Spatial Analysis of Dengue Clusters at Department, Municipality and Local Scales in the Southwest of Colombia, 2014–2019
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Description
2.2.1. Dengue Incidence Data for the Department Scale
2.2.2. Dengue Data for the Local Scale
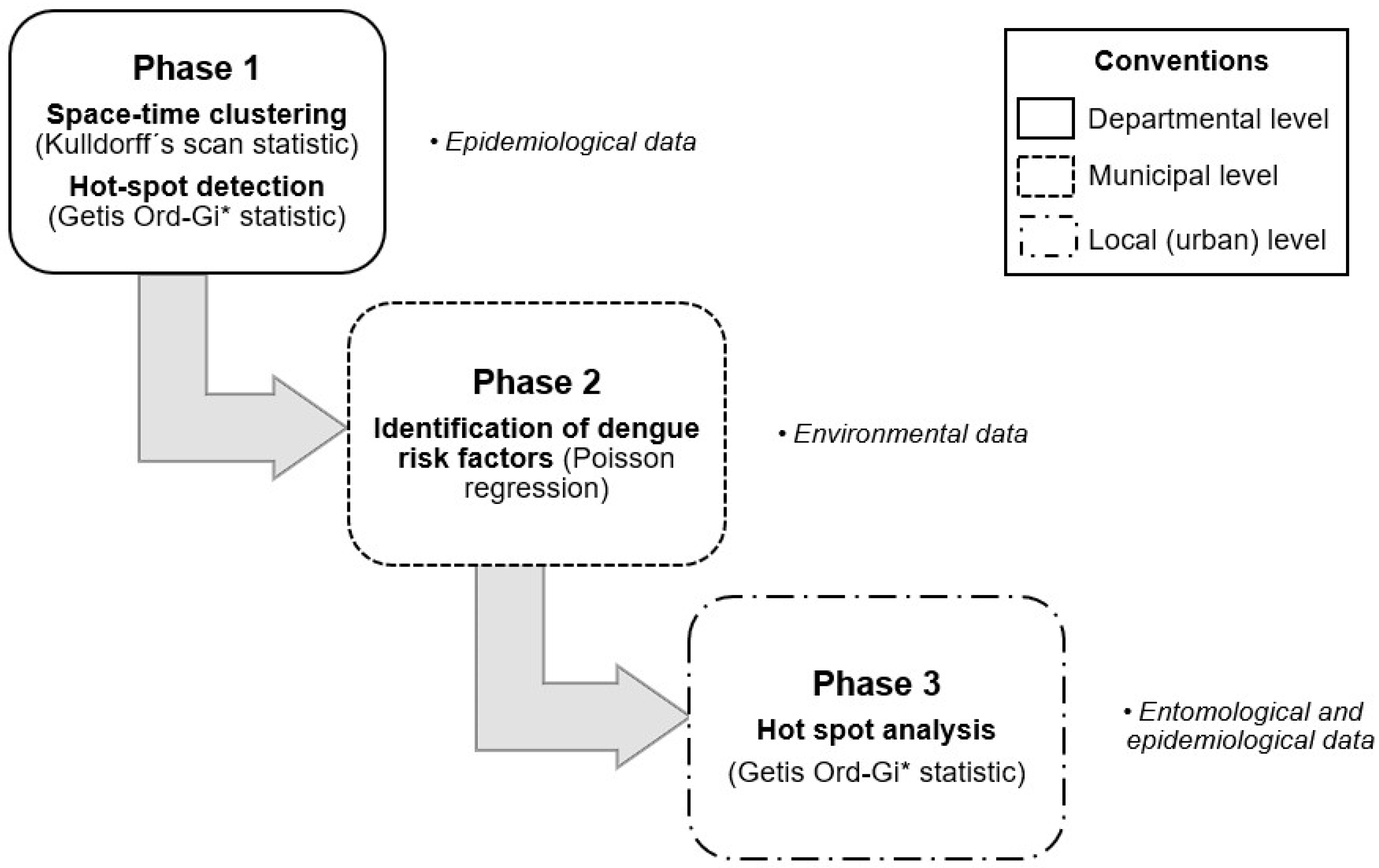
2.3. Study Design
2.3.1. Phase 1: Spatio-Temporal Analysis of Dengue Distribution for Cluster Detection, Targeting High-Dengue-Risk Clusters of Municipalities at the Departmental Scale (Objective 1)
- Space-time clustering: A space-time scan statistic for the detection of risk clusters following the Poisson model [23] was used for the years 2012 to 2018 when a spatial pattern of dengue cases existed. Targeting was carried out using the reported epidemiological information and included the following steps: (1) grouping of the epidemiological cases of the event reported to SIVIGILA according to the municipality of residence of the case or where the case originated, (2) information regarding the total number of cases of dengue and severe dengue taking into account the time of year and (3) location of the centroids in terms of latitude and longitude for each municipality in the department.
- The information was processed using Kulldorff’s method [23]. This method uses a model in which the number of events in a geographic area is distributed according to the Poisson model; under the null hypothesis, and without covariates, the expected number of cases in each area is proportional to its population size, or to the person-years in that area. Because the data are aggregated in census districts, the measurement was concentrated in terms of the central coordinates of those districts and was expanded along a third dimension that reflects the size of the population as it changes over time.
- The retrospective space-time analysis scanning for clusters with high rates using the discrete Poisson model was defined by a cylindrical window with a circular geographic base and with a height corresponding to time. The base was defined exactly as for the purely spatial scan statistic, while the height reflects the time frame of potential clusters. The cylindrical window was then moved in space and time, so that for each possible geographical location and size, it also visited each possible time frame. In effect, we obtained an infinite number of overlapping cylinders of different size and shape, jointly covering the entire study region, where each cylinder reflected a possible cluster [23].
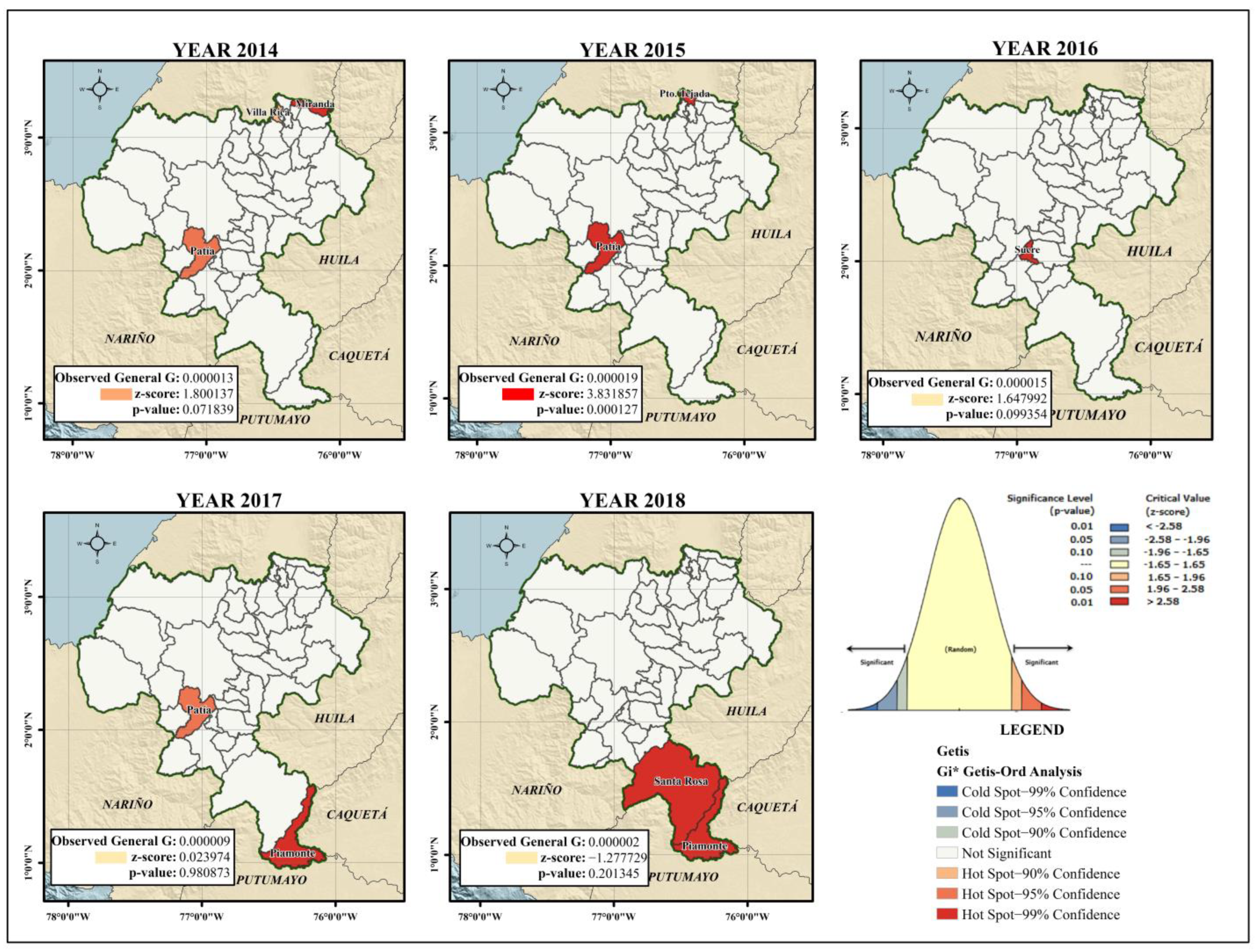
- Hotspot detection: Once the overall landscape of the Cauca department was defined by clustering risk, a more detailed analysis was carried out to distinguish the year-to-year epidemiological behaviour of each municipality using the High-Low Clustering technique (Getis-Ord General and Getis Ord-Gi* statistic). This technique identifies the areas where the highest incidence rates of dengue are geographically homogeneous and concentrated under statistical significance parameters. These areas, known as hot and cold spots, as they specifically consider the extreme high or low values, have been previously used by authors such as Khormi and Kumar [24] and Mutheneni et al. [12] to study the spatial patterns of dengue and their potential application in disease management programmes.
- The clusters that presented a pattern of distribution of the incidence rates of dengue per 10,000 inhabitants—that is, data that were not randomly distributed in the department of Cauca for the period between 2014 and 2018—were identified. The conceptualization of inverse distance was determined using the Euclidean distance without defining a distance threshold. Additionally, the statistical significance was based on the False Discovery Rate (FDR) correction.
- These spatial geostatistical techniques allowed us to characterize the geographic distribution of the incidence of dengue [25] and to thereby identify statistically significant hot spots and cold spots using the Getis-Ord Gi* statistic. This statistic allowed us to examine the local level of spatial clustering to identify and visualize the municipalities whose dengue rate values were extreme and geographically homogeneous.
2.3.2. Phase 2: Spatial Stratification of Dengue Risk Villages by Poisson Regression, to Determine the Effect of Environmental Factors That Influence the Spatial Variation of Dengue at the Municipal Scale (Objective 2)
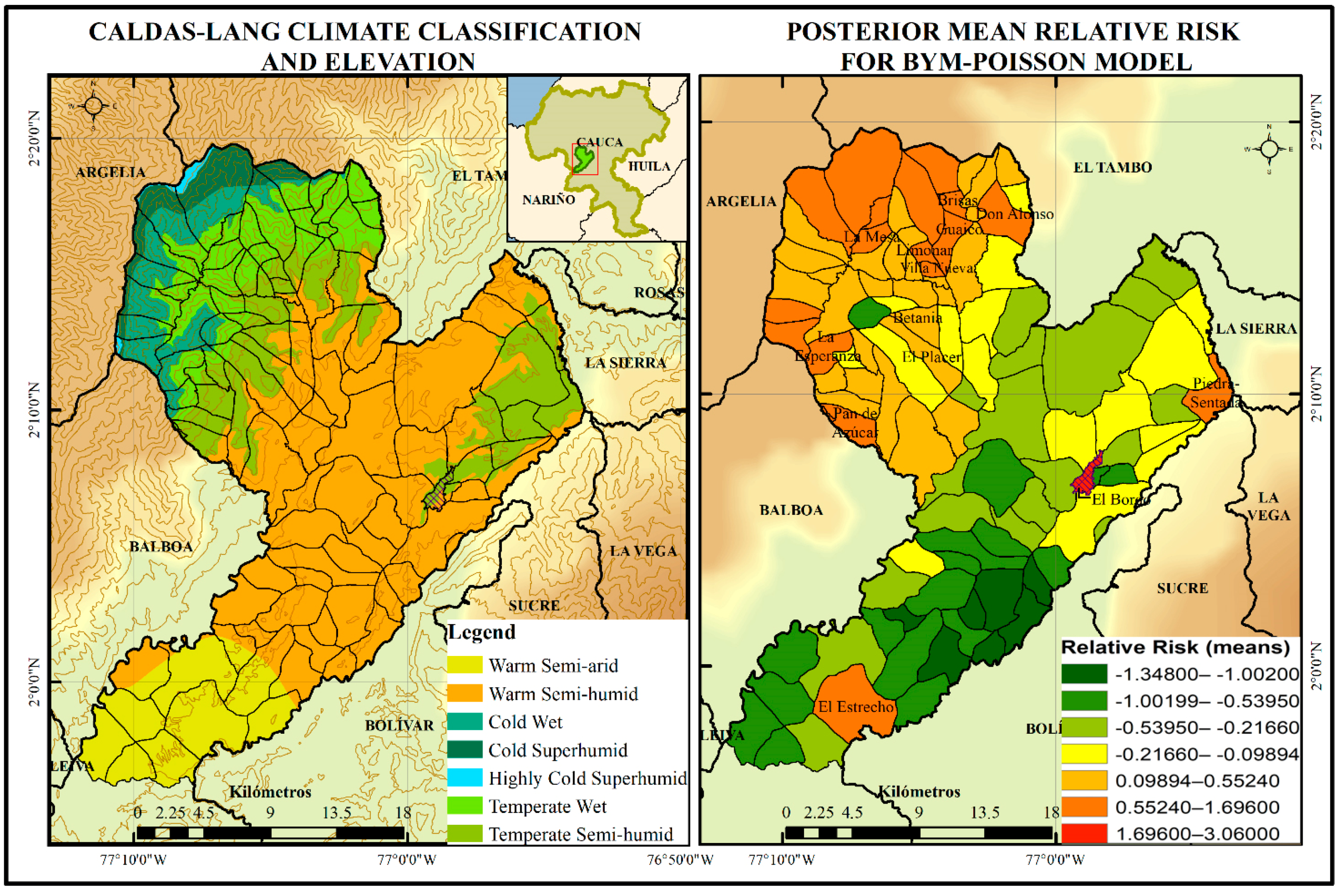
- Study site: Patía municipality was chosen for spatial and environmental factors analysis within the department after carrying out Phase 1; the municipality had one of the highest incidences of dengue fever in the time window (2012–2018). In relation to entomological indicators, it has been positive for pupae index throughout the years in several neighbourhoods of its municipal seat.
- The municipality is located in the department of Cauca and has an area of 723 km2. As of 2021, it had a population of 37,793 inhabitants, of whom 13,598 lived in the urban area. The municipal seat, called Bordo-Patía, is located at the coordinates 02°06′56″ N and 76°59′21″ W and at an altitude of 910 m.a.s.l. The average temperature of the municipality is between 25 and 27 °C (maximum temperature 33–38 °C and minimum temperature 15–19 °C), and the average annual precipitation is 2171 mm. The municipality includes zones of premontane rainforest, premontane dry forest, and tropical dry forest [26].
- The climate in Patía is conditioned by its geoforms. A warm climate is representative of the depression or valley of Patía, which covers most of its extent. A cold climate is associated with the western mountain range that passes through the north of the municipality. Precipitation in the municipality has a bimodal distribution and is divided into two wet periods (March–May and October–December, the latter of which is more intense) separated by two dry periods (January–February and June–September) [27].
- Environmental factors: The environmental variables that were considered in the Poisson regression were altitude, minimum and maximum temperature, and precipitation. To acquire the data associated with these variables, the NASA Earth Data platform and the Climate Engine climate database were used.
- Altitude data were obtained from the NASA website, https://search.asf.alaska.edu/#/ (accessed on 8 October 2020), which has a variety of high-resolution cartographic resources. To download the digital elevation model (DEM) from which the altitude data were derived, a user account was created to access the platform. After the area of interest was located, the dataset “ALOS PALSAR” was selected, which contains the global DEM at a resolution of 12.5 m obtained through the synthetic aperture radar of the Advanced Land Observation Satellite (ALOS).
- The climate data were acquired through the website https://app.climateengine.org/climateEngine (accessed on 24 March 2021); after logging into the platform, a series of parameters were defined, such as the dataset, the meteorological variables of precipitation, maximum and minimum temperature, and the time interval (2015–2019). The dataset selected for the study was Terraclimate, which has monthly information on all required variables with a spatial resolution of 4 km. It is the most detailed among the various datasets available in the Climate Engine platform, which covers pixel values between 5 × 5 km and 55 × 55 km. Additionally, this dataset has an array of data from WorldClim, CRUTs 4.0 and JRA 55, which were structured and validated using interpolation and reanalysis techniques [28].
- Poisson regression analysis: Prior to modelling, univariate Poisson regression models were developed to identify the environmental factors that influence the increase of and spatial variation in the dengue burden in the municipality; a Poisson regression model was run using ArcGis Pro© software, taking into consideration the statistically significant variables between altitude, temperature (maximum and minimum) and precipitation.
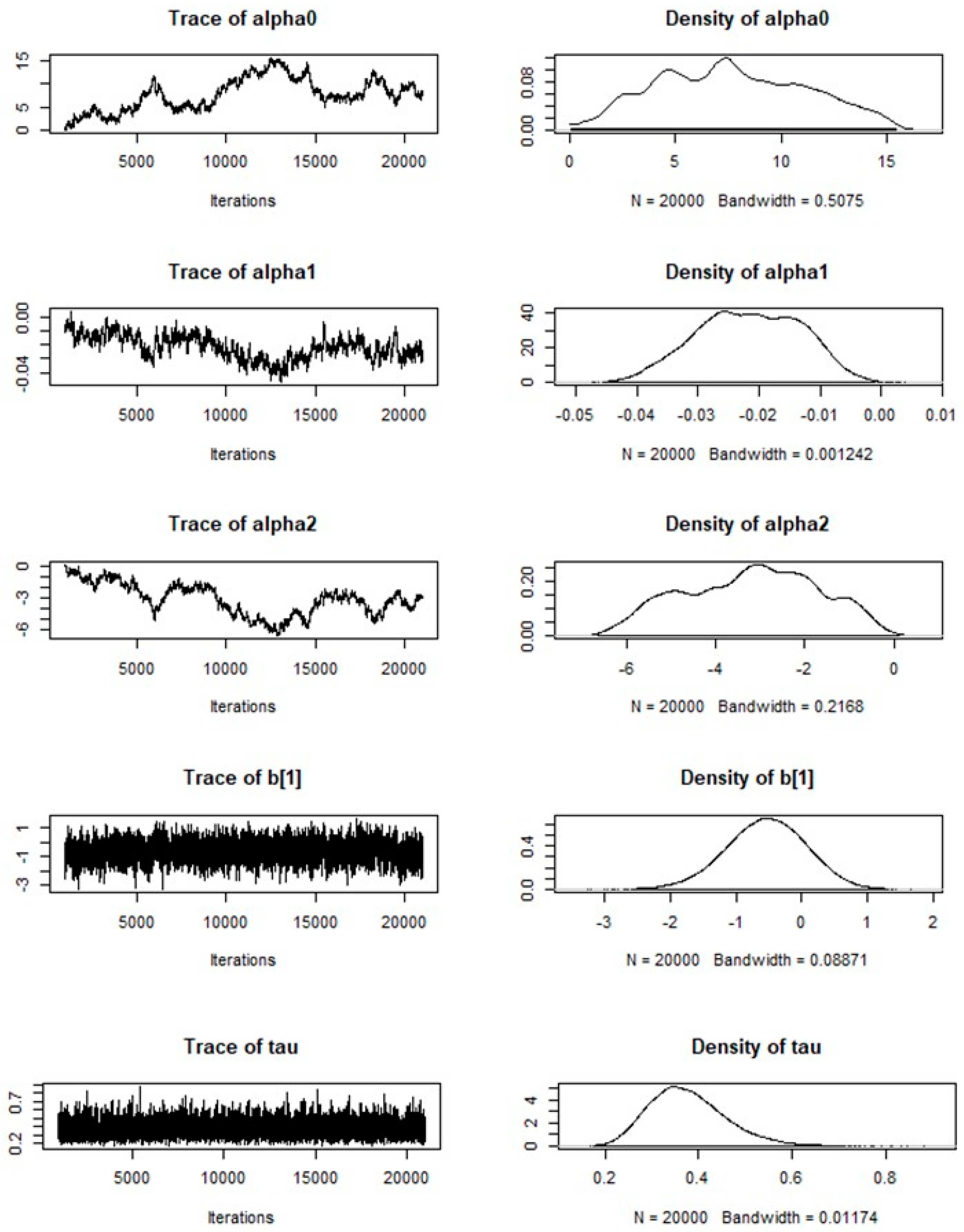
- To identify how the environmental factors influence the increase of and spatial variation in the dengue burden in a village within the municipality, a Poisson regression model was run using WinBUGS [29] and GeoBUGS [30] software packages, both useful for making inferences under a Bayesian framework using the Gibbs Sampling method.
- WinBUGS approaches Bayesian estimation problems by multiplying a priori distribution by the likelihood and then simulating samples from the a posteriori distribution using the Gibbs algorithm. Predictive maps for the risk of infection were obtained taking into consideration the statistically significant variables such as altitude and minimum temperature.
- Subsequently, a Hierarchical Bayesian Model (HBM) was built in two stages; the HBM uses multiple levels of analysis in an iterative way [31]. As described by Best [32], in the HBA the unexplained extra-variance found in spatial statistics is identified as either spatially correlated effects or heterogeneity effects.
- A purely spatial modelling in two stages was followed: at the first stage, a likelihood model for the observed and expected dengue disease counts was specified based on the environmental variables. At the second stage, a prior model over the space of possible relative risks (RR) was specified. The data included two covariates measuring the minimum temperature and elevation within the municipality, and a list of adjacent villages, using the intrinsic conditional autoregressive (CAR) prior proposed by Besag, York and Mollie [33]. This model considers the spatial correlation between neighbouring areas. The general model could be written as:
log (µi) = log Ei + α0 + α1xi/10 + bi,
- Where α0 is the log relative risk for dengue in the study region, xi is one of the covariates with associated regression coefficient α1, and bi is an area that represents the residual or unexplained relative risk of disease. To allow for spatial dependence between the random effects bi in nearby areas, the car.normal distribution was used. The set of posterior means of relative risks was then used to create a map to visualize high- or low-risk segments.
- The Bayesian interpretation of probability allows (proper prior) probabilities to be assigned subjectively to random events, in accordance with the natural history of the disease. The Markov Chain Monte Carlo (MCMC) implementation ran a sampling chain for 20,000 iterations, and the first 1000 iterations were discarded as pre-convergence “burn-in” [34]. Spatial autocorrelation was evaluated using Moran’s Index in the residuals. After running the HBM, we use the package CODA and R2WinBUGS to analyse the outputs [29,30,31,32].
2.3.3. Phase 3: Spatial Analysis for the Identification of Epidemiological and Entomological Clusters at the Local Scale, to Prioritize Vector-Control Activities (Objective 3)
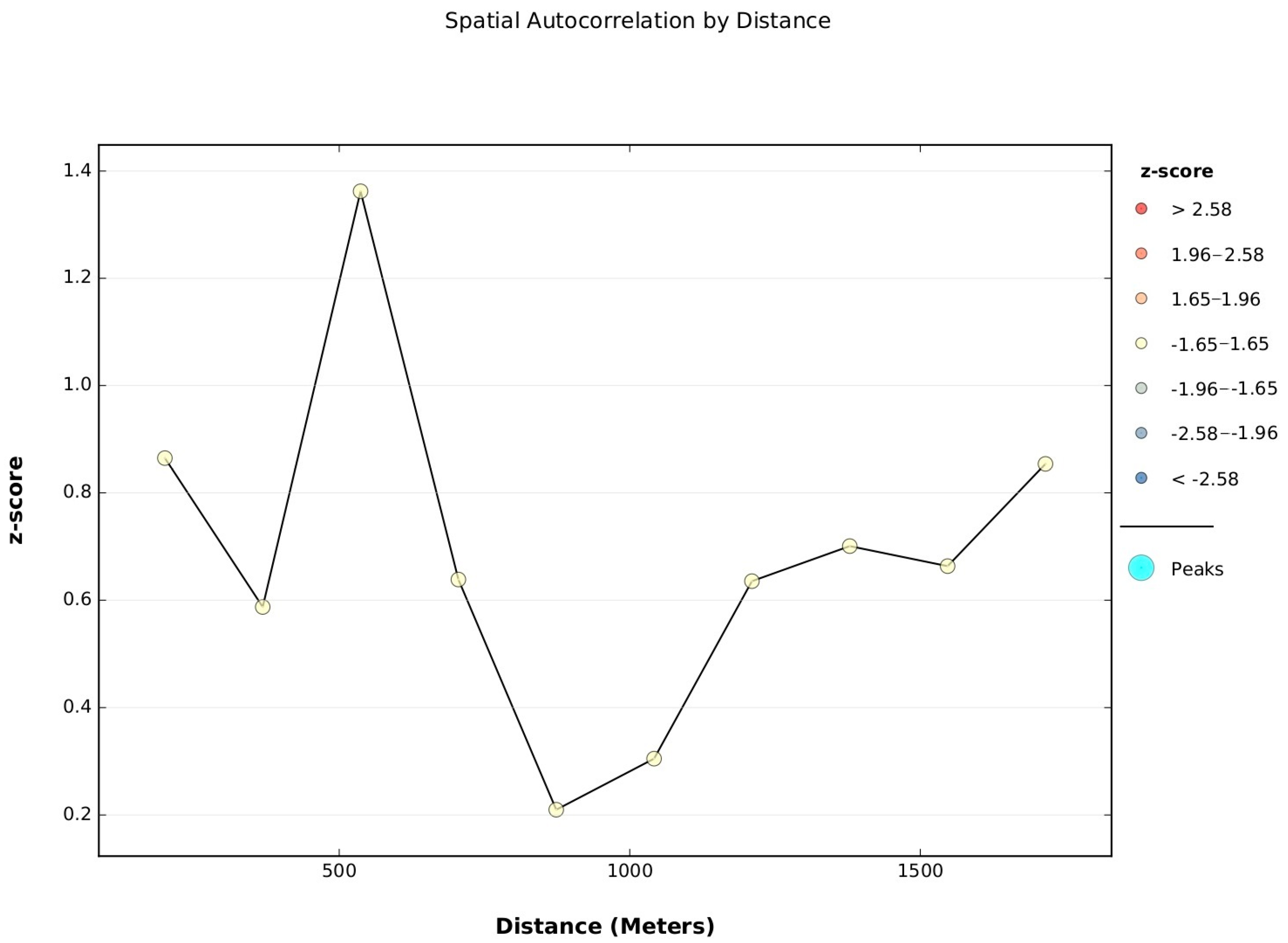
- Spatial analysis with Hot Spot analysis: Prior to hot spot analysis, the Getis-Ord General G statistic was used to identify significant risk clusters. The z-score and p-value are measures of statistical significance that lead to acceptance or rejection of the null hypothesis. For this technique, the null hypothesis states that the values associated with features are randomly distributed. For this analysis, a radius of 100 m was established as threshold distance within the neighbourhood area. This radius was selected based on the dispersal area of the vector [9,35,36] and the results of other similar studies [37,38,39,40]. The potential clusters with the highest burden of dengue per unit area were identified, based on the incidence rate in the neighbourhoods that permanently contribute to the cumulative burden of dengue cases in the municipal seat of Patía.
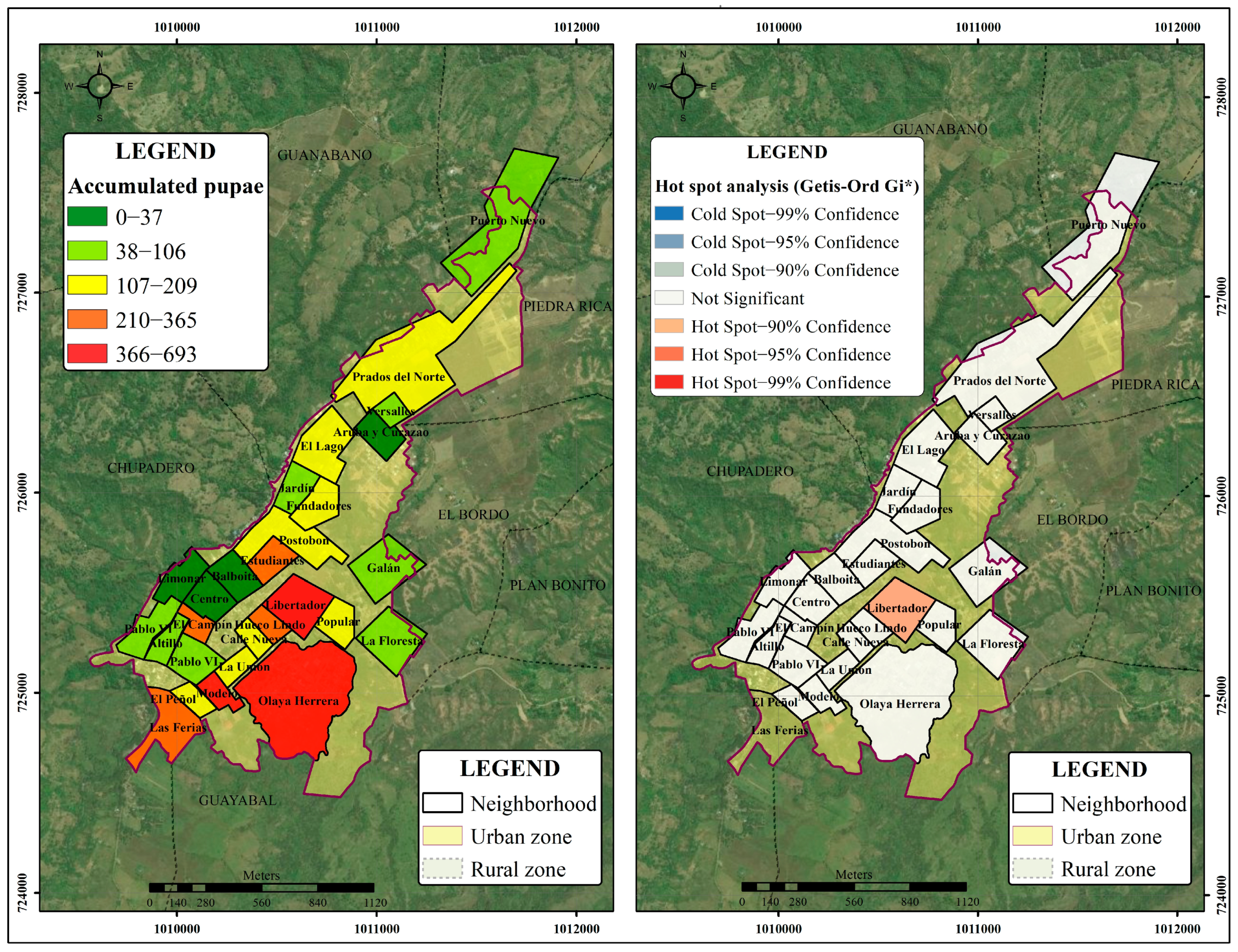
- Getis-Ord Gi* test for entomological data: The existence of spatial distribution patterns of the pupal stage of Ae. aegypti vector was evaluated through the study of entomological variables at the neighbourhood scale, such as the total number of pupae, the index of pupae per person and the Breteau index (BI) for spatial analysis in GIS software. Those indicators were selected because the sub-department of health of Cauca (Secretaría de Salud) collected systematically and continuously in conducting this type of entomological surveillance.
- First, an analysis of the spatial distribution of the vector was performed, with a measure of the degree of clustering (high or low) using the general G statistic of Getis-Ord to indicate whether the pattern was uniformly or randomly clustered based on the fixed distance band parameter. Subsequently, neighbourhoods with groupings or high and homogeneous concentrations of clusters were identified with the Getis-Ord Gi* statistic using 100 m as a distance threshold. The statistical significance was based on the False Discovery Rate (FDR) correction. The information on the variables used was obtained through the Basic Entomology Unit of the Department of Health of Cauca (data available by request).
- For both spatial analyses we consider “local” in the sense of considering neighbourhood areas within the municipality.
2.4. Ethics Statement
3. Results
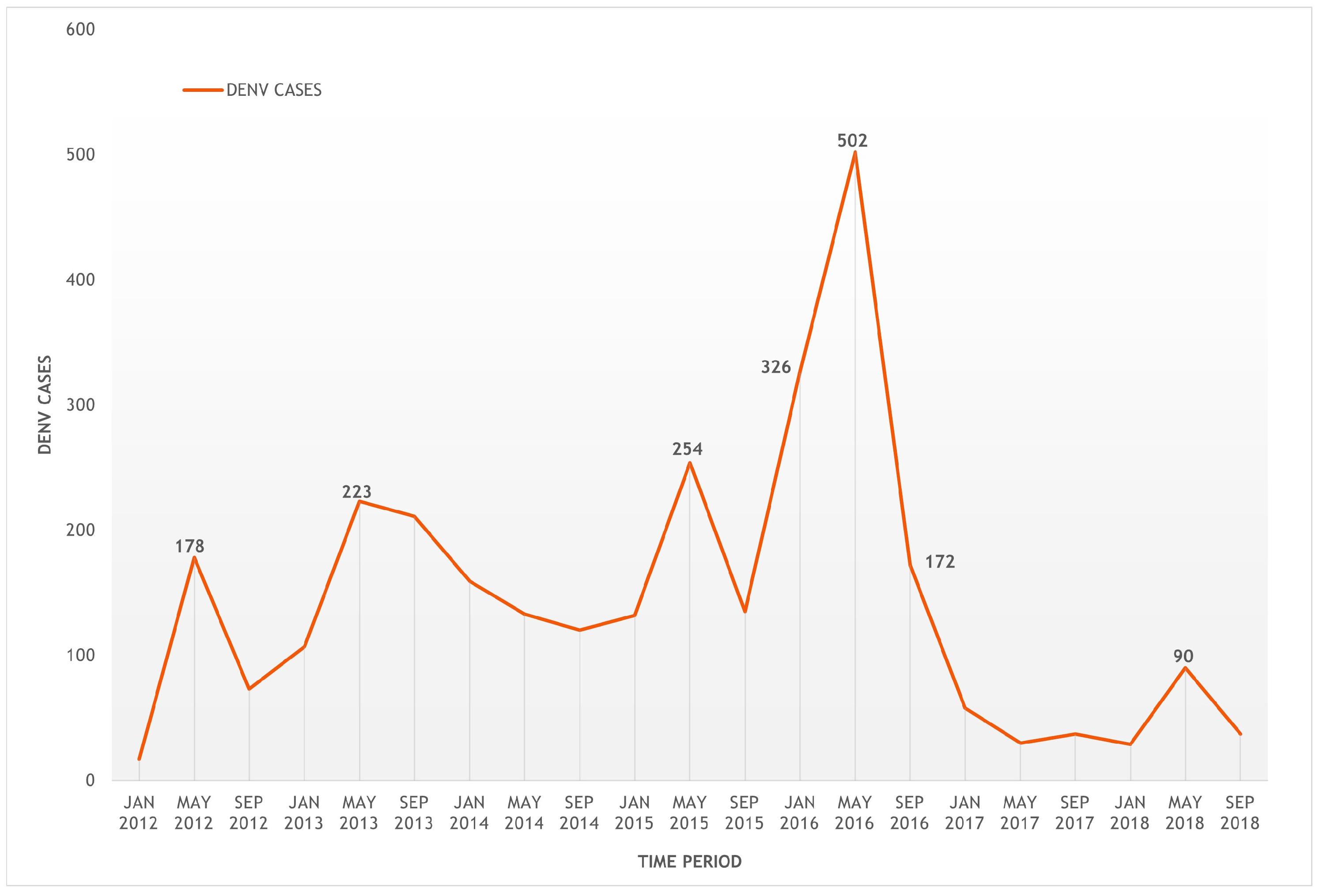
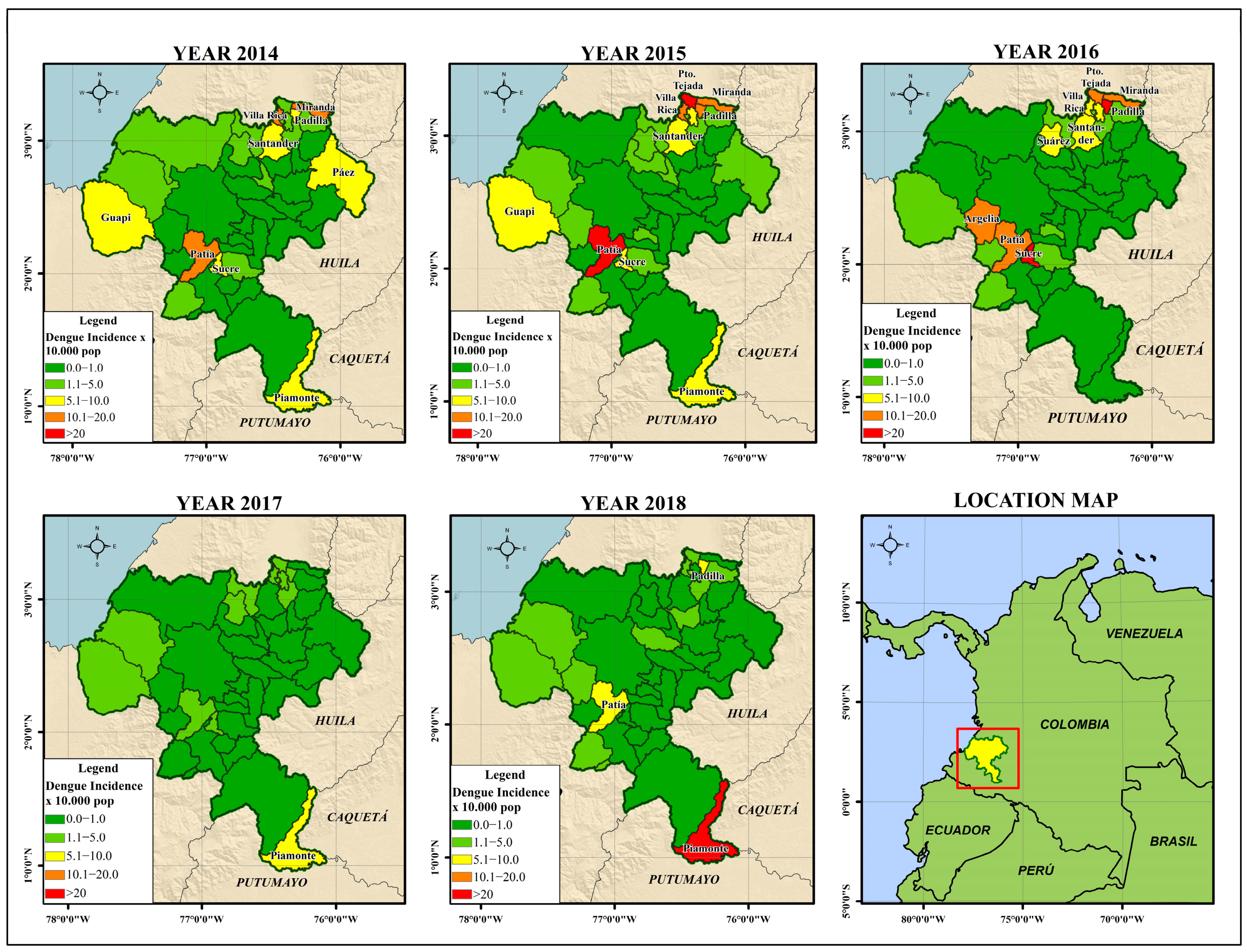
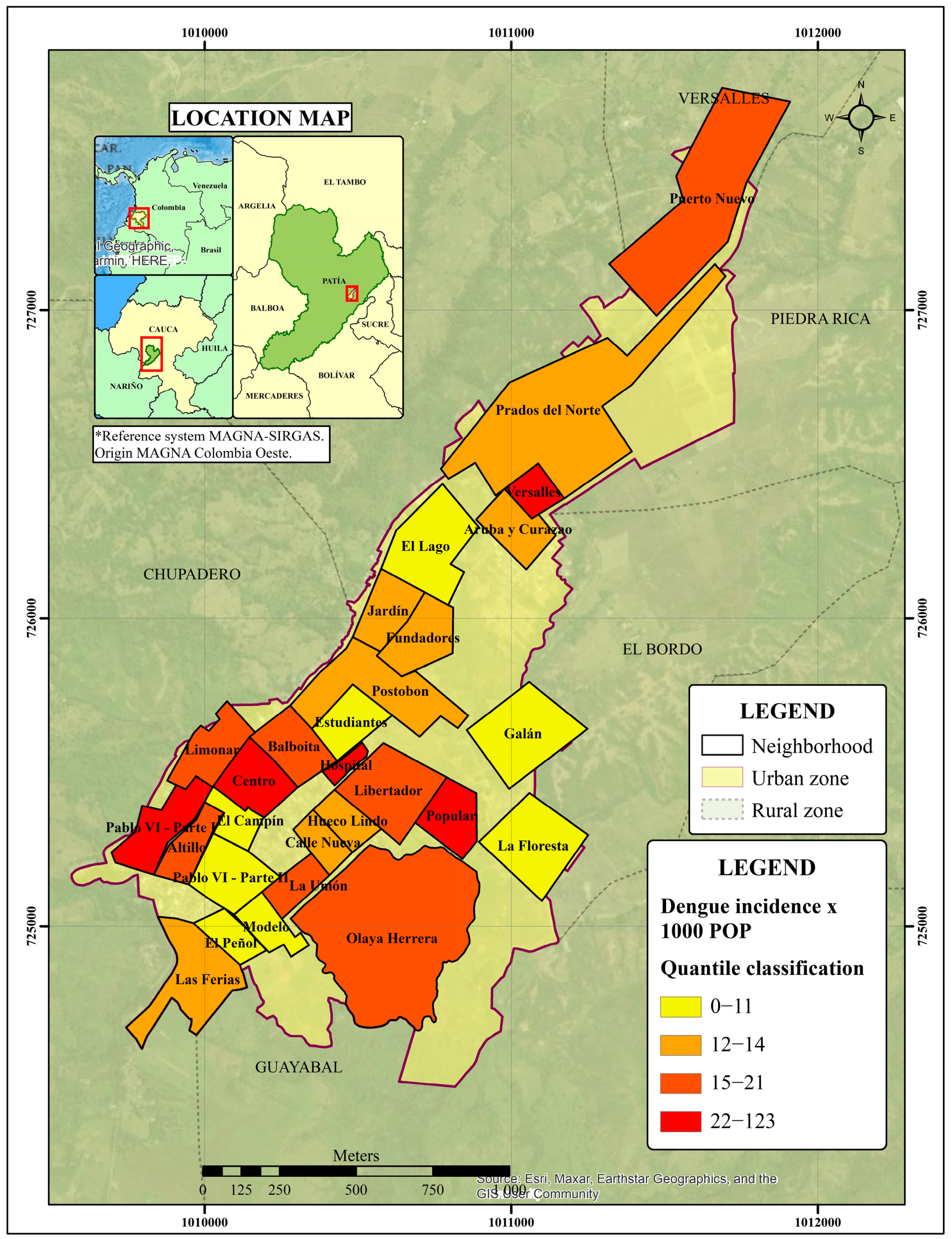
3.1. Geographic Distribution of Dengue Incidence
3.1.1. Identification of Clusters of High Risk for Dengue in Cauca
3.1.2. Getis-Ord Gi* Dengue Hotspot Detection
3.2. Spatial Variation of the Probability of Dengue Incidence as a Function of Environmental Variables in the Municipality of Patía
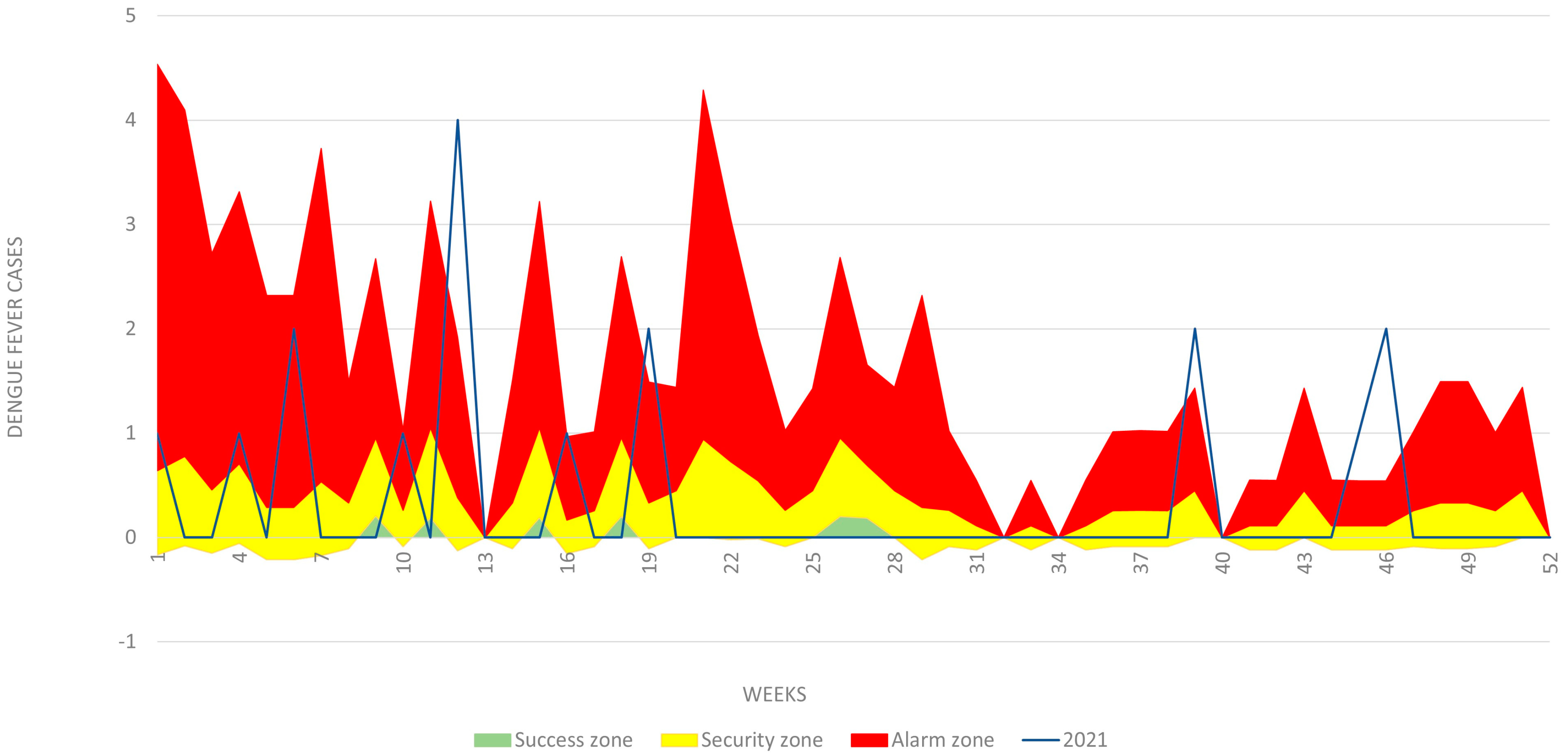
3.3. Determination of Disease and Entomological Cluster in the Municipal Seat of Patía
Spatial Pattern Analysis of Entomological Variables
4. Discussion
4.1. Epidemiological Behaviour of High-Risk Clusters for Dengue in Cauca
4.2. Importance of the Environmental Variables Elevation and Minimum Temperature in the Prediction of Dengue
4.3. Relationship between the Dengue Disease in the Municipal Seat of Patía and the Spatial Distribution Pattern of the Vector
4.4. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Padilla, J.C.; Lizarazo, F.E.; Murillo, O.L.; Mendigaña, F.A.; Pachón, E.; Vera, M.J. Transmission Scenarios of Major Vector-Borne Diseases in Colombia, 1990–2016. Biomédica 2017, 37, 27–40. [Google Scholar] [CrossRef]
- Ye, J.; Moreno, M. Comparing Different Spatio-Temporal Modeling Methods in Dengue Fever Data Analysis in Colombia during 2012–2015. Spat. Spat. Epidemiol. 2020, 34, 100360. [Google Scholar] [CrossRef] [PubMed]
- Organización Panamericana de la Salud/Organización Mundial de la Salud. Actualización Epidemiológica Dengue 11 de Noviembre de 2019. Available online: https://www3.paho.org/hq/index.php?option=com_docman&view=download&category_slug=dengue-2158&alias=50965-11-de-noviembre-de-2019-dengue-actualizacion-epidemiologica-1&Itemid=270&lang=es (accessed on 11 November 2019).
- Sistema Nacional de Vigilancia en Salud Pública-SIVIGILA, Instituto Nacional de Salud Boletín Epidemiológico Semanal, Semana Epidemiológica 32: 8 Al 14 de Agosto de 2021. Colombia; 2021. Available online: https://www.ins.gov.co/buscador-eventos/BoletinEpidemiologico/2021_Boletin_epidemiologico_semana_32.pdf (accessed on 20 August 2021).
- Sistema Nacional de Vigilancia en Salud Pública-SIVIGILA, Instituto Nacional de Salud. Boletín Epidemiológico Semanal, Semana Epidemiológica 52: 20 Al 26 de Diciembre de 2020. 2020. Available online: https://www.ins.gov.co/buscador-eventos/BoletinEpidemiologico/2020_Boletin_epidemiologico_semana_52.pdf (accessed on 30 December 2021).
- Suaza, J.; Barajas, J.; Galeano, E.; Uribe, S. Criaderos Naturales Para Aedes aegypti (Linnaeus, 1762) y Aedes albopictus (Sus, 1895) Vectores de Arbovirus En La Ciudad de Medellín (Antioquia, Colombia). Boletín del Mus. Entomológico Fr. Luis Gall. 2013, 5, 18–24. [Google Scholar]
- Ruiz, F.; González, A.; Vélez, A.; Gómez, G.; Zuleta, L.; Uribe, S.; Vélez, I. Presencia de Aedes (Stegomyia) aegypti (Linnaeus, 1762) y Su Infección Natural Con El Virus Del Dengue En Alturas No Registradas Para Colombia. Biomédica 2016, 36, 303–308. [Google Scholar] [CrossRef]
- Ministerio de la Protección Social & Instituto Nacional de Salud & Organización Panamericana de la Salud. Gestión Para La Vigilancia Entomológica y Control de La Transmisión Del Dengue. Colombia; 2011. Available online: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/DE/gestion-vigilancia-entomologica-dengue.pdf (accessed on 10 August 2020).
- Honório, N.; Costa, W.; Leite, P.; Gonçalves, J.; Lounibos, L.; Lourenço-de-Oliveira, R. Dispersal of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in an Urban Endemic Dengue Area in the State of Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz 2003, 98, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Padilla, J.C.; Rojas, D.P.; Roberto, S.-G. Dengue En Colombia: Epidemiología de la Reemergencia a la Hiperendemia. Rev. Salud. Bosque. 2015, 5, 81–83. [Google Scholar]
- Balaji, D.; Saravanabavan, V. Geo Spatial Variation of Dengue Risk Zone in Madurai City Using Autocorrelation Techniques. GeoJournal 2021, 86, 1481–1501. [Google Scholar] [CrossRef]
- Mutheneni, S.R.; Mopuri, R.; Naish, S.; Gunti, D.; Upadhyayula, S.M. Spatial Distribution and Cluster Analysis of Dengue Using Self Organizing Maps in Andhra Pradesh, India, 2011–2013. Parasite Epidemiol. Control 2018, 3, 52–61. [Google Scholar] [CrossRef]
- Mala, S.; Kumar, M. Geographic Information System Based Spatio-Temporal Dengue Fever Cluster Analysis and Mapping. Egypt. J. Remote Sens. Sp. Sci. 2019, 22, 297–304. [Google Scholar] [CrossRef]
- Oviedo, M.; Lage, R.; Romero, R.; Fonseca, C.; Amaral, J. Spatial and Statistical Methodologies to Determine the Distribution of Dengue in Brazilian Municipalities and Relate Incidence with the Health Vulnerability Index. Spat. Spat. Epidemiol. 2014, 11, 143–151. [Google Scholar] [CrossRef]
- Fonseca, F.; Carvalho, M. Spatial and Temporal Analysis of Epidemiological Data. 1996. In: GIS for Health and the Environment. Available online: http://www.idrc.ca/books/focus/766/nobre.html (accessed on 11 September 2020).
- Kitron, U. Landscape Ecology and Epidemiology of Vector-Borne Diseases: Tools for Spatial Analysis. J. Med. Entomol 1998, 35, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Departamento Nacional de Planeación Ficha Departamento de Cauca. Terridata. 2020. Available online: https://terridata.dnp.gov.co/ (accessed on 11 September 2020).
- Gobernación del Cauca Plan de Desarrollo Departamental 2020–2023. Colombia. 2020. Available online: https://obsgestioneducativa.com/download/plan-de-desarrollo-departamental-cauca-2020-2023/ (accessed on 22 November 2019).
- Zambrano, P. Protocolo de Vigilancia En Salud Pública. Dengue Código: 210–220–580. Vigil. Análisis Riesgo en Salud Pública Instituto Nacional de Salud; Instituto Nacional de Salud: Bogotá, Colombia, 2022; Volume 4. Available online: https://www.ins.gov.co/buscador-eventos/Lineamientos/Pro_Dengue.pdf (accessed on 15 June 2020).
- Rodrígues, T.; da Silveira, I.; Leite, W. Improving Geocoding Matching Rates of Structured Addresses in Rio de Janeiro, Brazil. Cad. Saúde Pública 2021, 37, e00039321. [Google Scholar] [CrossRef]
- Wieczorek, J.; Guo, Q.; Hujmans, R. The Point-Radius Method for Georeferencing Locality Descriptions and Calculating Associated Uncertainty. Int. J. Geogr. Inf. Sci. 2004, 18, 745–767. [Google Scholar] [CrossRef]
- Departamento Administrativo Nacional de Estadística Marco Geoestadístico Nacional. Geoportal DANE. Available online: https://geoportal.dane.gov.co/servicios/descarga-y-metadatos/descarga-mgn-marco-geoestadistico-nacional/ (accessed on 30 May 2020).
- Kulldorff, M. A Spatial Scan Statistic. Commun. Stat. 1997, 26, 1481–1496. [Google Scholar] [CrossRef]
- Khormi, H.M.; Kumar, L. Identifying and Visualizing Spatial Patterns and Hot Spots of Clinically-Confirmed Dengue Fever Cases and Female Aedes aegypti Mosquitoes in Jeddah, Saudi Arabia. Dengue Bull. 2011, 35, 15–34. [Google Scholar]
- Castillo, K.C.; Körbl, B.; Stewart, A.; Gonzalez, J.F.; Ponce, F. Application of Spatial Analysis to the Examination of Dengue Fever in Guayaquil, Ecuador. Procedia Environ. Sci. 2011, 7, 188–193. [Google Scholar] [CrossRef]
- Asociación Supradepartamental de Municipios de la Región del Alto Patía-Asopatía; Corporación Colombiana de Investigación Agropecuaria-Corpoica. Plan Básico de Ordenamiento Territorial Municipio de Patía (Cauca); Instituto Nacional de Salud: Bogotá, Colombia, 2001. Available online: http://www.asopatia.gov.co/ (accessed on 20 July 2020).
- Vergara, H.; Torres, P. Aspectos Generales Del Valle de Patía. Rev. Noved. Colomb. 2017, 12, 11–24. [Google Scholar]
- Abatzoglou, J.T.; Dobrowski, S.Z.; Parks, S.A.; Hegewisch, K.C. TerraClimate, a High-Resolution Global Dataset of Monthly Climate and Climatic Water Balance from 1958–2015. Sci. Data 2018, 5, 170191. [Google Scholar] [CrossRef]
- Spiegelhalter, D.J.; Thomas, A.; Best, N.G. WinBUGS Examples Volume 2. Imp. Coll. Med. Res. Counc. UK 2004, 6, 1–42. [Google Scholar]
- Thomas, A.; Best, N.; Lunn, D.; Spiegelhalter, D.; Way, R. GeoBUGS User Manual. GeoBugs 2004, 1, 45. [Google Scholar]
- Lawson, A. Using R for Bayesian Spatial and Spatio-Temporal Health Modeling; Kindle, Ed.; Chapman & Hall/CRC The R Series; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Best, N.; Arnold, R.; Thomas, A.; Waller, L.; Collon, E. Bayesian Models for Spatially Correlated Disease and Exposure Data. Bayesian Stat. 1999, 6, 131–156. [Google Scholar]
- Besag, J.; York, J.; Mollie, A. Bayesian Image Restoration, with Two Applications in Spatial Statistics (with Discussion). Ann. Inst. Stat. Math. 1991, 43, 1–59. [Google Scholar] [CrossRef]
- Stevenson, M.A.; Morris, R.S.; Lawson, A.B.; Wilesmith, J.W.; Ryan, J.B.M.; Jackson, R. Area-Level Risks for BSE in British Cattle before and after the July 1988 Meat and Bone Meal Feed Ban. Prev. Vet. Med. 2005, 69, 129–144. [Google Scholar] [CrossRef]
- Nelson, M. Aedes Aegypti: Biología y Ecología; Pan American Health Organization: Washington, DC, USA, 1986; pp. 1–62. [Google Scholar]
- Lourenço de Oliveira, R.; Castro, M.; Braks, M.; Lounibos, L. The Invasion of Urban Forest by Dengue Vectors in Rio de Janeiro. J. Vector Ecol. 2004, 29, 94–100. [Google Scholar]
- Rotela, C. Desarrollo de Modelos e Indicadores Remotos de Riesgo Epidemiológico de Dengue En Argentina; Universidad Nacional de Córdoba: Córdoba, Argentina, 2012; Available online: https://rdu.unc.edu.ar/handle/11086/11609 (accessed on 30 June 2020).
- Rojas, R.; Nazarena, M. Usos de Herramientas Geoespaciales En La Detección de Áreas Con Riesgo Epidemiológico a Partir de Variables Biofísicas y Casos de Dengue En Jujuy—Argentina; Universidad Nacional de Córdoba: Córdoba, Argentina, 2016; Available online: https://rdu.unc.edu.ar/handle/11086/2841 (accessed on 30 June 2020).
- Cuartas, D.E.; Martínez, G.; Caicedo, D.M.; Garcés, J.; Ariza-Araújo, Y.; Peña, M.; Méndez, F. Distribución Espacial de Criaderos Positivos y Potenciales de Aedes aegypti. Biomédica 2017, 37, 59–66. [Google Scholar] [CrossRef]
- Souza, R.; Carvalho, M. Análise Da Distribuição Espacial de Larvas de Aedes aegypti Na Ilha Do Governador, Rio de Janeiro, Brasil. Cad. Saude Publica 2000, 16, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Mutheneni, S.R.; Morse, A.P.; Caminade, C.; Upadhyayula, S.M. Dengue burden in India: Recent trends and importance of climatic parameters. Emerg. Microbes Infect. 2019, 6, 1–10. [Google Scholar] [CrossRef]
- Getis, A.; Ord, K. The Analysis of Spatial Association by Use of Distance Statistics. Geogr. Anal. 1992, 24, 189–206. [Google Scholar] [CrossRef]
- Dzul-Manzanilla, F.; Correa-Morales, F.; Che-Mendoza, A.; Palacio-Vargas, J.; Sánchez-Tejeda, G.; González-Roldan, J.F.; López-Gatell, H.; Flores-Suárez, A.E.; Gómez-Dantes, H.; Coelho, G.E.; et al. Identifying Urban Hotspots of Dengue, Chikungunya, and Zika Transmission in Mexico to Support Risk Stratification Efforts: A Spatial Analysis. Lancet Planet. Health 2021, 5, e277–e285. [Google Scholar] [CrossRef]
- Chaparro, P.; León-Quevedo, W.; Castañeda, C. Comportamiento de La Mortalidad Por Dengue En Colombia Entre 1985 y 2012. Biomédica 2016, 36, 125–134. [Google Scholar] [CrossRef]
- Hernández, M.; Arboleda, D.; Arce, S.; Benavides, A.; Tejada, P.; Ramírez, S.; Cubides, A. Metodología Para La Elaboración de Canales Endémicos y Tendencia de La Notificación Del Dengue, Valle Del Cauca, Colombia, 2009–2013. Biomédica 2016, 36, 98–107. [Google Scholar] [CrossRef]
- Molineros, L.F.; Pinzón, E.M.; Rengifo, N.E.; Daza, C.F.; Hernández-Carrillo, M.; Ortiz, M.E.; Lesmes, M.C. Seroprevalencia de Dengue En Municipios Con Transmisión Hiperendémica y Mesoendémica, Valle Del Cauca, Colombia. Rev. Cuba. Salud Pública 2020, 46, 1–20. [Google Scholar]
- Restrepo, B.; Arboleda, M.; Lopera, T. Estudio Seroepidemiológico de Dengue En La Región Del Urabá Antioqueño. Infectio 2004, 8, 255–262. [Google Scholar]
- Mena, N.; Troyo, A.; Bonilla-Carrión, R.; Calderón-Arguedas, O. Factores Asociados Con La Incidencia de Dengue En Costa Rica. Rev. Panam. Salud Pública 2011, 29, 234–242. [Google Scholar] [CrossRef]
- Gyawali, N.; Johnson, B.; Dixit, S.; Devine, G. Patterns of Dengue in Nepal from 2010–2019 in Relation to Elevation and Climate. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 741–749. [Google Scholar] [CrossRef]
- Vásquez, A. Factores Geográficos, Ecológicos y Sociodemográficos En La Ocurrencia de Dengue En Cundinamarca; Universidad Nacional de Colombia: Bogotá, Colombia, 2019. [Google Scholar]
- Organización Panamericana de la Salud/Organización Mundial de la Salud. Dengue y Dengue Hemorrágico En Las Américas: Guías Para Su Prevención y Control. 1995. Available online: https://iris.paho.org/handle/10665.2/36861 (accessed on 30 June 2021).
- Eslava, J. Climatología y Diversidad Climática En Colombia. Rev. Acad. Colomb. Cienc. 1993, 18, 508–538. [Google Scholar]
- Tuladhar, R.; Singh, A.; Varma, A.; Choudhary, D.K. Climatic Factors Influencing Dengue Incidence in an Epidemic Area of Nepal. BMC Res. 2019, 12, 131. [Google Scholar] [CrossRef]
- Chien, L.; Yu, H. Impact of Meteorological Factors on the Spatiotemporal Patterns of Dengue Fever Incidence. Environ. Int. 2014, 73, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Colón, F.; Fezzi, C.; Lake, I.; Hunter, P. Los Efectos Del Clima y El Cambio Climático Sobre El Dengue. PLoS Negl. Trop. Dis. 2013, 7, e2503. [Google Scholar] [CrossRef]
- Eastin, M.; Delmelle, E.; Casas, I.; Wexler, J.; Self, C. Intra and Interseasonal Autoregressive Prediction of Dengue Outbreaks Using Local Weather and Regional Climate for a Tropical Environment in Colombia. Am. J. Trop. Med. Hyg. 2014, 91, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Carrington, L.; Armijos, M.; Lambrechts, L.; Scott, T. Fluctuations at a Low Mean Temperature Accelerate Dengue Virus Transmission by Aedes aegypti. PLoS Negl. Trop. Dis. 2013, 7, e2190. [Google Scholar] [CrossRef]
- Carrington, L.; Armijos, M.; Lambrechts, L.; Barker, C.; Scott, T. Effects of Fluctuating Daily Temperatures at Critical Thermal Extremes on Aedes aegypti Life-History Traits. PLoS ONE 2013, 8, e58824. [Google Scholar] [CrossRef]
- García, J.; Loroño, M.; Farfán, J.; Flores, L.; Del Pilar Rosado, E.; Rivero, N.; Najera-Vazquez, R.; Gomez-Carro, S.; Lira-Zumbardo, V.; Gonzalez-Martinez, P.; et al. Dengue Virus Infected Aedes aegypti in the Home Environment. Am. J. Trop. Med. Hyg. 2008, 79, 940–950. [Google Scholar] [CrossRef]
- Johansson, M.; Cummings, D.; Glass, G. Multi-Year Climate Variability and Dengue-El Niño Southern Oscillation, Weather, and Dengue Transmission in Puerto Rico, Mexico, and Thailand: A Longitudinal Data Analysis. PLoS Med. 2009, 6, e1000168. [Google Scholar] [CrossRef] [PubMed]
- Rubio, Y.; Pérez, L.; Infante, M.; Comach, G.; Urdaneta, L. Influencia de Las Variables Climáticas En La Casuística de Dengue y La Abundancia de Aedes aegypti (Díptera: Culicidae) En Maracay, Venezuela. Bol. Malariol. Salud Ambient. 2011, 51, 145–158. [Google Scholar]
- Khormi, H.M.; Kumar, L. Modeling Dengue Fever Risk Based on Socioeconomic Parameters, Nationality and Age Groups: GIS and Remote Sensing Based Case Study. Sci. Total Environ. 2011, 409, 4713–4719. [Google Scholar] [CrossRef]
- Garelli, F.; Espinosa, M.; Gürtel, R. Spatial Analysis of Aedes aegypti Immatures in Northern Argentina: Clusters and Temporal Instability. Acta Trop. 2013, 128, 461–467. [Google Scholar] [CrossRef]
- LaCon, G.; Morrison, A.C.; Astete, H.; Stoddard, S.T.; Paz-Soldan, V.A.; Elder, J.P.; Halsey, E.S.; Scott, T.W.; Kitron, U.; Vazquez-Prokopec, G.M. Shifting Patterns of Aedes aegypti Fine Scale Spatial Clustering in Iquitos, Peru. PLoS Negl. Trop. Dis. 2014, 8, e3038. [Google Scholar] [CrossRef]
- Organización Panamericana de la Salud/Organización Mundial de la Salud. Documento Técnico Para La Implementación de Intervenciones Basado En Escenarios Operativos Genéricos Para El Control Del Aedes aegypti; Pan American Health Organization: Washington, DC, USA, 2019; Available online: https://iris.paho.org/handle/10665.2/51654 (accessed on 12 February 2020).
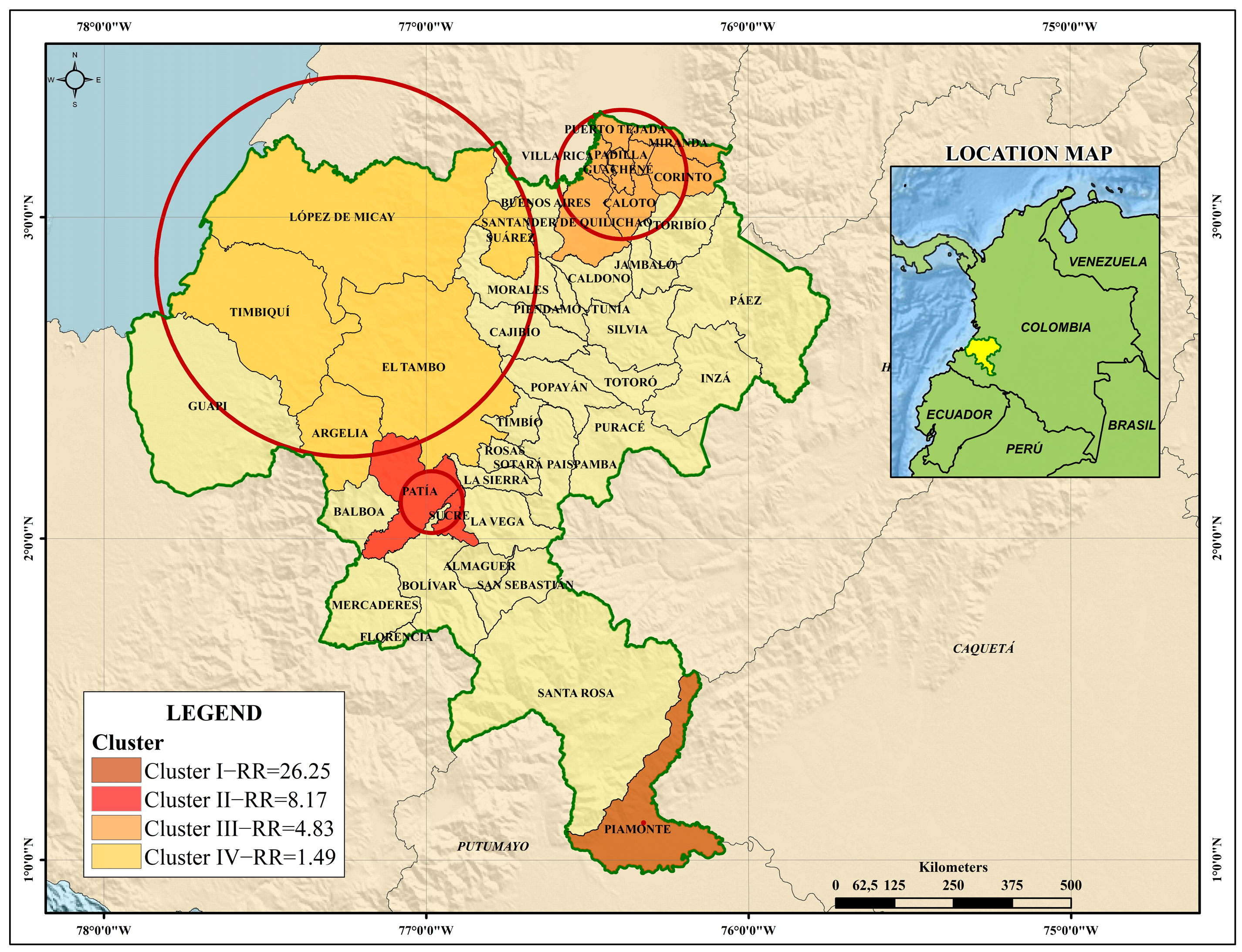

| Dengue Clusters in the Department of Cauca | ||||
|---|---|---|---|---|
| Cluster I * | Cluster II * | Cluster III * | Cluster IV | |
| Municipalities | Piamonte | Patía and Sucre | Caloto, Miranda, Corinto, Padilla, Puerto Tejada, Guachené, Santander de Quilichao and Villa Rica | López de Micay, Tim- biquí, Suárez, El Tambo and Argelia |
| Time frame | 2018 | 2014–2016 | 2013–2015 | 2016 |
| Population | 7343 | 45,103 | 274,554 | 134,841 |
| Registered cases | 56 | 293 | 857 | 59 |
| Expected cases | 2.18 | 39.65 | 237.22 | 39.77 |
| Relative risk | 26.25 | 8.17 | 4.83 | 1.49 |
| Likelihood ratio | 128.58 | 345.14 | 566.61 | 4.11 |
| Node | Mean | Standard Deviation | Monte Carlo Standard Error | 2.5% | Median | 97.5% |
|---|---|---|---|---|---|---|
| alpha0 | 7.695 | 3.47 | 0.2902 | 1.795 | 7.505 | 14.39 |
| alpha1 | −0.02165 | 0.008491 | 6.66 × 10−4 | −0.03802 | −0.02159 | −0.006483 |
| alpha2 | −3.194 | 1.483 | 0.1237 | −5.948 | −3.101 | −0.6001 |
| b [1] | −0.5435 | 0.6066 | 0.01957 | −1.777 | −0.5334 | 0.6039 |
| b [2] | 1.34 | 0.4015 | 0.01827 | 0.5572 | 1.337 | 2.132 |
| b [3] | −0.5395 | 0.2034 | 0.01239 | 2.672 | 3.057 | 3.464 |
| b [4] | −0.3136 | 0.5844 | 0.01519 | −1.499 | −0.3007 | 0.7992 |
| b [5] | −0.1096 | 0.5187 | 0.01175 | −1.165 | −0.09905 | 0.8628 |
| b [6] | −0.2194 | 0.5398 | 0.01347 | −1.334 | −0.2008 | 0.7841 |
| b [7] | −0.572 | 0.5891 | 0.01551 | −1.775 | −0.5546 | 0.5265 |
| b [8] | 0.6091 | 0.628 | 0.01041 | −0.7112 | 0.6408 | 1.763 |
| b [9] | 0.01779 | 0.5955 | 0.008791 | −1.202 | 0.03556 | 1.121 |
| b [10] | 0.2088 | 0.5201 | 0.01189 | −0.8448 | 0.2213 | 1.185 |
| b [11] | 0.3356 | 0.769 | 0.02283 | −1.213 | 0.3575 | 1.799 |
| b [12] | 0.2252 | 0.5891 | 0.005614 | −0.9738 | 0.2411 | 1.339 |
| b [13] | 0.6786 | 0.7378 | 0.02756 | −0.8057 | 0.686 | 2.082 |
| b [14] | −0.1665 | 0.5087 | 0.01047 | −1.227 | −0.1489 | 0.7755 |
| b [15] | −0.3246 | 0.5874 | 0.01131 | −1.532 | −0.3095 | 0.7713 |
| b [16] | 0.5209 | 0.8719 | 0.03325 | −1.256 | 0.5406 | 2.169 |
| b [17] | −0.2656 | 0.8166 | 0.01115 | −2.038 | −0.2108 | 1.168 |
| b [18] | 0.2778 | 0.5413 | 0.004316 | −0.8249 | 0.2923 | 1.291 |
| b [19] | 0.5524 | 0.9201 | 0.03325 | −1.301 | 0.5725 | 2.294 |
| b [20] | 0.3277 | 0.5034 | 0.004141 | −0.6865 | 0.3374 | 1.296 |
| b [21] | 0.5987 | 0.583 | 0.00776 | −0.5936 | 0.6208 | 1.676 |
| b [22] | 0.6985 | 0.7948 | 0.02767 | −0.9144 | 0.7118 | 2.201 |
| b [23] | 0.7003 | 0.7746 | 0.03044 | −0.8398 | 0.7047 | 2.194 |
| b [24] | 0.6407 | 0.8443 | 0.03292 | −1.052 | 0.6559 | 2.274 |
| b [25] | 0.6698 | 0.9354 | 0.03484 | −1.199 | 0.6845 | 2.46 |
| b [26] | 0.6066 | 0.9397 | 0.03487 | −1.268 | 0.633 | 2.4 |
| b [27] | 0.4907 | 0.8011 | 0.03154 | −1.127 | 0.5083 | 2.01 |
| b [28] | 0.2674 | 0.7399 | 0.02962 | −1.217 | 0.2898 | 1.654 |
| b [29] | 0.4655 | 0.7695 | 0.02498 | −1.113 | 0.4954 | 1.902 |
| b [30] | 1.099 | 0.5708 | 0.01492 | −0.0659 | 1.119 | 2.17 |
| b [31] | 0.5501 | 0.6691 | 0.01684 | −0.813 | 0.5687 | 1.815 |
| b [32] | 0.4066 | 0.778 | 0.01396 | −1.227 | 0.4386 | 1.83 |
| b [33] | −0.0915 | 0.7589 | 0.7589 | −1.718 | −0.03475 | 1.237 |
| b [34] | 0.4784 | 0.5976 | 0.009939 | −0.7641 | 0.4966 | 1.595 |
| b [35] | 0.849 | 0.7001 | 0.02512 | −0.5624 | 0.8676 | 2.169 |
| b [36] | 0.9551 | 0.5268 | 0.005398 | −0.1324 | 0.9757 | 1.924 |
| b [37] | 0.606 | 0.6236 | 0.02026 | −0.6453 | 0.6139 | 1.809 |
| b [38] | 0.8178 | 0.5307 | 0.008372 | −0.2679 | 0.8293 | 1.818 |
| b [39] | 0.4421 | 0.6346 | 0.02222 | −0.8558 | 0.4562 | 1.663 |
| b [40] | 0.2733 | 0.6547 | 0.02012 | −1.051 | 0.2935 | 1.513 |
| b [41] | 0.7322 | 0.5801 | 0.01306 | −0.4673 | 0.7522 | 1.81 |
| b [42] | 0.3273 | 0.5643 | 0.006039 | −0.8295 | 0.3457 | 1.381 |
| b [43] | −0.341 | 0.706 | 0.01081 | −1.842 | −0.3008 | 0.9222 |
| b [44] | 0.05025 | 0.6627 | 0.006465 | −1.334 | 0.07333 | 1.279 |
| b [45] | −0.1234 | 0.6103 | 0.01085 | −1.353 | −0.1065 | 1.033 |
| b [46] | −0.0973 | 0.5937 | 0.008167 | −1.32 | −0.07968 | 1.005 |
| b [47] | −0.009787 | 0.5173 | 0.005345 | −1.068 | 0.005371 | 0.9668 |
| b [48] | 0.1815 | 0.5157 | 0.00985 | −0.861 | 0.1892 | 1.171 |
| b [49] | 0.1697 | 0.6075 | 0.005576 | −1.071 | 0.1897 | 1.316 |
| b [50] | 0.3164 | 0.5631 | 0.006296 | −0.8412 | 0.336 | 1.368 |
| b [51] | 0.09894 | 0.709 | 0.01129 | −1.346 | 0.1211 | 1.418 |
| b [52] | 0.1512 | 0.6355 | 0.01506 | −1.137 | 0.1628 | 1.357 |
| b [53] | 0.07944 | 0.5645 | 0.004516 | −1.074 | 0.09702 | 1.143 |
| b [54] | 0.377 | 0.6513 | 0.02601 | −0.9065 | 0.3821 | 1.643 |
| b [55] | −0.6758 | 0.5308 | 0.0199 | −1.748 | −0.661 | 0.3264 |
| b [56] | 0.1952 | 0.5787 | 0.01601 | −1.003 | 0.2111 | 1.279 |
| b [57] | 0.2195 | 0.6124 | 0.01644 | −1.024 | 0.236 | 1.374 |
| b [58] | 0.4243 | 0.6111 | 0.02113 | −0.7872 | 0.4305 | 1.605 |
| b [59] | 0.6599 | 0.834 | 0.02483 | −1.075 | 0.6953 | 2.181 |
| b [60] | 0.1436 | 0.6062 | 0.01453 | −1.076 | 0.1599 | 1.293 |
| b [61] | 0.1939 | 0.6635 | 0.01304 | −1.185 | 0.2181 | 1.416 |
| b [62] | −0.01998 | 0.7152 | 0.02696 | −1.478 | −0.01188 | 1.346 |
| b [63] | 0.2854 | 0.6176 | 0.009941 | −0.9863 | 0.3086 | 1.432 |
| b [64] | 0.3602 | 0.6933 | 0.0105 | −1.06 | 0.3934 | 1.629 |
| b [65] | 0.8406 | 0.7516 | 0.01541 | −0.7825 | 0.9024 | 2.152 |
| b [66] | −0.3531 | 0.5254 | 0.01345 | −1.404 | −0.3417 | 0.6417 |
| b [67] | −0.3 | 0.6403 | 0.01245 | −1.612 | −0.2808 | 0.8996 |
| b [68] | −0.2166 | 0.5635 | 0.0104 | −1.381 | −0.2001 | 0.8314 |
| b [69] | −0.4795 | 0.6148 | 0.01522 | −1.742 | −0.4533 | 0.6633 |
| b [70] | −0.7309 | 0.6564 | 0.01606 | −2.078 | −0.7153 | 0.5015 |
| b [71] | −0.3847 | 0.6053 | 0.01633 | −1.613 | −0.3669 | 0.7566 |
| b [72] | −0.2605 | 0.6073 | 0.0147 | −1.523 | −0.2339 | 0.8679 |
| b [73] | −0.08215 | 0.6319 | 0.009134 | −1.407 | −0.04773 | 1.057 |
| b [74] | 1.696 | 0.4305 | 0.005831 | 0.802 | 1.714 | 2.492 |
| b [75] | −0.0793 | 0.5685 | 0.009272 | −1.267 | −0.05448 | 0.9694 |
| b [76] | −0.2972 | 0.6727 | 0.01103 | −1.692 | −0.2665 | 0.9358 |
| b [77] | −0.04271 | 0.5972 | 0.009422 | −1.281 | −0.01751 | 1.048 |
| b [78] | −0.2496 | 0.5543 | 0.00934 | −1.413 | −0.2206 | 0.7576 |
| b [79] | −0.1282 | 0.5572 | 0.01277 | −1.305 | −0.1067 | 0.8917 |
| b [80] | −0.4082 | 0.5174 | 0.01142 | −1.498 | −0.3859 | 0.5412 |
| b [81] | −0.7311 | 0.6199 | 0.01116 | −2.031 | −0.6984 | 0.3958 |
| b [82] | −0.7444 | 0.5425 | 0.01338 | −1.871 | −0.7151 | 0.2382 |
| b [83] | −0.2832 | 0.9397 | 0.01321 | −2.357 | −0.1985 | 1.312 |
| b [84] | −1.256 | 0.6806 | 0.01606 | −2.659 | −1.228 | −0.009965 |
| b [85] | −0.8898 | 0.655 | 0.01436 | −2.256 | −0.8608 | 0.3195 |
| b [86] | −1.294 | 0.5551 | 0.01508 | −2.444 | −1.27 | −0.27 |
| b [87] | −1.348 | 0.7438 | 0.01577 | −2.915 | −1.31 | 0.007691 |
| b [88] | −0.4158 | 0.6708 | 0.0184 | −1.799 | −0.3965 | 0.8323 |
| b [89] | −0.05354 | 0.6186 | 0.01769 | −1.314 | −0.04162 | 1.119 |
| b [90] | −1.061 | 0.5791 | 0.01582 | −2.244 | −1.046 | 0.03023 |
| b [91] | −1.265 | 0.7097 | 0.01687 | −2.74 | −1.23 | 0.02105 |
| b [92] | −0.8122 | 0.5955 | 0.01683 | −2.022 | −0.7983 | 0.3181 |
| b [93] | −0.8615 | 0.6813 | 0.01718 | −2.263 | −0.8314 | 0.4095 |
| b [94] | −0.8309 | 0.5883 | 0.01738 | −2.03 | −0.8136 | 0.2922 |
| b [95] | −0.7791 | 0.6103 | 0.01846 | −2.038 | −0.76 | 0.3611 |
| b [96] | −1.085 | 0.6701 | 0.01851 | −2.488 | −1.057 | 0.1453 |
| b [97] | −0.2666 | 0.6203 | 0.01904 | −1.56 | −0.25 | 0.9118 |
| b [98] | −1.002 | 0.7106 | 0.01762 | −2.467 | −0.976 | 0.3117 |
| b [99] | −0.7158 | 0.573 | 0.01842 | −1.878 | −0.7047 | 0.3791 |
| b [100] | −0.9311 | 0.6718 | 0.01815 | −2.343 | −0.8914 | 0.2785 |
| b [101] | −0.5415 | 0.8362 | 0.01919 | −2.312 | −0.4927 | 0.9519 |
| b [102] | −0.3021 | 0.6052 | 0.01899 | −1.546 | −0.2837 | 0.8592 |
| b [103] | −0.5478 | 0.6663 | 0.0198 | −1.923 | −0.5185 | 0.6852 |
| b [104] | −0.8285 | 0.7359 | 0.02025 | −2.37 | −0.7977 | 0.5246 |
| b [105] | 3.06 | 0.2034 | 0.01239 | 2.672 | 3.057 | 3.464 |
| tau | 0.3771 | 0.08271 | 0.001286 | 0.2407 | 0.3688 | 0.5622 |
| Dengue Cases in the Municipality of Patia (2015–2019) | |||||
|---|---|---|---|---|---|
| SIVIGILA Notified a | CAUCA Notified b | Provenience | Dismiss | Final Data | |
| Year | |||||
| 2015 | 101 | 97 | 109 | 12 | 97 |
| 2016 | 65 | 86 | 91 | 3 | 88 |
| 2017 | 14 | 14 | 23 | 9 | 14 |
| 2018 | 24 | 24 | 31 | 6 | 25 |
| 2019 | 17 | 22 | 28 | 6 | 22 |
| Total | 221 | 243 | 282 | 36 | 246 |
| Neighbourhoods | Population | Year 2017 | Year 2018 | Year 2019 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. Cases | Total Pupae | Breteau Index | Pupae/Person | No. Cases | Total Pupae | Breteau Index | Pupae/Person | No. Cases | Total Pupae | Breteau Index | Pupae/Person | ||
| Altillo | 185 | 0 | 34 | 7.22 | 0.13 | 0 | 18 | 2.78 | 0.06 | 0 | 54 | 6.18 | 0.22 |
| Aruba y Curazao | 178 | 0 | 0 | 0.00 | 0.00 | 0 | 0 | 0.00 | 0.00 | 2 | 0 | 0.00 | 0.00 |
| Balboita | 365 | 0 | 9 | 2.38 | 0.02 | 0 | 0 | 0.00 | 0.00 | 1 | 17 | 2.08 | 0.04 |
| Calle Nueva | 291 | 0 | 67 | 3.57 | 0.12 | 0 | 35 | 1.92 | 0.10 | 0 | 20 | 0.69 | 0.02 |
| Centro | 263 | 0 | 2 | 1.59 | 0.01 | 0 | 0 | 0.00 | 0.00 | 0 | 35 | 4.17 | 0.15 |
| El Campín | 155 | 0 | 175 | 5.93 | 0.31 | 0 | 18 | 2.50 | 0.06 | 0 | 172 | 8.04 | 0.26 |
| El Lago | 523 | 0 | 9 | 4.17 | 0.04 | 0 | 12 | 7.14 | 0.09 | 1 | 102 | 12.50 | 0.28 |
| El Peñol | 416 | 0 | 42 | 5.36 | 0.10 | 0 | 33 | 5.41 | 0.12 | 0 | 60 | 4.25 | 0.12 |
| Estudiantes | 368 | 0 | 80 | 2.78 | 0.09 | 0 | 32 | 4.17 | 0.06 | 0 | 135 | 5.09 | 0.14 |
| Fundadores | 419 | 0 | 81 | 5.29 | 0.22 | 0 | 17 | 4.78 | 0.06 | 0 | 88 | 4.17 | 0.15 |
| Galán | 199 | 0 | 49 | 18.18 | 0.54 | 1 | 17.5 | 5.56 | 0.11 | 0 | 0 | 0.00 | 0.00 |
| Hospital | 8 | 0 | 0 | 0.00 | 0.00 | 0 | 0 | 0.00 | 0.00 | 0 | 0 | 0.00 | 0.00 |
| Hueco Lindo | 359 | 1 | 102.5 | 4.29 | 0.19 | 0 | 45 | 2.38 | 0.14 | 0 | 96 | 5.00 | 0.13 |
| Jardín | 303 | 0 | 63 | 1.85 | 0.09 | 0 | 0 | 0.00 | 0.00 | 0 | 29 | 2.78 | 0.03 |
| La Floresta | 281 | 0 | 43 | 15.38 | 0.41 | 0 | 12 | 7.14 | 0.10 | 0 | 0 | 0.00 | 0.00 |
| La Unión | 348 | 1 | 98 | 6.21 | 0.21 | 1 | 33 | 7.14 | 0.10 | 0 | 55 | 4.76 | 0.09 |
| Las Ferias | 86 | 0 | 163 | 14.29 | 0.49 | 0 | 15 | 2.78 | 0.07 | 0 | 108 | 9.44 | 0.21 |
| Libertador | 583 | 1 | 410 | 15.93 | 0.47 | 0 | 145 | 8.33 | 0.33 | 0 | 138 | 4.70 | 0.15 |
| Limonar | 163 | 0 | 0 | 0.00 | 0.00 | 0 | 0 | 0.00 | 0.00 | 0 | 14 | 2.50 | 0.05 |
| Modelo | 369 | 0 | 124 | 8.25 | 0.22 | 0 | 69 | 9.52 | 0.22 | 0 | 282 | 11.55 | 0.42 |
| Olaya Herrera | 1461 | 0 | 98 | 3.27 | 0.09 | 3 | 124.5 | 7.92 | 0.17 | 0 | 224 | 7.32 | 0.18 |
| Pablo VI–Parte I | 187 | 0 | 0 | 0.00 | 0.00 | 0 | 0 | 0.00 | 0.00 | 0 | 61 | 5.95 | 0.12 |
| Pablo VI–Parte II | 513 | 0 | 0 | 0.00 | 0.00 | 0 | 0 | 0.00 | 0.00 | 0 | 0 | 0.00 | 0.00 |
| Popular | 294 | 2 | 110 | 7.25 | 0.21 | 0 | 0 | 0.00 | 0.00 | 1 | 99 | 5.17 | 0.14 |
| Postobon | 717 | 0 | 114 | 6.23 | 0.16 | 2 | 15 | 1.43 | 0.03 | 0 | 60 | 3.66 | 0.07 |
| Prados del Norte | 327 | 1 | 33 | 2.38 | 0.12 | 0 | 42 | 11.21 | 0.20 | 1 | 77 | 5.14 | 0.14 |
| Puerto Nuevo | 198 | 0 | 60 | 5.81 | 0.23 | 0 | 0 | 0.00 | 0.00 | 0 | 21 | 1.96 | 0.06 |
| Versalles | 130 | 0 | 48 | 5.56 | 0.17 | 0 | 18 | 3.33 | 0.08 | 1 | 33 | 4.58 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marceló-Díaz, C.; Lesmes, M.C.; Santamaría, E.; Salamanca, J.A.; Fuya, P.; Cadena, H.; Muñoz-Laiton, P.; Morales, C.A. Spatial Analysis of Dengue Clusters at Department, Municipality and Local Scales in the Southwest of Colombia, 2014–2019. Trop. Med. Infect. Dis. 2023, 8, 262. https://doi.org/10.3390/tropicalmed8050262
Marceló-Díaz C, Lesmes MC, Santamaría E, Salamanca JA, Fuya P, Cadena H, Muñoz-Laiton P, Morales CA. Spatial Analysis of Dengue Clusters at Department, Municipality and Local Scales in the Southwest of Colombia, 2014–2019. Tropical Medicine and Infectious Disease. 2023; 8(5):262. https://doi.org/10.3390/tropicalmed8050262
Chicago/Turabian StyleMarceló-Díaz, Catalina, María Camila Lesmes, Erika Santamaría, José Alejandro Salamanca, Patricia Fuya, Horacio Cadena, Paola Muñoz-Laiton, and Carlos Andrés Morales. 2023. "Spatial Analysis of Dengue Clusters at Department, Municipality and Local Scales in the Southwest of Colombia, 2014–2019" Tropical Medicine and Infectious Disease 8, no. 5: 262. https://doi.org/10.3390/tropicalmed8050262
APA StyleMarceló-Díaz, C., Lesmes, M. C., Santamaría, E., Salamanca, J. A., Fuya, P., Cadena, H., Muñoz-Laiton, P., & Morales, C. A. (2023). Spatial Analysis of Dengue Clusters at Department, Municipality and Local Scales in the Southwest of Colombia, 2014–2019. Tropical Medicine and Infectious Disease, 8(5), 262. https://doi.org/10.3390/tropicalmed8050262









