Chagas Disease Diagnostic Testing in Two Academic Hospitals in New Orleans, Louisiana: A Call to Action
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting
2.3. Data Extraction
2.4. Ethics
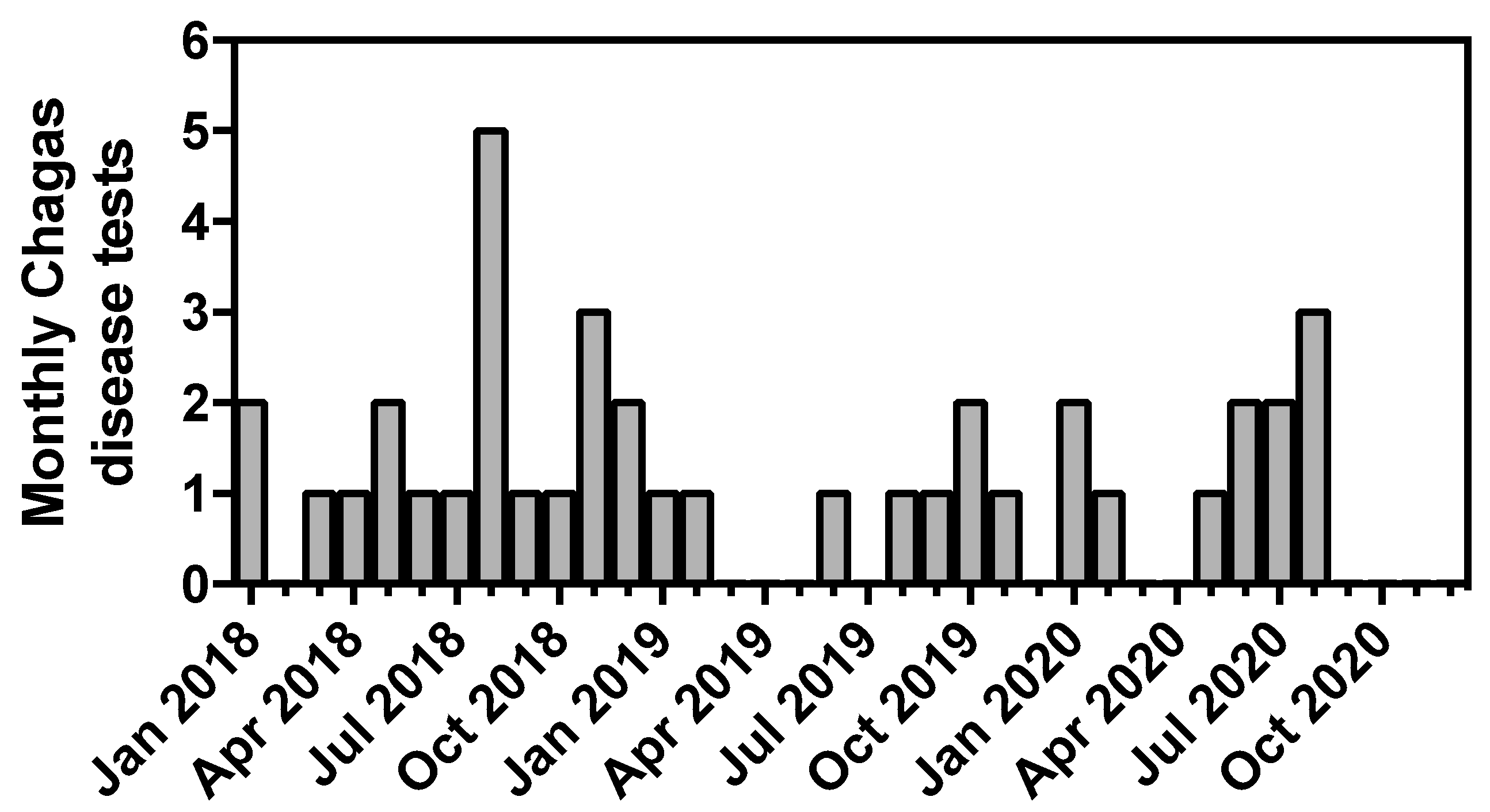
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, B.Y.; Bacon, K.M.; Bottazzi, M.E.; Hotez, P.J. Global economic burden of Chagas disease: A computational simulation model. Lancet Infect. Dis. 2013, 13, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Lidani, K.C.F.; Andrade, F.A.; Bavia, L.; Damasceno, F.S.; Beltrame, M.H.; Messias-Reason, I.J.; Sandri, T.L. Chagas Disease: From Discovery to a Worldwide Health Problem. Front. Public Health 2019, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Bern, C.; Montgomery, S.P. An estimate of the burden of Chagas disease in the United States. Clin. Infect. Dis. 2009, 49, e52–e54. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Chagas disease in Latin America: An epidemiological update based on 2010 estimates. Wkly. Epidemiol. Rec. 2015, 90, 33–43. [Google Scholar]
- Forsyth, C.; Meymandi, S.; Moss, I.; Cone, J.; Cohen, R.; Batista, C. Proposed multidimensional framework for understanding Chagas disease healthcare barriers in the United States. PLoS Negl. Trop. Dis. 2019, 13, e0007447. [Google Scholar] [CrossRef]
- Bern, C.; Messenger, L.A.; Whitman, J.D.; Maguire, J.H. Chagas Disease in the United States: A Public Health Approach. Clin. Microbiol. Rev. 2019, 33, e00023-19. [Google Scholar] [CrossRef]
- Mahoney West, H.; Milliren, C.E.; Vragovic, O.; Kohler, J.R.; Yarrington, C. Perceived barriers to Chagas disease screening among a diverse group of prenatal care providers. PLoS ONE 2021, 16, e0246783. [Google Scholar] [CrossRef]
- Marcus, R.; Henao-Martinez, A.F.; Nolan, M.; Livingston, E.; Klotz, S.A.; Gilman, R.H.; Miranda-Schaeubinger, M.; Meymandi, S. Recognition and screening for Chagas disease in the USA. Ther. Adv. Infect. Dis. 2021, 8, 20499361211046086. [Google Scholar] [CrossRef]
- Manne-Goehler, J.; Umeh, C.A.; Montgomery, S.P.; Wirtz, V.J. Estimating the Burden of Chagas Disease in the United States. PLoS Negl. Trop. Dis. 2016, 10, e0005033. [Google Scholar] [CrossRef]
- Krogstad, J.M.; Passel, J.S.; Cohn, D.V. 5 Facts about Illegal Immigration in the U.S. Available online: https://www.pewresearch.org/fact-tank/2019/06/12/5-facts-about-illegal-immigration-in-the-u-s/ (accessed on 5 December 2020).
- Garcia, M.N.; Woc-Colburn, L.; Aguilar, D.; Hotez, P.J.; Murray, K.O. Historical Perspectives on the Epidemiology of Human Chagas Disease in Texas and Recommendations for Enhanced Understanding of Clinical Chagas Disease in the Southern United States. PLoS Negl. Trop. Dis. 2015, 9, e0003981. [Google Scholar] [CrossRef]
- Klein, M.D.; Proaño, A.; Noazin, S.; Sciaudone, M.; Gilman, R.H.; Bowman, N.M. Risk factors for vertical transmission of Chagas disease: A systematic review and meta-analysis. Int. J. Infect. Dis. 2021, 105, 357–373. [Google Scholar] [CrossRef]
- Carlier, Y.; Sosa-Estani, S.; Luquetti, A.O.; Buekens, P. Congenital Chagas disease: An update. Memórias Inst. Oswaldo Cruz 2015, 110, 363–368. [Google Scholar] [CrossRef]
- Nunes, M.C.P.; Beaton, A.; Acquatella, H.; Bern, C.; Bolger, A.F.; Echeverria, L.E.; Dutra, W.O.; Gascon, J.; Morillo, C.A.; Oliveira-Filho, J.; et al. Chagas Cardiomyopathy: An Update of Current Clinical Knowledge and Management: A Scientific Statement from the American Heart Association. Circulation 2018, 138, e169–e209. [Google Scholar] [CrossRef]
- Matsuda, N.M.; Miller, S.M.; Evora, P.R. The chronic gastrointestinal manifestations of Chagas disease. Clinics 2009, 64, 1219–1224. [Google Scholar] [CrossRef]
- Py, M.O. Neurologic manifestations of Chagas disease. Curr. Neurol. Neurosci. Rep. 2011, 11, 536–542. [Google Scholar] [CrossRef]
- Bern, C.; Montgomery, S.P.; Katz, L.; Caglioti, S.; Stramer, S.L. Chagas disease and the US blood supply. Curr. Opin. Infect. Dis. 2008, 21, 476–482. [Google Scholar] [CrossRef]
- Association for the Advancement of Blood & Biotherapies. Chagas Biovigilance Network. Available online: https://www.aabb.org/news-resources/resources/hemovigilance/chagas-biovigilance-network (accessed on 30 April 2023).
- Bennett, C.; Straily, A.; Haselow, D.; Weinstein, S.; Taffner, R.; Yaglom, H.; Komatsu, K.; Venkat, H.; Brown, C.; Byers, P.; et al. Chagas Disease Surveillance Activities—Seven States, 2017. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 738–741. [Google Scholar] [CrossRef]
- Dorn, P.L.; Perniciaro, L.; Yabsley, M.J.; Roellig, D.M.; Balsamo, G.; Diaz, J.; Wesson, D. Autochthonous transmission of Trypanosoma cruzi, Louisiana. Emerg. Infect. Dis. 2007, 13, 605–607. [Google Scholar] [CrossRef]
- Louisiana Department of Health. Chagas Disease—American Trypanosomiasis; Louisiana Office of Public Health—Infectious Disease Epidemiology Section: Baton Rouge, LA, USA, 2018; pp. 1–3.
- Lynn, M.K.; Bossak, B.H.; Sandifer, P.A.; Watson, A.; Nolan, M.S. Contemporary autochthonous human Chagas disease in the USA. Acta Trop. 2020, 205, 105361. [Google Scholar] [CrossRef]
- Ghersi, B.M.; Peterson, A.C.; Gibson, N.L.; Dash, A.; Elmayan, A.; Schwartzenburg, H.; Tu, W.; Riegel, C.; Herrera, C.; Blum, M.J. In the heart of the city: Trypanosoma cruzi infection prevalence in rodents across New Orleans. Parasit Vectors 2020, 13, 577. [Google Scholar] [CrossRef]
- Majeau, A.; Pronovost, H.; Sanford, A.; Cloherty, E.; Anderson, A.N.; Balsamo, G.; Gee, L.; Straif-Bourgeois, S.C.; Herrera, C. Raccoons as an Important Reservoir for Trypanosoma cruzi: A Prevalence Study from Two Metropolitan Areas in Louisiana. Vector-Borne Zoonotic Dis. 2020, 20, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Waleckx, E.; Suarez, J.; Richards, B.; Dorn, P.L. Triatoma sanguisuga blood meals and potential for Chagas disease, Louisiana, USA. Emerg. Infect. Dis. 2014, 20, 2141–2143. [Google Scholar] [CrossRef] [PubMed]
- Hsu, R.C.; Burak, J.; Tiwari, S.; Chakraborti, C.; Sander, G.E. Chagas Cardiomyopathy in New Orleans and the Southeastern United States. Ochsner J. 2016, 16, 304–308. [Google Scholar] [PubMed]
- Kelly, E.A.; Bern, C.; Whitman, J.D. Review of Chagas Disease Diagnostic Testing at a Major University Hospital in California. In Proceedings of the ASTMH, Virtual Meeting, 15–19 November 2020. [Google Scholar]
- Hyson, P.; Barahona, L.V.; Pedraza-Arevalo, L.C.; Schultz, J.; Mestroni, L.; da Consolacao Moreira, M.; Taylor, M.; Franco-Paredes, C.; Benamu, E.; Ramanan, P.; et al. Experiences with Diagnosis and Treatment of Chagas Disease at a United States Teaching Hospital-Clinical Features of Patients with Positive Screening Serologic Testing. Trop. Med. Infect. Dis. 2021, 6, 93. [Google Scholar] [CrossRef]
- The Data Center. Who Lives in New Orleans and Metro Parishes Now? Available online: https://www.datacenterresearch.org/data-resources/who-lives-in-new-orleans-now/ (accessed on 11 October 2021).
- Irish, A.; Whitman, J.D.; Clark, E.H.; Marcus, R.; Bern, C. Updated Estimates and Mapping for Prevalence of Chagas Disease among Adults, United States. Emerg. Infect. Dis. 2022, 28, 1313–1320. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Congenital transmission of Chagas disease—Virginia, 2010. MMWR Morb. Mortal. Wkly. Rep. 2012, 61, 477–479. [Google Scholar]
- Alarcon, A.; Morgan, M.; Montgomery, S.P.; Scavo, L.; Wong, E.C.; Hahn, A.; Jantausch, B. Diagnosis and Treatment of Congenital Chagas Disease in a Premature Infant. J. Pediatr. Infect. Dis. Soc. 2016, 5, e28–e31. [Google Scholar] [CrossRef]
- Murillo, J.; Bofill, L.M.; Bolivar, H.; Torres-Viera, C.; Urbina, J.A.; Benhayon, D.; Torres, J.R. Congenital Chagas’ disease transmission in the United States: Diagnosis in adulthood. IDCases 2016, 5, 72–75. [Google Scholar] [CrossRef]
- Verani, J.R.; Montgomery, S.P.; Schulkin, J.; Anderson, B.; Jones, J.L. Survey of obstetrician-gynecologists in the United States about Chagas disease. Am. J. Trop. Med. Hyg. 2010, 83, 891–895. [Google Scholar] [CrossRef]
- Edwards, M.S.; Abanyie, F.A.; Montgomery, S.P. Survey of Pediatric Infectious Diseases Society Members About Congenital Chagas Disease. Pediatr. Infect. Dis. J. 2018, 37, e24–e27. [Google Scholar] [CrossRef]
- Di Pentima, M.C.; Hwang, L.Y.; Skeeter, C.M.; Edwards, M.S. Prevalence of antibody to Trypanosoma cruzi in pregnant Hispanic women in Houston. Clin. Infect. Dis. 1999, 28, 1281–1285. [Google Scholar] [CrossRef]
- Edwards, M.S.; Rench, M.A.; Todd, C.W.; Czaicki, N.; Steurer, F.J.; Bern, C.; Montgomery, S.P. Perinatal Screening for Chagas Disease in Southern Texas. J. Pediatric. Infect. Dis. Soc. 2015, 4, 67–70. [Google Scholar] [CrossRef]
- Yarrington, C.D.; Hamer, D.A.; Barnett, E.; Camelo, I.; Perez, J.H.; Poorvu, M.; Koehler, J.R.; Hochberg, N.S. Simple and effective screening for Chagas disease at the prenatal intake visit. Am. J. Obstet. Gynecol. 2019, 221, 687. [Google Scholar] [CrossRef]
- Stillwaggon, E.; Perez-Zetune, V.; Bialek, S.R.; Montgomery, S.P. Congenital Chagas Disease in the United States: Cost Savings through Maternal Screening. Am. J. Trop. Med. Hyg. 2018, 98, 1733–1742. [Google Scholar] [CrossRef]
- Forsyth, C.J.; Manne-Goehler, J.; Bern, C.; Whitman, J.; Hochberg, N.S.; Edwards, M.; Marcus, R.; Beatty, N.L.; Castro-Sesquen, Y.E.; Coyle, C.; et al. Recommendations for Screening and Diagnosis of Chagas Disease in the United States. J. Infect. Dis. 2022, 225, 1601–1610. [Google Scholar] [CrossRef]
- Hakim, J.M.C.; Waltmann, A.; Tinajeros, F.; Kharabora, O.; Malaga Machaca, E.; Calderon, M.; Menduina, M.D.C.; Wang, J.; Rueda, D.; Zimic, M.; et al. Amplicon sequencing reveals complex infection in infants congenitally infected with Trypanosoma cruzi and informs the dynamics of parasite transmission. J. Infect. Dis. 2023, jiad125. [Google Scholar] [CrossRef]
- Carlier, Y.; Altcheh, J.; Angheben, A.; Freilij, H.; Luquetti, A.O.; Schijman, A.G.; Segovia, M.; Wagner, N.; Albajar Vinas, P. Congenital Chagas disease: Updated recommendations for prevention, diagnosis, treatment, and follow-up of newborns and siblings, girls, women of childbearing age, and pregnant women. PLoS Negl. Trop. Dis. 2019, 13, e0007694. [Google Scholar] [CrossRef]
- Mahoney West, H.; Milliren, C.E.; Manne-Goehler, J.; Davis, J.; Gallegos, J.; Perez, J.H.; Kohler, J.R. Effect of clinician information sessions on diagnostic testing for Chagas disease. PLoS Negl. Trop. Dis. 2022, 16, e0010524. [Google Scholar] [CrossRef]
| Hospital | UMCNO | CHNOLA | Total |
|---|---|---|---|
| Number of tests ordered | 33 | 6 | 39 |
| Total patients | 23 | 4 | 27 |
| Male patients (%) | 16 (70%) | 3 (75%) | 19 (70%) |
| Age of patients (median and range) | 41 (20–75) | 16.5 (4–17) | 40 (4–75) |
| Hispanic patients (%) | 18 (78%) | 2 (50%) * | 20 (74%) * |
| Presumed positive patients (%) | 2 (8.7%) | 0 (0%) | 2 (7.4%) ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Proaño, A.; Dumonteil, E.; Herrera, C. Chagas Disease Diagnostic Testing in Two Academic Hospitals in New Orleans, Louisiana: A Call to Action. Trop. Med. Infect. Dis. 2023, 8, 277. https://doi.org/10.3390/tropicalmed8050277
Proaño A, Dumonteil E, Herrera C. Chagas Disease Diagnostic Testing in Two Academic Hospitals in New Orleans, Louisiana: A Call to Action. Tropical Medicine and Infectious Disease. 2023; 8(5):277. https://doi.org/10.3390/tropicalmed8050277
Chicago/Turabian StyleProaño, Alvaro, Eric Dumonteil, and Claudia Herrera. 2023. "Chagas Disease Diagnostic Testing in Two Academic Hospitals in New Orleans, Louisiana: A Call to Action" Tropical Medicine and Infectious Disease 8, no. 5: 277. https://doi.org/10.3390/tropicalmed8050277
APA StyleProaño, A., Dumonteil, E., & Herrera, C. (2023). Chagas Disease Diagnostic Testing in Two Academic Hospitals in New Orleans, Louisiana: A Call to Action. Tropical Medicine and Infectious Disease, 8(5), 277. https://doi.org/10.3390/tropicalmed8050277









