Feasibility, Uptake, and Results of COVID-19 Antigen Rapid Diagnostic Tests among Refugees and Migrants in a Pilot Project in North-West Syria
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population Settings
2.3. Ag-RDT Project Settings
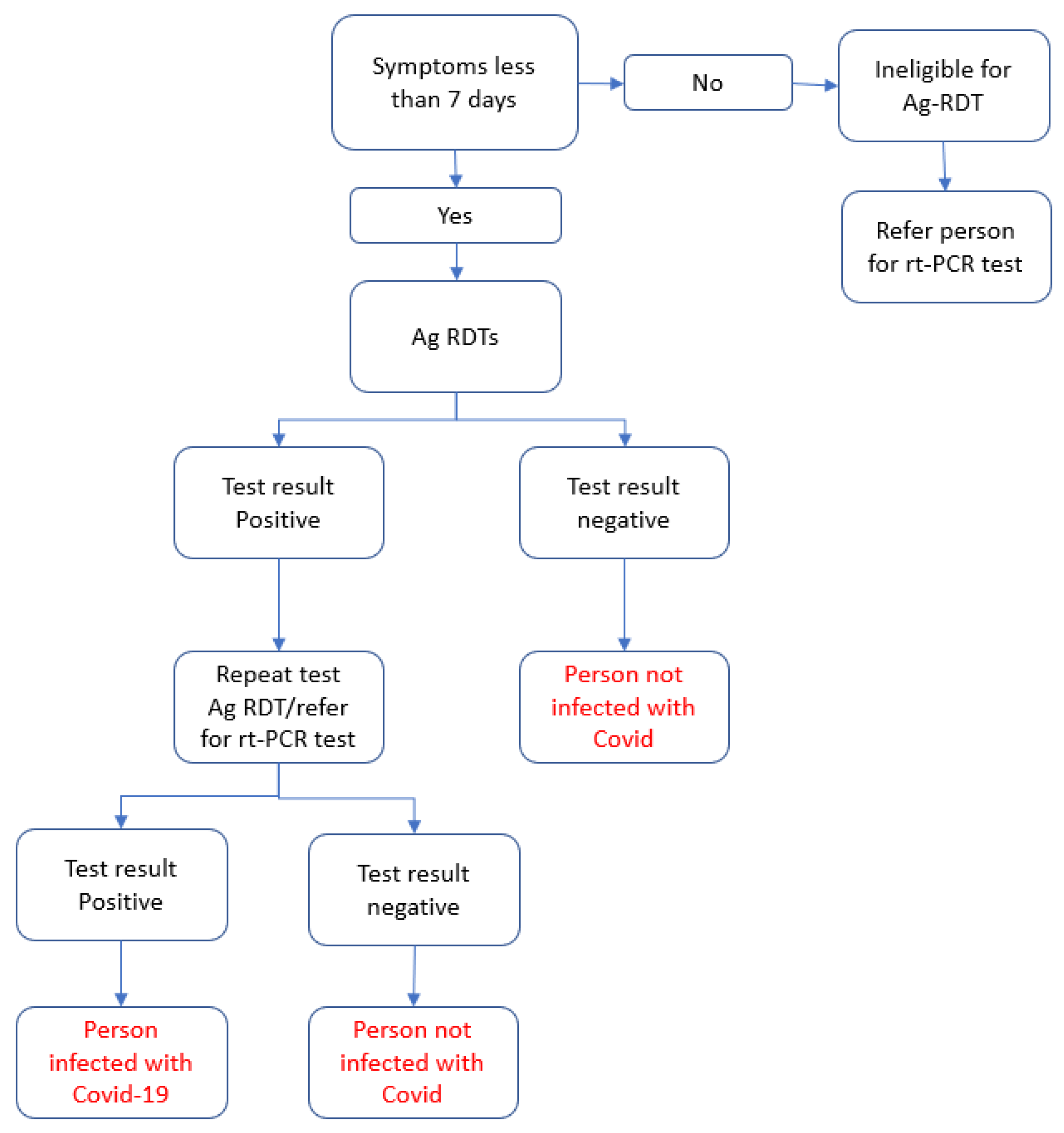
2.4. Eligibility Criteria
2.5. Data Recording and Reporting
2.6. Project Supervision
2.7. Study Sample
2.8. Data Variables
2.9. Data Analysis and Interpretation
2.10. Ethical Issues
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Bank. MENA-Crisis-Tracker-2-7-2022. Office of the Chief Economist. 2022. Available online: https://thedocs.worldbank.org/en/doc/a32a611d09ad47947f154d81c635b658-0280032022/original/MENA-Crisis-Tracker-2-7-2022.pdf (accessed on 2 February 2023).
- Karamouzian, M.; Madani, N. COVID-19 response in the Middle East and north Africa: Challenges and paths forward. Lancet Glob. Health 2020, 8, e886–e887. [Google Scholar] [CrossRef] [PubMed]
- Flores, G.; Abbasi, A.; Korachais, C.; Lavado, R. Unaffordability of COVID-19 tests: Assessing age-related inequalities in 83 countries. Int. J. Equity Health 2022, 21, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Esposito, A.; Palmisano, A.; Scotti, G.M.; Morelli, M.J.; Vignale, D.; De Cobelli, F.; Tonon, G.; Tacchetti, C. Why is chest CT important for early diagnosis of COVID-19? Prevalence matters. medRxiv 2020. [Google Scholar] [CrossRef]
- Ly, T.D.A.; Nguyen, N.N.; Hoang, V.T.; Goumballa, N.; Louni, M.; Canard, N.; Dao, T.L.; Medkour, H.; Borg, A.; Bardy, K.; et al. Screening of SARS-CoV-2 among homeless people, asylum-seekers and other people living in precarious conditions in Marseille, France, March-April 2020. Int. J. Infect. Dis. 2021, 105, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kheirallah, K.A.; Ababneh, B.F.; Bendak, H.; Alsuwaidi, A.R.; Elbarazi, I. Exploring the Mental, Social, and Lifestyle Effects of a Positive COVID-19 Infection on Syrian Refugees in Jordan: A Qualitative Study. Int. J. Environ. Res. Public Health 2022, 19, 12588. [Google Scholar] [CrossRef]
- Marc Lynch. (PDF) COVID-19: Lebanon’s Experience and Response. In: POMEPS Studies [Internet]. 2020. Available online: https://www.researchgate.net/publication/340819777_COVID-19_Lebanon’s_Experience_and_Response (accessed on 7 January 2023).
- Peeling, R.W.; Heymann, D.L. Innovations in COVID-19 testing: The road from pandemic response to control. Lancet Infect. Dis. 2021, 21, 1334–1335. [Google Scholar] [CrossRef]
- Peeling, R.W.; Heymann, D.L.; Teo, Y.Y.; Garcia, P.J. Diagnostics for COVID-19: Moving from pandemic response to control. Lancet 2022, 399, 757–768. [Google Scholar] [CrossRef]
- Brümmer, L.E.; Katzenschlager, S.; Gaeddert, M.; Erdmann, C.; Schmitz, S.; Bota, M.; Grilli, M.; Larmann, J.; Weigand, M.A.; Pollock, N.R.; et al. Accuracy of novel antigen rapid diagnostics for SARS-CoV-2: A living systematic review and meta-analysis. PLoS Med. 2021, 18, e1003735. [Google Scholar] [CrossRef]
- Foundation of Innovative New Diagnostics. FIND | Diagnosis for All. 2023. Available online: https://www.finddx.org/ (accessed on 23 January 2023).
- UNOCHA. North-West Syria|Situation Reports. 2023. Available online: https://reports.unocha.org/en/country/syria/ (accessed on 23 January 2023).
- ARCS. Statement on the Continuation of UN-Led Cross-Border Humanitarian Assistance in Northwest Syria|American Relief Coalition for Syria. 2023. Available online: https://arcsyria.org/article/statement-continuation-un-led-cross-border-humanitarian-assistance-northwest-syria (accessed on 23 January 2023).
- Alanba, A.-K. ارتفاع يومي بإصابات الكوليرا وكورونا شمال (Daily increase in Cholera and COVID-19 infections in North-West Syria). 23 December 2022. Available online: https://www.alanba.com.kw/1160966 (accessed on 7 January 2023).
- OCHA. COVID-19 Monthly Update Northwest Syria (As of 31 December 2022)-Syrian Arab Republic|ReliefWeb. 2022. Available online: https://reliefweb.int/report/syrian-arab-republic/covid-19-monthly-update-northwest-syria-31-december-2022 (accessed on 13 April 2023).
- Lindner, A.K.; Nikolai, O.; Rohardt, C.; Kausch, F.; Wintel, M.; Gertler, M.; Burock, S.; Hörig, M.; Bernhard, J.; Tobian, F.; et al. Diagnostic accuracy and feasibility of patient self-testing with a SARS-CoV-2 antigen-detecting rapid test. J. Clin. Virol. 2021, 141, 104874. [Google Scholar] [CrossRef]
- WHO. Use of SARS-CoV-2 Antigen-Detection Rapid Diagnostic Tests for COVID-19 Self-Testing; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Vink, M.; Iglói, Z.; Fanoy, E.B.; van Beek, J.; Boelsums, T.; de Graaf, M.; Voeten, H.A.; Molenkamp, R.; Koopmans, M.P.; Mevissen, F.E. Community-based SARS-CoV-2 testing in low-income neighbourhoods in Rotterdam: Results from a pilot study. J. Glob. Health 2022, 12, 05042. [Google Scholar] [CrossRef]
- Finch, L.S.; Harris, A.; Lester, C.; Veal, D.; Jones, K.; Fulton, J.; Jones, L.; Lee, M.; Walker, T.; Rossiter, M.; et al. Implementation study of SARS-CoV-2 antigen lateral flow tests in men’s professional (Premiership) rugby union sports squads in England during the COVID-19 pandemic. J. Infect. 2022, 84, e3–e5. [Google Scholar] [CrossRef]
- Krüger, L.J.; Lindner, A.K.; Gaeddert, M.; Tobian, F.; Klein, J.; Steinke, S.; Lainati, F.; Schnitzler, P.; Nikolai, O.; Mockenhaupt, F.P.; et al. A Multicenter Clinical Diagnostic Accuracy Study of SureStatus, an Affordable, WHO Emergency Use-Listed, Rapid, Point-Of-Care Antigen-Detecting Diagnostic Test for SARS-CoV-2. Microbiol. Spectr. 2022, 10, e0122922. [Google Scholar] [CrossRef]
- Byrne, R.L.; Aljayyoussi, G.; Kontogianni, K.; Clerkin, K.; McIntyre, M.; Wardale, J.; Williams, C.T.; CONDOR Steering Group; Body, R.; Adams, E.R.; et al. Head-to head comparison of anterior nares and nasopharyngeal swabs for SARS-CoV-2 1 antigen detection in a community drive-through test centre in the UK Running title: Anterior nares or NP swabs for SARS-CoV-2 RDTs. medRxiv 2022. [Google Scholar] [CrossRef]
- Keskin, A.U.; Ciragil, P.; Topkaya, A.E. Clinical Accuracy of Instrument-Read SARS-CoV-2 Antigen Rapid Diagnostic Tests (Ag-IRRDTs). Int. J. Microbiol. 2022, 2022, 9489067. [Google Scholar] [CrossRef]
- Baro, B.; Rodo, P.; Ouchi, D.; Bordoy, A.E.; Amaro, E.N.S.; Salsench, S.V.; Molinos, S.; Alemany, A.; Ubals, M.; Corbacho-Monné, M.; et al. Performance characteristics of five antigen-detecting rapid diagnostic test (Ag-RDT) for SARS-CoV-2 asymptomatic infection: A head-to-head benchmark comparison. J. Infect. 2021, 82, 269–275. [Google Scholar] [CrossRef]
- La Scola, B.; Le Bideau, M.; Andreani, J.; Hoang, V.T.; Grimaldier, C.; Colson, P.; Gautret, P.; Raoult, D. Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1059–1061. [Google Scholar] [CrossRef]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef]
- Quicke, K.; Gallichote, E.; Sexton, N.; Young, M.; Janich, A.; Gahm, G.; Carlton, E.J.; Ehrhart, N.; Ebel, G.D. Longitudinal Surveillance for SARS-CoV-2 RNA Among Asymptomatic Staff in Five Colorado Skilled Nursing Facilities: Epidemiologic, Virologic and Sequence Analysis. medRxiv 2020. [Google Scholar] [CrossRef]
- Mina, M.J.; Parker, R.; Larremore, D.B. Rethinking COVID-19 Test Sensitivity—A Strategy for Containment. N. Engl. J. Med. 2020, 383, e120. [Google Scholar] [CrossRef]
- Wachinger, J.; Olaru, I.D.; Horner, S.; Schnitzler, P.; Heeg, K.; Denkinger, C.M. The potential of SARS-CoV-2 antigen-detection tests in the screening of asymptomatic persons. Clin. Microbiol. Infect. 2021, 27, e1–e1700. [Google Scholar] [CrossRef]
- Sagna, A.S.; Seydi, A.G.; Gueye, M.M.; Fall, M.T.A.; Mansuy, J.M. Contribution of COVID Antigenic RDT to the Management Strategy of COVID-19 Pandemic in a Senegalese Company. J. Occup. Environ. Med. 2022, 64, e257. [Google Scholar] [CrossRef] [PubMed]
- WHO. Monthly COVID-19 Bulletin October 2022. 2022. Available online: https://www.emro.who.int/images/stories/Monthly_COVID-19_Bulletin_October_2022.pdf?ua=1 (accessed on 20 March 2023).
| Participants | Consented to an Ag-RDT | COVID-19-Positive Results among Those Who Underwent an Ag-RDT | ||||
|---|---|---|---|---|---|---|
| Demographic and Clinical Characteristics | N | (%) | N | (%) | N | (%) |
| Total | 27,888 | 100.0% | 24,956 | 89.5% | 121 | 0.5% |
| Gender | ||||||
| Female | 15,913 | 57.1% | 14,474 | 90.3% | 60 | 0.4% |
| Male | 11,959 | 42.9% | 10,467 | 86.7% | 61 | 0.6% |
| Other | 16 | 0.1% | 15 | 93.8% | 0 | 0.0% |
| Age (years) | ||||||
| <5 | 685 | 2.5% | 557 | 81.3% | 6 | 1.1% |
| 5–17 | 2217 | 7.9% | 2038 | 91.9% | 21 | 1.0% |
| 18–34 | 13,569 | 48.7% | 12,087 | 89.1% | 44 | 0.4% |
| 35–49 | 7002 | 25.1% | 6311 | 90.1% | 34 | 0.5% |
| 50–64 | 3338 | 12.0% | 2999 | 89.8% | 12 | 0.4% |
| >64 | 1077 | 3.9% | 964 | 89.5% | 4 | 0.4% |
| Participants | Consented to an Ag-RDT | COVID-19-Positive Results among Those Who Underwent an Ag-RDT | ||||
|---|---|---|---|---|---|---|
| Demographic and Clinical Characteristics | N | (%) | N | (%) | N | (%) |
| Total | 27,888 | 100.0% | 24,956 | 89.5% | 121 | 0.5% |
| Reason for Testing * | ||||||
| Febrile illness | 307 | 1.1% | 288 | 93.8% | 2 | 0.7% |
| Respiratory illness | 198 | 0.7% | 177 | 89.4% | 4 | 2.3% |
| Other (seeking assurance) | 1095 | 3.9% | 1087 | 99.3% | 0 | 0.0% |
| Healthcare worker | 731 | 2.6% | 671 | 91.8% | 13 | 1.9% |
| Is a refugee | 12,419 | 44.5% | 10,811 | 87.1% | 10 | 0.1% |
| Is a migrant | 29 | 0.1% | 28 | 96.6% | 0 | 0.0% |
| Has COVID-19 symptoms | 12,026 | 43.1% | 11,054 | 91.9% | 97 | 0.9% |
| Contact of a COVID-19-infected person | 1672 | 6.0% | 1393 | 83.3% | 10 | 0.7% |
| Recent travel | 93 | 0.3% | 90 | 96.8% | 1 | 1.1% |
| Identified at a TB clinic | 123 | 0.4% | 119 | 96.7% | 0 | 0.0% |
| Admitted to ICU | 307 | 1.1% | 21 | 100.0% | 0 | 0.0% |
| Vaccination Status | ||||||
| No doses | 17,166 | 61.6% | 15,379 | 89.6% | 79 | 0.5% |
| One dose | 4802 | 17.2% | 4246 | 88.4% | 15 | 0.4% |
| Two doses | 4504 | 16.2% | 4049 | 89.9% | 18 | 0.4% |
| More than two doses | 1416 | 5.1% | 1282 | 90.5% | 9 | 0.7% |
| Duration of Symptoms | N = 12,026 ** | (%) | N = 11,054 $ | (%) | ||
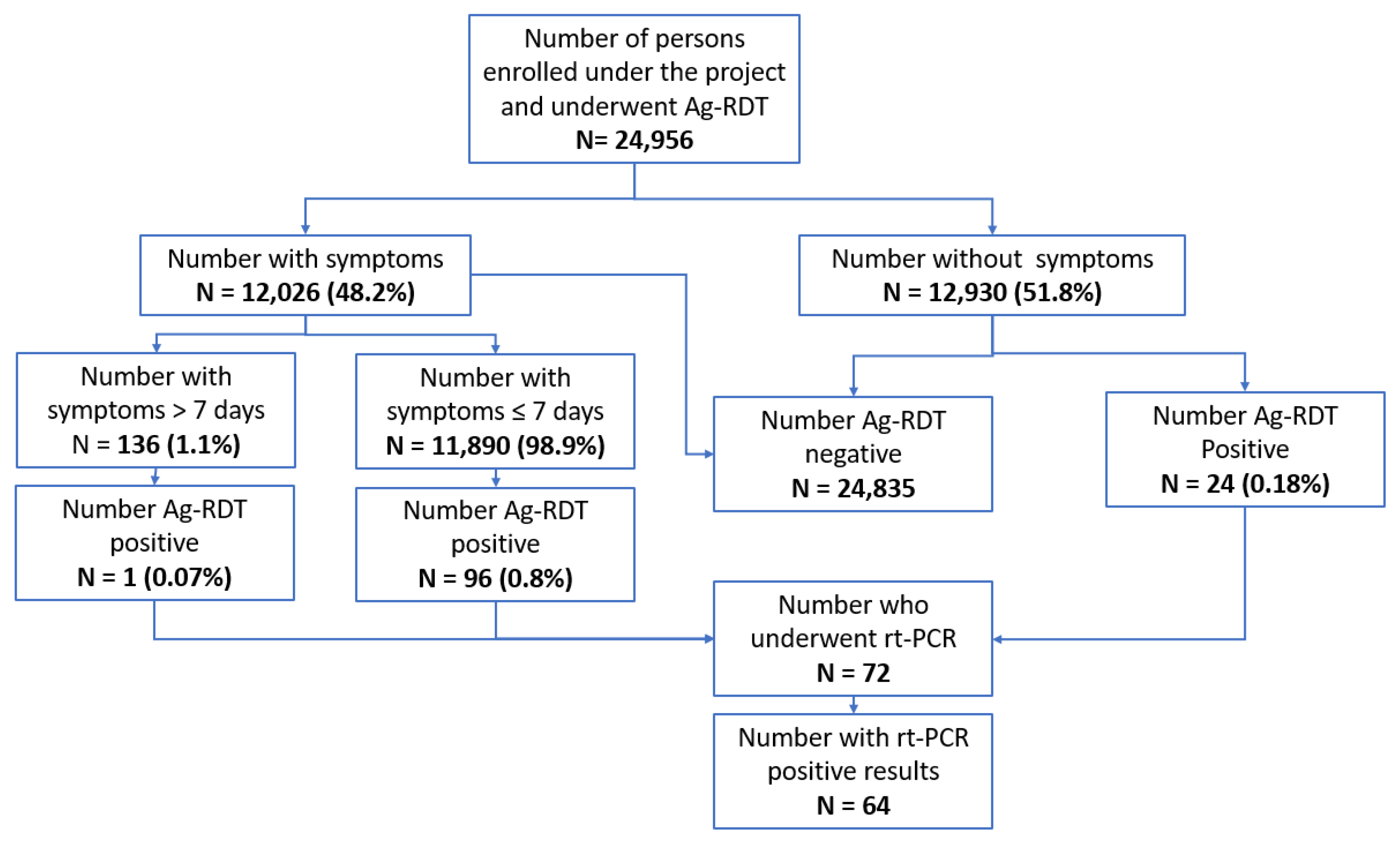
| ≤7 days | 11,890 | 98.9% | 10,928 | 91.9% | 96 | 0.9% |
| >7 days | 136 | 1.1% | 126 | 92.6% | 1 | 0.8% |
| Severity of Symptoms | N = 12,026 ** | (%) | N = 11,054 $ | (%) | ||
| Mild | 11,777 | 97.9% | 10,818 | 91.9% | 67 | 0.6% |
| Participants | Uptake of Ag-RDT | COVID-19-Positive Results among Those Who Underwent an Ag-RDT | ||||
|---|---|---|---|---|---|---|
| Demographic and Clinical Characteristics | N | (%) | N | (%) | N | (%) |
| Total | 27,888 | 100.0% | 24,956 | 89.5% | 121 | 0.5% |
| Testing facility | ||||||
| Idleb—PHC | 8732 | 31.3% | 7435 | 85.1% | 24 | 0.3% |
| Idleb—hospital | 8152 | 29.2% | 6998 | 85.8% | 38 | 0.5% |
| Idleb–camps/mobile clinic | 20 | 0.1% | 16 | 80.0% | 0 | 0.0% |
| Afrin—PHC | 3845 | 13.8% | 3438 | 89.4% | 37 | 1.1% |
| Afrin—hospital | 278 | 1.0% | 228 | 82.0% | 5 | 2.5% |
| Afrin—camp/mobile clinic | 441 | 1.6% | 433 | 98.2% | 0 | 0.0% |
| Jarablus/Albab—PHC | 3112 | 11.2% | 3105 | 99.8% | 7 | 0.2% |
| Jarablus/Albab—hospital | 2274 | 8.2% | 2269 | 99.8% | 9 | 0.4% |
| Jarablus/Albab—camp/mobile clinic | 1034 | 3.7% | 1034 | 100.0% | 1 | 0.1% |
| Demographic and Clinical Characteristics | Bivariable Analysis | Multivariable Analysis * | |||
|---|---|---|---|---|---|
| OR | 95% CI | Adj. OR | 95% CI | p-Value | |
| Gender | |||||
| Female | Reference | Reference | |||
| Male | 0.69 | (0.645, 0.753) | 0.78 | (0.72, 0.84) | <0.001 |
| Others | 1.49 | (0.3, 26.9) | 0.97 | (0.11, 8.18) | 0.983 |
| Age group (years) | |||||
| <5 | 0.53 | (0.43, 0.65) | 0.62 | (0.50, 0.77) | <0.001 |
| 5–17 | 1.39 | (1.19, 1.64) | 1.7 | (1.43, 2.01) | <0.011 |
| 18–34 | Ref | Ref | |||
| 35–49 | 1.11 | (1.01, 1.23) | 1.06 | (0.96, 1.17) | 0.209 |
| 50–64 | 1.08 | (0.95, 1.23) | 1.04 | (0.91–1.18) | 0.524 |
| >64 | 1.04 | (0.85, 1.28) | 1.14 | (0.42, 1.40) | 0.204 |
| Reason for testing | |||||
| Has symptoms | 1.6 | (1.479, 1.738) | 2.63 | (1.94, 3.55) | <0.001 |
| Contact of a COVID-19 case | 1.6 | (1.479, 1.738) | 1.4 | (1.00, 1.95) | 0.045 |
| Healthcare worker | 1.32 | (1.01, 1.72) | 2.86 | (1.94, 4.22) | <0.001 |
| Member of an IDP | 0.63 | (0.58, 0.68) | 2.32 | (1.70, 3.15) | <0.001 |
| Is a migrant | 3.29 | (0.44, 24.2) | 2.92 | (0.35, 23.9) | 0.317 |
| Identified at a TB clinic | 3.5 | (1.29, 9.5) | 12.27 | (4.32, 34.8) | <0.001 |
| Febrile illness | 1.79 | (1.12, 2.85) | 1.41 | (0.78, 2.54) | 0.243 |
| Respiratory illness | 0.99 | (0.63, 1.55) | 1.55 | (0.90, 2.65) | 0.107 |
| Recent travel | 3.53 | (1.12, 11.17) | 1.46 | (0.41, 5.23) | 0.559 |
| Vaccination status | |||||
| No doses | Ref | ||||
| One dose | 0.88 | (0.8, 0.98) | 1.03 | (0.93, 1.14) | 0.564 |
| Two doses | 1.03 | (0.92, 1.15) | 1.15 | (1.02, 1.29) | 0.016 |
| More than two doses | 1.11 | (0.93, 1.34) | 1.3 | (1.07, 1.58) | 0.006 |
| Testing district | |||||
| Idleb | 0.011 | (0.006, 0.019) | 0.008 | (0.004, 0.016) | <0.001 |
| Afrin | 0.017 | (0.009, 0.029) | 0.015 | (0.008, 0.027) | <0.001 |
| Jarablus | Ref | Ref | |||
| RT-PCR Result | ||||
|---|---|---|---|---|
| Ag-RDT Result | Positive | Negative | Not Available | Total |
| Positive | 64 | 6 | 2 | 72 |
| Negative | 16 | 149 | 1 | 166 |
| Not available | 0 | 1 | 0 | 1 |
| Total | 80 | 156 | 3 | 239 |
| Domain | Challenge |
|---|---|
| Identification of eligible participants. | Excluding participants who had experienced symptoms for more than 7 days. |
| Obtaining informed consent. | The most common reason for refusing a test was fear of pain and discomfort based on a previous unpleasant experience when taking a test, followed by the belief that the COVID-19 pandemic was over. Other possible reasons included conspiracy theories, fear of isolation, and stigma. |
| Conducting the test. | No major challenges were detected. |
| Acceptability of positive/negative results. | No major challenges were detected. |
| Referral for RT-PCR testing. | Only a small number of RT-PCR tests were performed in collaboration with the Assistance Coordination Unit (ACU), the representing local NGO for COVID-19 RT-PCRs, as policies in NWS had restricted the overall use of COVID-19 PCR tests due to the low prevalence of COVID-19 at that time. |
| Supply chain management issues with Ag-RDTs or ancillary items. | No major challenges were detected. |
| Recording and reporting. | This was carried out using mobile phones, which was challenging, especially during the data cleaning stage. The use of laptops was a more convenient choice. |
| Mobility. | Initially, it was challenging for CHWs to move to implementing sites. However, this challenge was mitigated by the implementing partner by scheduling rides with their fleet of cars. |
| Domain | Facilitators |
|---|---|
| Overall positive perceptions from the NWS community in relation to Ag-RDTs when compared with PCR and other tests. |
|
| Engagement of CHWs with previous experience of the COVID-19 response. |
|
| Convenience in performing confirmatory PCRs when possible. |
|
| The common culture between health workers and participants |
|
| Increasing the motivation of participants to take an Ag-RDT. | This was achieved through on-the-spot education and awareness sessions during the implementation of the project |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghawji, H.; AlYousfi, M.N.; Satyanarayana, S.; Wilson, N.; Tomeh, L.; Alkhellov, H.; Hasan, S.; Sarin, S.; Kao, K. Feasibility, Uptake, and Results of COVID-19 Antigen Rapid Diagnostic Tests among Refugees and Migrants in a Pilot Project in North-West Syria. Trop. Med. Infect. Dis. 2023, 8, 281. https://doi.org/10.3390/tropicalmed8050281
Ghawji H, AlYousfi MN, Satyanarayana S, Wilson N, Tomeh L, Alkhellov H, Hasan S, Sarin S, Kao K. Feasibility, Uptake, and Results of COVID-19 Antigen Rapid Diagnostic Tests among Refugees and Migrants in a Pilot Project in North-West Syria. Tropical Medicine and Infectious Disease. 2023; 8(5):281. https://doi.org/10.3390/tropicalmed8050281
Chicago/Turabian StyleGhawji, Hassan, Mohamad Nihad AlYousfi, Srinath Satyanarayana, Nevin Wilson, Laila Tomeh, Hussam Alkhellov, Sali Hasan, Sanjay Sarin, and Kekeletso Kao. 2023. "Feasibility, Uptake, and Results of COVID-19 Antigen Rapid Diagnostic Tests among Refugees and Migrants in a Pilot Project in North-West Syria" Tropical Medicine and Infectious Disease 8, no. 5: 281. https://doi.org/10.3390/tropicalmed8050281
APA StyleGhawji, H., AlYousfi, M. N., Satyanarayana, S., Wilson, N., Tomeh, L., Alkhellov, H., Hasan, S., Sarin, S., & Kao, K. (2023). Feasibility, Uptake, and Results of COVID-19 Antigen Rapid Diagnostic Tests among Refugees and Migrants in a Pilot Project in North-West Syria. Tropical Medicine and Infectious Disease, 8(5), 281. https://doi.org/10.3390/tropicalmed8050281






