic heart disease”[tiab] OR “IHD”[tiab] OR “heart disease”[tiab] OR “Cardiovascular diseases”[tiab] OR “CVS” OR “stroke”[tiab] OR “peripheral artery disease”[tiab] | 318,922 |
| #4 | Diabetes | “Diabetes Mellitus”[Majr] OR “Hyperglycemia”[Majr] OR Diabetes[Title/Abstract] OR Hyperglycem*[Title/Abstract] | 142,826 |
| #5 | Hypertension | “hypertension”[Title/Abstract] OR “blood pressure”[Title/Abstract] OR “Hypertension”[Majr] OR “Blood Pressure”[Majr] | 101,201 |
| #6 | NCDs | “noncommunicable disease”[Title/Abstract] OR “non-communicable disease”[Title/Abstract] OR NCDs[Title/Abstract] OR “Noncommunicable Diseases”[Majr] | 3829 |
| #7 | African countries | “Africa”[Majr] OR Africa*[tiab] OR Nigeria[tiab] OR Ethiopia[tiab] OR Egypt[tiab] OR “DR Congo” [tiab] OR Tanzania[tiab] OR “South Africa”[tiab] OR Kenya[tiab] OR Uganda[tiab] OR Algeria[tiab] OR Sudan[tiab] OR Morocco[tiab] OR Angola[tiab] OR Mozambique[tiab] OR Ghana[tiab] OR Madagascar[tiab] OR Cameroon[tiab] OR ” Côte d’Ivoire”[tiab] OR “Ivory Coast”[tiab] OR Niger[tiab] OR “Burkina Faso” [tiab] OR Mali[tiab] OR Malawi[tiab] OR Zambia[tiab] OR Senegal[tiab] OR Chad[tiab] OR Somalia[tiab] OR Zimbabwe[tiab] OR Guinea[tiab] OR Rwanda[tiab] OR Benin[tiab] OR Burundi[tiab] OR Tunisia[tiab] OR “South Sudan”[tiab] OR Togo[tiab] OR “Sierra Leone”[tiab] OR Libya[tiab] OR Congo[tiab] OR Liberia[tiab] OR “Central African Republic” [tiab] OR Mauritania[tiab] OR Eritrea[tiab] OR Namibia[tiab] OR Gambia[tiab] OR Botswana[tiab] OR Gabon[tiab] OR Lesotho[tiab] OR “Guinea-Bissau”[tiab] OR “Equatorial Guinea” [tiab] OR Mauritius[tiab] OR Eswatini[tiab] OR Djibouti[tiab] OR Comoros[tiab] OR “Cabo Verde”[tiab] OR “Sao Tome & Principe” [tiab] OR Seychelles[tiab] | 108,079 |
| #8 | To evaluate the effect of NCDs on COVID-19 severity | (((“COVID-19”[tiab] OR “COVID-19”[MeSH Terms] OR “SARS-CoV-2”[tiab] OR “SARS-CoV-2”[MeSH Terms] OR “coronavirus”[MeSH Terms] OR “coronavirus”[tiab]) AND (severity[tiab])) AND (“noncommunicable disease”[tiab] OR “non-communicable disease”[tiab] OR NCDs[tiab] OR “Noncommunicable Diseases”[Majr] OR “Diabetes Mellitus”[Majr] OR “Hyperglycemia”[Majr] OR Diabetes[tiab] OR Hyperglycem*[tiab] OR “Coronary Artery Disease”[Majr] OR “Cardiovascular Diseases”[Majr] OR “Coronary artery disease”[tiab] OR “CAD”[tiab] OR “coronary heart disease”[tiab] OR “CHD”[tiab] OR “ischemic heart disease”[tiab] OR “IHD”[tiab] OR “heart disease”[tiab] OR “Cardiovascular diseases”[tiab] OR “CVS” OR “hypertension”[Title/Abstract] OR “blood pressure”[tiab] OR “Hypertension”[Majr] OR “Blood Pressure”[Majr])) AND (“Africa”[Majr] OR Africa*[tiab] OR Nigeria[tiab] OR Ethiopia[tiab] OR Egypt[tiab] OR “DR Congo”[tiab] OR Tanzania[tiab] OR “South Africa”[tiab] OR Kenya[tiab] OR Uganda[tiab] OR Algeria[tiab] OR Sudan[tiab] OR Morocco[tiab] OR Angola[tiab] OR Mozambique[tiab] OR Ghana[tiab] OR Madagascar[tiab] OR Cameroon[tiab] OR ” Côte d’Ivoire”[tiab] OR “Ivory Coast”[tiab] OR Niger[tiab] OR “Burkina Faso”[tiab] OR Mali[tiab] OR Malawi[tiab] OR Zambia[tiab] OR Senegal[tiab] OR Chad[tiab] OR Somalia[tiab] OR Zimbabwe[tiab] OR Guinea[tiab] OR Rwanda[tiab] OR Benin[tiab] OR Burundi[tiab] OR Tunisia[tiab] OR “South Sudan”[tiab] OR Togo[tiab] OR “Sierra Leone”[tiab] OR Libya[tiab] OR Congo[tiab] OR Liberia[tiab] OR “Central African Republic”[tiab] OR Mauritania[tiab] OR Eritrea[tiab] OR Namibia[tiab] OR Gambia[tiab] OR Botswana[tiab] OR Gabon[tiab] OR Lesotho[tiab] OR “Guinea-Bissau”[tiab] OR “Equatorial Guinea”[tiab] OR Mauritius[tiab] OR Eswatini[tiab] OR Djibouti[tiab] OR Comoros[tiab] OR “Cabo Verde”[tiab] OR “Sao Tome & Principe”[tiab] OR Seychelles[tiab]) | 124 |
Appendix C
Data extraction instrument.
| Item | Description | Response |
| Study ID | 1. Author | |
| 2. Year | ||
| 3. Title | ||
| 4. Journal | ||
| Reason for inclusion or exclusion | 5. Did the study or source of information present COVID-19 and NCD comorbidity? |
|
| 6. Did the study or source of information present severity of COVID-19? |
| |
| 7. Was the study or source of information from or about any African country? |
| |
| 8. Was the literature finding a review? |
| |
| 9. Was the search finding an expert opinion? |
| |
| 10. Were there other reasons to exclude the article? |
| |
| 11. If yes for #10, specify reason | List the reasons | |
| Characteristics of study population/articles | 12. Sample size | Specify number |
| 13. Gender balance | Male to female ratio | |
| 14. Study population type |
| |
| 15. Age groups of patients (years) |
| |
| 16. Which single comorbidity did the patients have? |
| |
| 17. Were there multiple comorbidities? |
| |
| 18. Patient type |
| |
| 19. Vaccination status |
| |
| Research methods used in the study (project)/article (Published paper) | 20. What type of article was it? |
|
| 21. Study design |
| |
| 22. What kind of study? |
| |
| 23. If observation |
| |
| 24. If experimental |
| |
| 25. Study type |
| |
| 26. Data collection tool/procedure applied |
| |
| 27. Data analysis |
| |
| 28. Study area coverage |
| |
| 29. Study setting (centers) |
| |
| Note | 30. Short note or summary of the article | Description |
References
- WHO. Naming the Coronavirus Disease (COVID-19) and the Virus That Causes It. 2020. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-i (accessed on 6 July 2022).
- Africa-CDC. Coronavirus Disease 2019 (COVID-19): Latest Updates on the COVID-19 Crisis from Africa CDC. 2023. Available online: https://africacdc.org/covid-19/ (accessed on 13 March 2023).
- WHO. Weekly Epidemiological Update on COVID-19—4 January 2023. 2023. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19 (accessed on 4 January 2023).
- WHO-UNDP. Responding to Non-Communicable Diseases during and beyond the COVID-19 Pandemic; World Health Organization & United Nations Development Programme: Geneva, Switzerland, 2020. [Google Scholar]
- Wildman, J.M.; Morris, S.; Pollard, T.; Gibson, K.; Moffatt, S. “I wouldn’t survive it, as simple as that”: Syndemic vulnerability among people living with chronic non-communicable disease during the COVID-19 pandemic. SSM Qual. Res. Health 2022, 2, 100032. [Google Scholar] [CrossRef] [PubMed]
- Formenti, B.; Natalia, G.; Crosato, V.; Marchese, V.; Tomasoni, R.L.; Castelli, F. The impact of COVID-19 on communicable and non-communicable diseases in Africa: A narrative review. Infez. Med. 2022, 30, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Delobelle, P.A.; Abbas, M.; Datay, I.; De Sa, A.; Levitt, N.; Schouw, D.; Reid, S. Non-communicable disease care and management in two sites of the Cape Town Metro during the first wave of COVID-19: A rapid appraisal. Afr. J. Prim. Health Care Fam. Med. 2022, 14, 3215. [Google Scholar] [CrossRef] [PubMed]
- Owopetu, O.; Fasehun, L.K.; Abakporo, U. COVID-19: Implications for NCDs and the continuity of care in Sub-Saharan Africa. Glob. Health Promot. 2021, 28, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Kiragu, Z.W.; Gathecha, G.; Mwangi, M.K.; Ndegwa, Z.; Pastakia, S.; Nyagah, D.; Cizungu, R.N.; Takah Mutwiri, M.; Ndolo, M.; Wirtz, V.J. Access to Medicines for Non-Communicable Diseases (NCDS) during COVID-19 in Kenya: A Descriptive Commentary. Health Syst. Reform 2021, 7, e1984865. [Google Scholar] [CrossRef] [PubMed]
- Quaglio, G.; Cavallin, F.; Nsubuga, J.B.; Lochoro, P.; Maziku, D.; Tsegaye, A.; Azzimonti, G.; Kamunga, A.M.; Manenti, F.; Putoto, G. The impact of the COVID-19 pandemic on health service use in sub-Saharan Africa. Public Health Action 2022, 12, 34–39. [Google Scholar] [CrossRef] [PubMed]
- WHO-Africa. COVID-19 Hits Life-Saving Health Services in Africa. 2020. Available online: https://www.afro.who.int/news/covid-19-hits-life-saving-health-services-africa (accessed on 7 July 2022).
- Amouzou, A.; Maïga, A.; Faye, C.M.; Chakwera, S.; Melesse, D.Y.; Mutua, M.K.; Thiam, S.; Abdoulaye, I.B.; Afagbedzi, S.K.; Ag Iknane, A.; et al. Health service utilisation during the COVID-19 pandemic in sub-Saharan Africa in 2020: A multicountry empirical assessment with a focus on maternal, newborn and child health services. BMJ Glob. Health 2022, 7, e008069. [Google Scholar] [CrossRef] [PubMed]
- Shapira, G.; Ahmed, T.; Drouard, S.H.P.; Amor Fernandez, P.; Kandpal, E.; Nzelu, C.; Wesseh, C.S.; Mohamud, N.A.; Smart, F.; Mwansambo, C.; et al. Disruptions in maternal and child health service utilization during COVID-19: Analysis from eight sub-Saharan African countries. Health Policy Plan. 2021, 36, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Holtz, L. COVID-19’s impact on overall health care services in Africa. Brook. Inst. 2021. Available online: https://www.brookings.edu/blog/africa-in-focus/2021/10/12/covid-19s-impact-on-overall-health-care-services-in-africa/ (accessed on 7 January 2022).
- Remais, J.V.; Zeng, G.; Li, G.; Tian, L.; Engelgau, M.M. Convergence of non-communicable and infectious diseases in low- and middle-income countries. Int. J. Epidemiol. 2013, 42, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Tessema, G.A.; Kinfu, Y.; Dachew, B.A.; Tesema, A.G.; Assefa, Y.; Alene, K.A.; Aregay, A.F.; Ayalew, M.B.; Bezabhe, W.M.; Bali, A.G.; et al. The COVID-19 pandemic and healthcare systems in Africa: A scoping review of preparedness, impact and response. BMJ Glob. Health 2021, 6, e007179. [Google Scholar] [CrossRef] [PubMed]
- WHO-Africa. Noncommunicable Diseases Increase Risk of Dying from COVID-19 in Africa. 2020. Available online: https://www.afro.who.int/news/noncommunicable-diseases-increase-risk-dying-covid-19-africa (accessed on 7 July 2022).
- Singh, R.; Rathore, S.S.; Khan, H.; Karale, S.; Chawla, Y.; Iqbal, K.; Bhurwal, A.; Tekin, A.; Jain, N.; Mehra, I.; et al. Association of Obesity With COVID-19 Severity and Mortality: An Updated Systemic Review, Meta-Analysis, and Meta-Regression. Front. Endocrinol. 2022, 13, 780872. [Google Scholar] [CrossRef] [PubMed]
- Moschovis, P.P.; Lu, M.; Hayden, D.; Yonker, L.M.; Lombay, J.; Taveras, E.; Boudreau, A.A.; Triant, V.A.; Foulkes, A.S.; Bassett, I.; et al. Effect modification by age of the association between obstructive lung diseases, smoking and COVID-19 severity. BMJ Open Respir. Res. 2021, 8, e001038. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; He, Y.; Hu, Q.; Yang, S.; Li, J.; Liu, Y.; Hu, J. Association between smoking and COVID-19 severity: A multicentre retrospective observational study. Medicine 2022, 101, e29438. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.K.; Charles, W.N.; Sklavounos, A.; Dutt, A.; Seed, P.T.; Khajuria, A. The effect of smoking on COVID-19 severity: A systematic review and meta-analysis. J. Med. Virol. 2021, 93, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- SeyedAlinaghi, S.; Mehrtak, M.; MohsseniPour, M.; Mirzapour, P.; Barzegary, A.; Habibi, P.; Moradmand-Badie, B.; Afsahi, A.M.; Karimi, A.; Heydari, M.; et al. Genetic susceptibility of COVID-19: A systematic review of current evidence. Eur. J. Med. Res. 2021, 26, 46. [Google Scholar] [CrossRef] [PubMed]
- PROSPERO. International Prospective Register of Systematic Reviews-PROSPERO. 2022. Available online: https://www.crd.york.ac.uk/prospero/#searchadvanced (accessed on 7 July 2022).
- OSF. Open Science Framework-OSF. 2022. Available online: https://osf.io/registries/discover?page=4&provider=OSF%20Registries&q=covid-19%20and%20NCD%20in%20Africa&type=Registered%20Report%20Protocol%20Preregistration&view_only=true (accessed on 7 July 2022).
- JBI. Joanna Briggs Institute-JBI. 2022. Available online: https://jbi.global/systematic-review-register (accessed on 7 July 2022).
- Peters, M.D.J.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for conducting systematic scoping reviews. JBI Evid. Implement. 2015, 13, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Guérin, P.J.; McLean, A.R.D.; Rashan, S.; Lawal, A.; Watson, J.A.; Strub-Wourgaft, N.; White, N.J. Definitions matter: Heterogeneity of COVID-19 disease severity criteria and incomplete reporting compromise meta-analysis. Cold Spring Harbor Laboratory. medRxiv 2021, 1, 16–18. [Google Scholar] [CrossRef]
- NIH. Clinical Spectrum of SARS-CoV-2 Infection. 2021. Available online: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ (accessed on 7 July 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).