Trends in ELISA-Based Flavivirus IgG Serosurveys: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources and Search Strategy
2.4. Study Selection
2.5. Data Collection
2.6. Quality Assessment
2.7. Statistical Analysis
3. Results
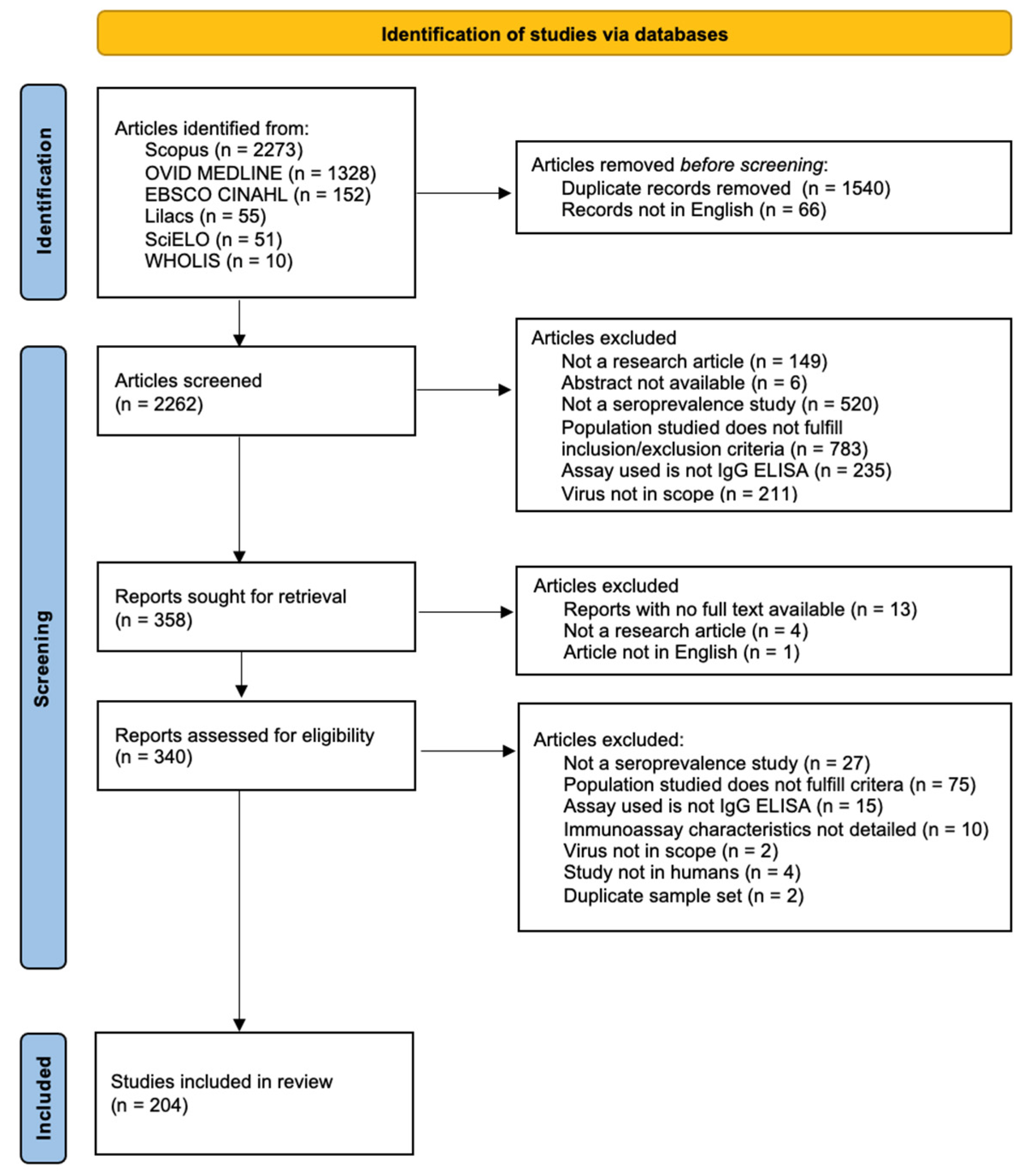
3.1. Study Selection
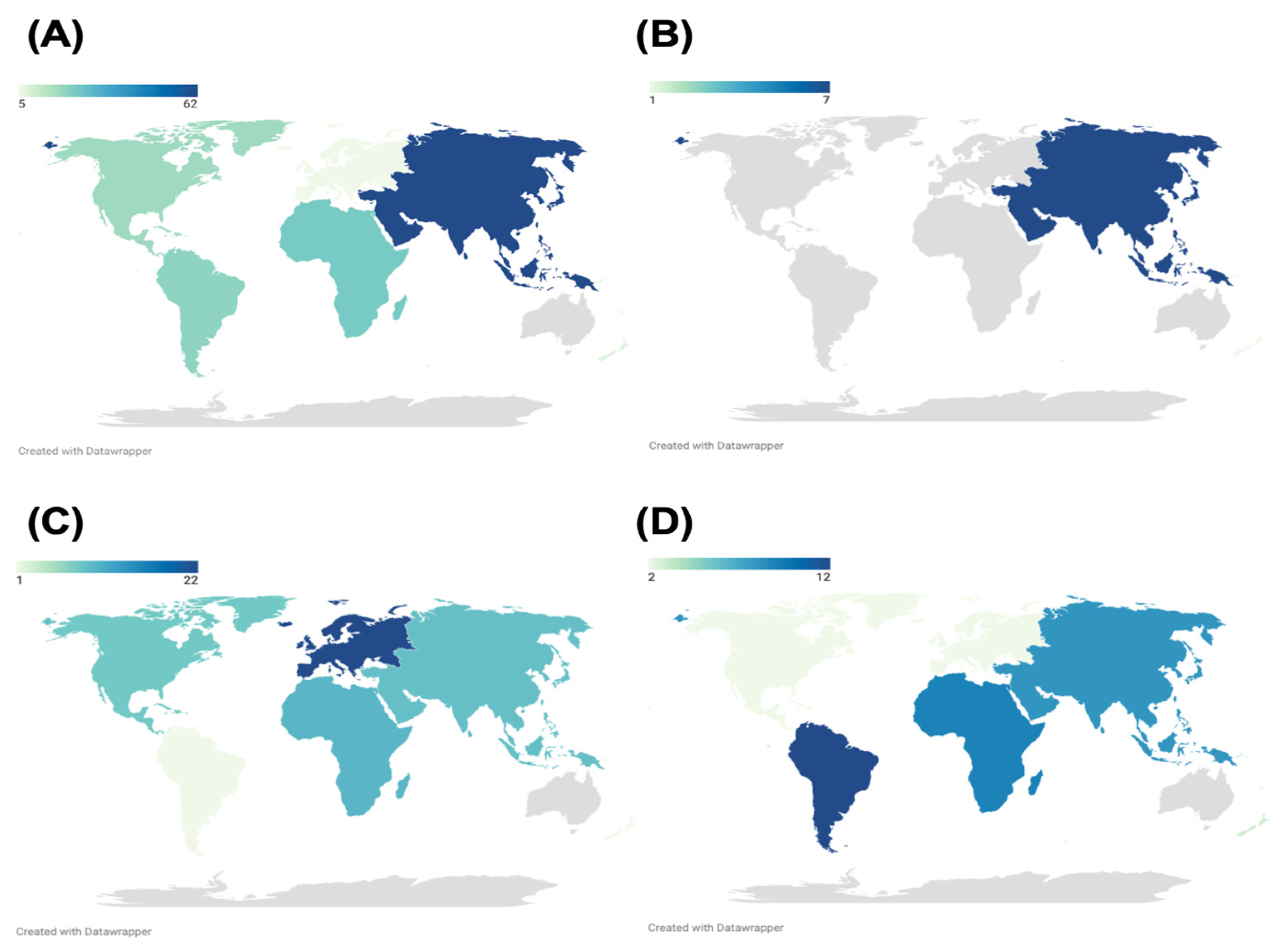
3.2. Distribution of Flavivirus Seroprevalence Studies
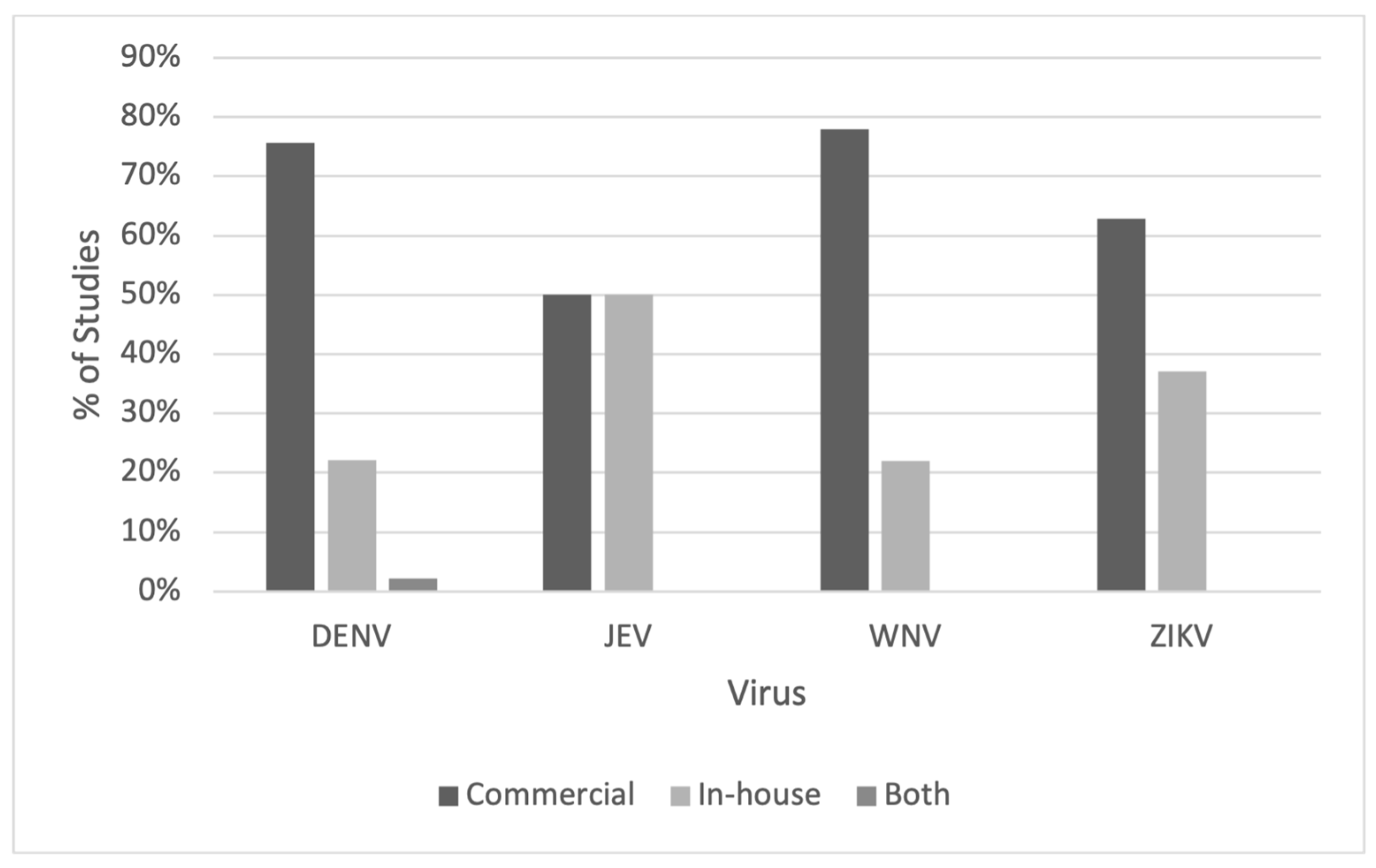
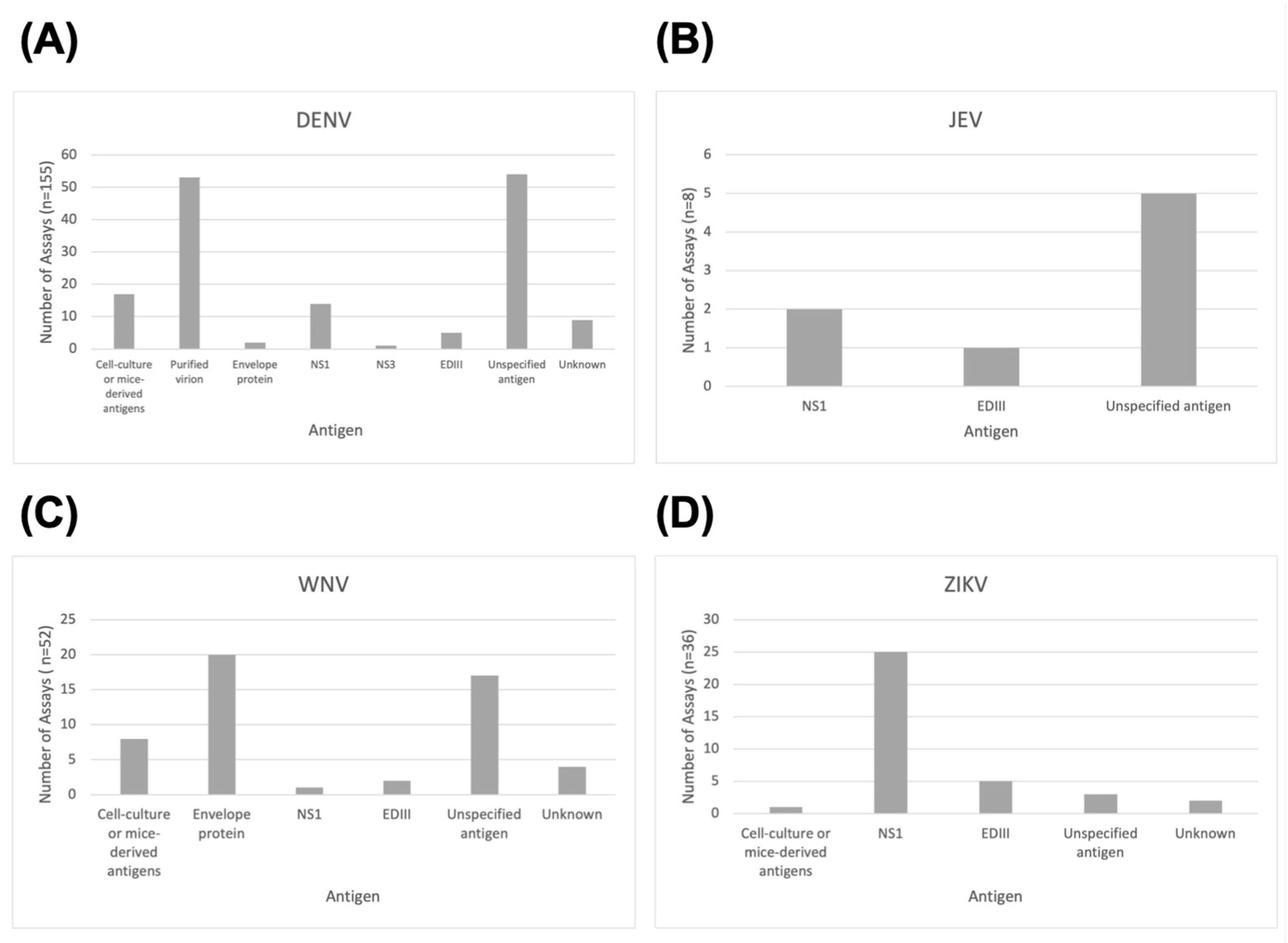
3.3. Types of ELISA and ELISA Antigens Used
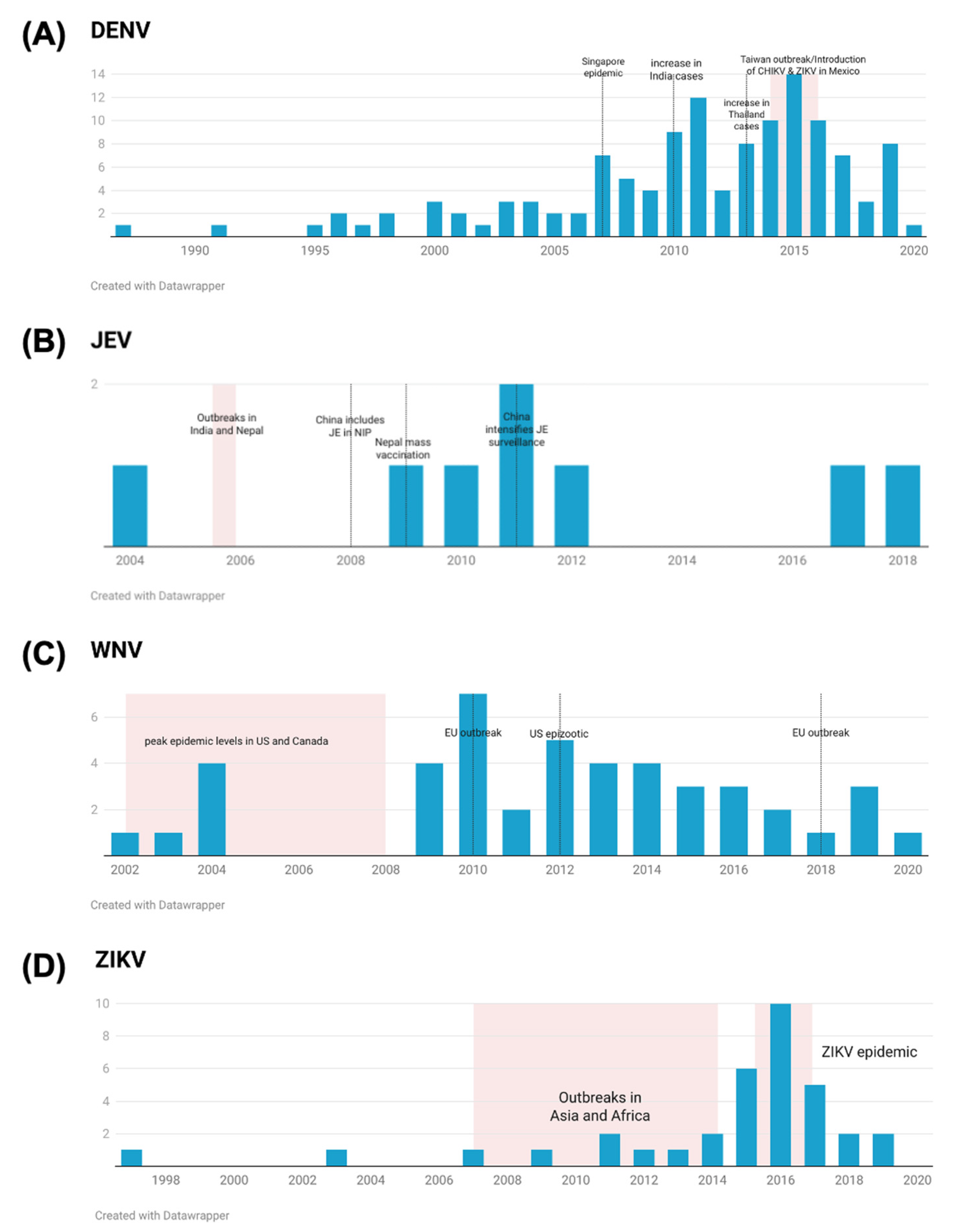
3.4. Serosurvey Trends over Time
4. Discussion
4.1. Regional Distribution of Flavivirus Serosurveys
4.2. Temporal Distribution of Flavivirus Serosurveys
4.3. IgG ELISAs Used in Flavivirus Serosurveys
4.4. ELISA Formats and Antigens in Flavivirus Serosurveys
4.5. Challenges in ELISA-Based Flavivirus Serosurveillance
4.6. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindenbach, B.D.; Murray, C.L.; Heinz-Jürgel, T.; Rice, C.M. Flaviviridae. In Fields Virology; Knipe, D.M., Howley, P.M., Eds.; Wolters Kluwer/Lippincott Williams & Wilkins Health: Philadelphia, PA, USA, 2013; pp. 712–746. [Google Scholar]
- Kaaijk, P.; Luytjes, W. Are We Prepared for Emerging Flaviviruses in Europe? Challenges for Vaccination. Hum. Vaccin. Immunother. 2017, 14, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Pierson, T.C.; Diamond, M.S. The Continued Threat of Emerging Flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Costa, F.; Garcia-Blanco, M.A.; Ko, A.I.; Ribeiro, G.S.; Saade, G.; Shi, P.-Y.; Vasilakis, N. Zika Virus: History, Emergence, Biology, and Prospects for Control. Antiviral. Res. 2016, 130, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Desprès, P. Serological Diagnosis of Flavivirus-Associated Human Infections. Diagnostics 2020, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.M.; Mohammed, A.T.; Vu, T.T.; Khattab, M.; Doheim, M.F.; Ashraf Mohamed, A.; Abdelhamed, M.M.; Shamandy, B.E.; Dawod, M.T.; Alesaei, W.A.; et al. Prevalence and Burden of Dengue Infection in Europe: A Systematic Review and Meta-analysis. Rev. Med. Virol. 2020, 30, e2093. [Google Scholar] [CrossRef]
- Cleton, N.; Koopmans, M.; Braks, M.; Van Maanen, K.; Reusken, C. Japanese encephalitis in Southern Europe. Tijdschr Diergeneeskd 2014, 139, 20–25. [Google Scholar]
- Khawar, W.; Bromberg, R.; Moor, M.; Lyubynska, N.; Mahmoudi, H. Seven Cases of Zika Virus Infection in South Florida. Cureus 2017, 9, e1099. [Google Scholar] [CrossRef]
- Hasan, S.; Jamdar, S.; Alalowi, M.; Al Ageel Al Beaiji, S. Dengue Virus: A Global Human Threat: Review of Literature. J. Int. Soc. Prevent. Communit. Dent. 2016, 6, 1. [Google Scholar] [CrossRef]
- Daep, C.A.; Muñoz-Jordán, J.L.; Eugenin, E.A. Flaviviruses, an Expanding Threat in Public Health: Focus on Dengue, West Nile, and Japanese Encephalitis Virus. J. Neurovirol. 2014, 20, 539–560. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The Global Distribution and Burden of Dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Suwanmanee, S.; Luplertlop, N. Dengue and Zika Viruses: Lessons Learned from the Similarities between These Aedes Mosquito-Vectored Arboviruses. J. Microbiol. 2017, 55, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Paixão, E.S.; Barreto, F.; da Glória Teixeira, M.; da Conceição, N.; Costa, M.; Rodrigues, L.C. History, Epidemiology, and Clinical Manifestations of Zika: A Systematic Review. Am. J. Public Health 2016, 106, 606–612. [Google Scholar] [CrossRef]
- Halani, S.; Tombindo, P.E.; O’Reilly, R.; Miranda, R.N.; Erdman, L.K.; Whitehead, C.; Bielecki, J.M.; Ramsay, L.; Ximenes, R.; Boyle, J.; et al. Clinical Manifestations and Health Outcomes Associated with Zika Virus Infections in Adults: A Systematic Review. PLoS Negl. Trop. Dis. 2021, 15, e0009516. [Google Scholar] [CrossRef]
- Moore, C.A.; Staples, J.E.; Dobyns, W.B.; Pessoa, A.; Ventura, C.V.; da Fonseca, E.B.; Ribeiro, E.M.; Ventura, L.O.; Neto, N.N.; Arena, J.F.; et al. Characterizing the Pattern of Anomalies in Congenital Zika Syndrome for Pediatric Clinicians. JAMA Pediatr. 2017, 171, 288. [Google Scholar] [CrossRef] [PubMed]
- WHO. Zika Epidemiology Update—February 2022. Available online: https://www.who.int/publications/m/item/zika-epidemiology-update---february-2022 (accessed on 4 August 2022).
- Solomon, T.; Dung, N.M.; Kneen, R.; Gainsborough, M.; Brown, D.W.; Khanh, V.T. Japanese Encephalitis. J. Neurol. Neurosurg. Psychiatry 2000, 68, 405–415. [Google Scholar] [CrossRef]
- WHO. Japanese Encephalitis. Available online: https://www.who.int/news-room/fact-sheets/detail/japanese-encephalitis (accessed on 4 August 2022).
- Habarugira, G.; Suen, W.W.; Hobson-Peters, J.; Hall, R.A.; Bielefeldt-Ohmann, H. West Nile Virus: An Update on Pathobiology, Epidemiology, Diagnostics, Control and “One Health” Implications. Pathogens 2020, 9, 589. [Google Scholar] [CrossRef]
- Sejvar, J.J. West Nile Virus Infection. Microbiol. Spectr. 2016, 4, 237–269. [Google Scholar] [CrossRef]
- Yan, G.; Pang, L.; Cook, A.R.; Ho, H.J.; Win, M.S.; Khoo, A.L.; Wong, J.G.X.; Lee, C.K.; Yan, B.; Jureen, R.; et al. Distinguishing Zika and Dengue Viruses through Simple Clinical Assessment, Singapore. Emerg. Infect. Dis. 2018, 24, 1565–1568. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.P.S.; John, A.L.S. Cross-Reactive Immunity Among Flaviviruses. Front. Immunol. 2020, 11, 334. [Google Scholar] [CrossRef]
- World Health Organization. Vaccines and Immunization: Dengue. Available online: https://www.who.int/news-room/questions-and-answers/item/dengue-vaccines (accessed on 6 April 2023).
- Fritzell, C.; Rousset, D.; Adde, A.; Kazanji, M.; Van Kerkhove, M.D.; Flamand, C. Current Challenges and Implications for Dengue, Chikungunya and Zika Seroprevalence Studies Worldwide: A Scoping Review. PLoS Negl. Trop. Dis. 2018, 12, e0006533. [Google Scholar] [CrossRef] [PubMed]
- Undurraga, E.A.; Halasa, Y.A.; Shepard, D.S. Use of Expansion Factors to Estimate the Burden of Dengue in Southeast Asia: A Systematic Analysis. PLoS Negl. Trop. Dis. 2013, 7, e2056. [Google Scholar] [CrossRef]
- Alera, M.T.; Srikiatkhachorn, A.; Velasco, J.M.; Tac-An, I.A.; Lago, C.B.; Clapham, H.E.; Fernandez, S.; Levy, J.W.; Thaisomboonsuk, B.; Klungthong, C.; et al. Incidence of Dengue Virus Infection in Adults and Children in a Prospective Longitudinal Cohort in the Philippines. PLoS Negl. Trop. Dis. 2016, 10, e0004337. [Google Scholar] [CrossRef]
- Nagy, A.; Csonka, N.; Takács, M.; Mezei, E.; Barabás, É. West Nile and Usutu Virus Seroprevalence in Hungary: A Nationwide Serosurvey among Blood Donors in 2019. PLoS ONE 2022, 17, e0266840. [Google Scholar] [CrossRef]
- Yen, T.-Y.; Trovoada dos Santos, M.d.J.; Tseng, L.-F.; Chang, S.-F.; Cheng, C.-F.; Carvalho, A.V.d.A.; Shu, P.-Y.; Lien, J.-C.; Tsai, K.-H. Seroprevalence of Antibodies against Dengue Virus among Pregnant Women in the Democratic Republic of Sao Tome and Principe. Acta Tropica 2016, 155, 58–62. [Google Scholar] [CrossRef]
- Flamand, C.; Bailly, S.; Fritzell, C.; Berthelot, L.; Vanhomwegen, J.; Salje, H.; Paireau, J.; Matheus, S.; Enfissi, A.; Fernandes-Pellerin, S.; et al. Impact of Zika Virus Emergence in French Guiana: A Large General Population Seroprevalence Survey. J. Infect. Dis. 2019, 220, 1915–1925. [Google Scholar] [CrossRef]
- Biggs, J.R.; Sy, A.K.; Brady, O.J.; Kucharski, A.J.; Funk, S.; Tu, Y.-H.; Reyes, M.A.J.; Quinones, M.A.; Jones-Warner, W.; Ashall, J.; et al. Serological Evidence of Widespread Zika Transmission across the Philippines. Viruses 2021, 13, 1441. [Google Scholar] [CrossRef] [PubMed]
- Otu, A.A.; Udoh, U.A.; Ita, O.I.; Hicks, J.P.; Egbe, W.O.; Walley, J. A Cross-Sectional Survey on the Seroprevalence of Dengue Fever in Febrile Patients Attending Health Facilities in Cross River State, Nigeria. PLoS ONE 2019, 14, e0215143. [Google Scholar] [CrossRef] [PubMed]
- Sam, I.-C.; Montoya, M.; Chua, C.L.; Chan, Y.F.; Pastor, A.; Harris, E. Low Seroprevalence Rates of Zika Virus in Kuala Lumpur, Malaysia. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 678–684. [Google Scholar] [CrossRef]
- Lukman, N.; Salim, G.; Kosasih, H.; Susanto, N.H.; Parwati, I.; Fitri, S.; Alisjahbana, B.; Widjaja, S.; Williams, M. Comparison of the Hemagglutination Inhibition Test and IgG ELISA in Categorizing Primary and Secondary Dengue Infections Based on the Plaque Reduction Neutralization Test. BioMed Res. Int. 2016, 2016, 1–5. [Google Scholar] [CrossRef]
- Balingit, J.C.; Phu Ly, M.H.; Matsuda, M.; Suzuki, R.; Hasebe, F.; Morita, K.; Moi, M.L. A Simple and High-Throughput ELISA-Based Neutralization Assay for the Determination of Anti-Flavivirus Neutralizing Antibodies. Vaccines 2020, 8, 297. [Google Scholar] [CrossRef] [PubMed]
- Maezono, K.; Kobayashi, S.; Tabata, K.; Yoshii, K.; Kariwa, H. Development of a Highly Specific Serodiagnostic ELISA for West Nile Virus Infection Using Subviral Particles. Sci. Rep. 2021, 11, 9213. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S. A Short History, Principles, and Types of ELISA, and Our Laboratory Experience with Peptide/Protein Analyses Using ELISA. Peptides 2015, 72, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Moher, D. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Moola, S.; Lisy, K.; Riitano, D.; Tufanaru, C. Methodological Guidance for Systematic Reviews of Observational Epidemiological Studies Reporting Prevalence and Cumulative Incidence Data. Int. J. Evid.-Based Healthc. 2015, 13, 147–153. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 1 February 2023).
- World Health Organization. Guidance on Conducting Serosurveys in Support of Measles and Rubella Elimination in the WHO European Region; WHO: Geneva, Switzerland, 2013; p. 25. [Google Scholar]
- Guzman, M.G.; Gubler, D.J.; Izquierdo, A.; Martinez, E.; Halstead, S.B. Dengue Infection. Nat. Rev. Dis. Primers 2016, 2, 16055. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention Dengue. Areas of Risk Around the World. Available online: https://www.cdc.gov/dengue/areaswithrisk/around-the-world.html (accessed on 11 February 2023).
- Gwee, X.W.S.; Chua, P.E.Y.; Pang, J. Global Dengue Importation: A Systematic Review. BMC Infect. Dis. 2021, 21, 1078. [Google Scholar] [CrossRef]
- Gainor, E.M.; Harris, E.; LaBeaud, A.D. Uncovering the Burden of Dengue in Africa: Considerations on Magnitude, Misdiagnosis, and Ancestry. Viruses 2022, 14, 233. [Google Scholar] [CrossRef]
- Guo, Z.; Jing, W.; Liu, J.; Liu, M. The Global Trends and Regional Differences in Incidence of Zika Virus Infection and Implications for Zika Virus Infection Prevention. PLOS Negl. Trop. Dis. 2022, 16, e0010812. [Google Scholar] [CrossRef]
- Gubler, D.J.; Vasilakis, N.; Musso, D. History and Emergence of Zika Virus. J. Infect. Dis. 2017, 216, S860–S867. [Google Scholar] [CrossRef]
- Sejvar, J.J. West Nile Virus: An Historical Overview. Ochsner J. 2003, 5, 6–10. [Google Scholar]
- Young, J.J.; Haussig, J.M.; Aberle, S.W.; Pervanidou, D.; Riccardo, F.; Sekulić, N.; Bakonyi, T.; Gossner, C.M. Epidemiology of Human West Nile Virus Infections in the European Union and European Union Enlargement Countries, 2010 to 2018. Eurosurveillance 2021, 26, 2001095. [Google Scholar] [CrossRef] [PubMed]
- Ronca, S.E.; Ruff, J.C.; Murray, K.O. A 20-Year Historical Review of West Nile Virus since Its Initial Emergence in North America: Has West Nile Virus Become a Neglected Tropical Disease? PLoS Negl. Trop. Dis. 2021, 15, e0009190. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. West Nile Virus. Available online: https://www.who.int/news-room/fact-sheets/detail/west-nile-virus (accessed on 11 February 2023).
- Mencattelli, G.; Ndione, M.H.D.; Rosà, R.; Marini, G.; Diagne, C.T.; Diagne, M.M.; Fall, G.; Faye, O.; Diallo, M.; Faye, O.; et al. Epidemiology of West Nile Virus in Africa: An Underestimated Threat. PLoS Negl. Trop. Dis. 2022, 16, e0010075. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.L.; Hills, S.L.; Fischer, M.; Jacobson, J.A.; Hoke, C.H.; Hombach, J.M.; Marfin, A.A.; Solomon, T.; Tsai, T.F.; Tsu, V.D.; et al. Estimated Global Incidence of Japanese Encephalitis: A Systematic Review. Bull. World Health Organ. 2011, 89, 766–774E. [Google Scholar] [CrossRef]
- Vannice, K.S.; Hills, S.L.; Schwartz, L.M.; Barrett, A.D.; Heffelfinger, J.; Hombach, J.; Letson, G.W.; Solomon, T.; Marfin, A.A. The Future of Japanese Encephalitis Vaccination: Expert Recommendations for Achieving and Maintaining Optimal JE Control. NPJ Vaccines 2021, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-H.; Tsai, Y.-T.; Wang, S.-F.; Wang, W.-H.; Chen, Y.-H. Dengue Vaccine: An Update. Expert. Rev. Anti Infect. Ther. 2021, 19, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ling, L.; Zhang, Z.; Marin-Lopez, A. Current Advances in Zika Vaccine Development. Vaccines 2022, 10, 1816. [Google Scholar] [CrossRef]
- Konishi, E.; Kitai, Y. Detection by ELISA of Antibodies to Japanese Encephalitis Virus Nonstructural 1 Protein Induced in Subclinically Infected Humans. Vaccine 2009, 27, 7053–7058. [Google Scholar] [CrossRef]
- Spickler, A.R. Japanese Encephalitis. Available online: http://www.cfsph.iastate.edu/DiseaseInfo/factsheets.php (accessed on 7 March 2023).
- Ler, T.S.; Ang, L.W.; Yap, G.S.L.; Ng, L.C.; Tai, J.C.; James, L.; Goh, K.T. Epidemiological Characteristics of the 2005 and 2007 Dengue Epidemics in Singapore—Similarities and Distinctions. Western Pac. Surveill Response J. 2011, 2, 24–29. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Regional Office for South-East Asia Dengue Bulletin; WHO Regional Office for South-East Asia: New Delhi, India, 2007; Volume 31. [Google Scholar]
- Wang, S.-F.; Chang, K.; Loh, E.-W.; Wang, W.-H.; Tseng, S.-P.; Lu, P.-L.; Chen, Y.-H.; Chen, Y.-M.A. Consecutive Large Dengue Outbreaks in Taiwan in 2014–2015. Emerg. Microbes Infect. 2016, 5, e123. [Google Scholar] [CrossRef]
- Saita, S.; Maeakhian, S.; Silawan, T. Temporal Variations and Spatial Clusters of Dengue in Thailand: Longitudinal Study before and during the Coronavirus Disease (COVID-19) Pandemic. Trop. Med. Infect. Dis. 2022, 7, 171. [Google Scholar] [CrossRef] [PubMed]
- Mutheneni, S.R.; Morse, A.P.; Caminade, C.; Upadhyayula, S.M. Dengue Burden in India: Recent Trends and Importance of Climatic Parameters. Emerg. Microbes Infect. 2017, 6, 1–10. [Google Scholar] [CrossRef]
- Amaya-Larios, I.Y.; Martínez-Vega, R.A.; Diaz-Quijano, F.A.; Sarti, E.; Puentes-Rosas, E.; Chihu, L.; Ramos-Castañeda, J. Risk of Dengue Virus Infection According to Serostatus in Individuals from Dengue Endemic Areas of Mexico. Sci. Rep. 2020, 10, 19017. [Google Scholar] [CrossRef] [PubMed]
- Dzul-Manzanilla, F.; Correa-Morales, F.; Che-Mendoza, A.; Palacio-Vargas, J.; Sánchez-Tejeda, G.; González-Roldan, J.F.; López-Gatell, H.; Flores-Suárez, A.E.; Gómez-Dantes, H.; Coelho, G.E.; et al. Identifying Urban Hotspots of Dengue, Chikungunya, and Zika Transmission in Mexico to Support Risk Stratification Efforts: A Spatial Analysis. Lancet Planet. Health 2021, 5, e277–e285. [Google Scholar] [CrossRef]
- Moore, S.M. The Current Burden of Japanese Encephalitis and the Estimated Impacts of Vaccination: Combining Estimates of the Spatial Distribution and Transmission Intensity of a Zoonotic Pathogen. PLoS Negl. Trop. Dis. 2021, 15, e0009385. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Historical Data by Year—West Nile Virus Seasonal Surveillance. Available online: https://www.ecdc.europa.eu/en/west-nile-fever/surveillance-and-disease-data/historical (accessed on 11 February 2023).
- Danis, K.; Papa, A.; Theocharopoulos, G.; Dougas, G.; Athanasiou, M.; Detsis, M.; Baka, A.; Lytras, T.; Mellou, K.; Bonovas, S.; et al. Outbreak of West Nile Virus Infection in Greece, 2010. Emerg. Infect. Dis. 2011, 17, 1868–1872. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Dengue Guidelines, for Diagnosis, Treatment, Prevention and Control; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Panbio Diagnostics. DENGUE IgG INDIRECT ELISA. Available online: http://www.rootbio.com/admin/download/2009216131816609.pdf (accessed on 17 February 2023).
- Euroimmun. Anti-Zika Virus ELISA (IgG). Available online: https://www.euroimmun.com/documents/Indications/Infections/Zika-virus/EI_2668_D_UK_A.pdf (accessed on 17 February 2023).
- Medialdea-Carrera, R.; Levy, F.; Castanha, P.; Carvalho de Sequeira, P.; Brasil, P.; Lewis-Ximenez, L.L.; Turtle, L.; Solomon, T.; Bispo de Filippis, A.M.; Brown, D.W.; et al. A Systematic Evaluation of IgM and IgG Antibody Assay Accuracy in Diagnosing Acute Zika Virus Infection in Brazil: Lessons Relevant to Emerging Infections. J. Clin. Microbiol. 2021, 59, e0289320. [Google Scholar] [CrossRef]
- Low, S.L.; Leo, Y.S.; Lai, Y.L.; Lam, S.; Tan, H.H.; Wong, J.C.C.; Tan, L.K.; Ng, L.C. Evaluation of Eight Commercial Zika Virus IgM and IgG Serology Assays for Diagnostics and Research. PLoS ONE 2021, 16, e0244601. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. 510(k) Summary of Safety and Effectiveness: West Nile Virus ELISA IgG Catalog No. EL0300G. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf3/K031953.pdf (accessed on 17 February 2023).
- Gómez-Vicente, E.; Garcia, R.; Calatrava, E.; Olivares Duran, M.J.; Gutiérrez-Bautista, J.F.; Rodriguez-Granger, J.; Cobo, F.; Navarro Mari, J.M.; Sampedro-Martinez, A. Comparative Evaluation of Chemiluminescent Immunoassay and Enzyme-Linked Immunosorbent Assays for the Diagnosis of West Nile Virus Infections. APMIS 2022, 130, 215–220. [Google Scholar] [CrossRef]
- Amin, M.; Zaim, M.; Edalat, H.; Basseri, H.R.; Yaghoobi-Ershadi, M.R.; Rezaei, F.; Azizi, K.; Salehi-Vaziri, M.; Ghane, M.; Yousefi, S.; et al. Seroprevalence Study on West Nile Virus (WNV) Infection, a Hidden Viral Disease in Fars Province, Southern Iran. J. Arthropod. Borne Dis. 2020, 14, 173–184. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Diagnostic Testing|Japanese Encephalitis|CDC. Available online: https://www.cdc.gov/japaneseencephalitis/healthcareproviders/healthcareproviders-diagnostic.html (accessed on 17 February 2023).
- Schroeder, H.W.; Cavacini, L. Structure and Function of Immunoglobulins. J. Allergy Clin. Immunol. 2010, 125, S41–S52. [Google Scholar] [CrossRef]
- Sathe, A.; Cusick, J.K. Biochemistry, Immunoglobulin, M. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Chao, D.-Y.; Galula, J.U.; Shen, W.-F.; Davis, B.S.; Chang, G.-J.J. Nonstructural Protein 1-Specific Immunoglobulin M and G Antibody Capture Enzyme-Linked Immunosorbent Assays in Diagnosis of Flaviviral Infections in Humans. J. Clin. Microbiol. 2015, 53, 557–566. [Google Scholar] [CrossRef]
- Cuzzubbo, A.J.; Vaughn, D.W.; Nisalak, A.; Solomon, T.; Kalayanarooj, S.; Aaskov, J.; Dung, N.M.; Devine, P.L. Comparison of PanBio Dengue Duo Enzyme-Linked Immunosorbent Assay (ELISA) and MRL Dengue Fever Virus Immunoglobulin M Capture ELISA for Diagnosis of Dengue Virus Infections in Southeast Asia. Clin. Diagn. Lab. Immunol. 1999, 6, 705–712. [Google Scholar] [CrossRef]
- Furuya, A.K.M.; Hunt, D.; George, K.S.; Dupuis, A.P.; Kramer, L.D.; Shi, P.-Y.; Wong, S. Use of the Immunoglobulin G Avidity Assay to Differentiate between Recent Zika and Past Dengue Virus Infections. Clin. Sci. 2019, 133, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Roehrig, J.T. Antigenic Structure of Flavivirus Proteins. In Advances in Virus Research; Elsevier: Amsterdam, The Netherlands, 2003; Volume 59, pp. 141–175. ISBN 978-0-12-039859-1. [Google Scholar]
- Lindenbach, B.D.; Rice, C.M. Molecular Biology of Flaviviruses. In Advances in Virus Research; Elsevier: Amsterdam, The Netherlands, 2003; Volume 59, pp. 23–61. ISBN 978-0-12-039859-1. [Google Scholar]
- Endale, A.; Medhin, G.; Darfiro, K.; Kebede, N.; Legesse, M. Magnitude of Antibody Cross-Reactivity in Medically Important Mosquito-Borne Flaviviruses: A Systematic Review. IDR 2021, 14, 4291–4299. [Google Scholar] [CrossRef]
- Hou, B.; Chen, H.; Gao, N.; An, J. Cross-Reactive Immunity among Five Medically Important Mosquito-Borne Flaviviruses Related to Human Diseases. Viruses 2022, 14, 1213. [Google Scholar] [CrossRef]
- Muller, D.A.; Young, P.R. The Flavivirus NS1 Protein: Molecular and Structural Biology, Immunology, Role in Pathogenesis and Application as a Diagnostic Biomarker. Antivir. Res. 2013, 98, 192–208. [Google Scholar] [CrossRef]
- Rothan, H.A.; Bidokhti, M.R.; Byrareddy, S.N. Current Concerns and Perspectives on Zika Virus Co-Infection with Arboviruses and HIV. J. Autoimmun. 2018, 89, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Wang, X.; Yang, L.; Lv, J.; Meng, R.; Dai, W.; Li, Q.; Liu, H.; Zhang, B.; Zhang, D. Identification of a Neutralizing Monoclonal Antibody That Recognizes a Unique Epitope on Domain III of the Envelope Protein of Tembusu Virus. Viruses 2020, 12, 647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jia, R.; Shen, H.; Wang, M.; Yin, Z.; Cheng, A. Structures and Functions of the Envelope Glycoprotein in Flavivirus Infections. Viruses 2017, 9, 338. [Google Scholar] [CrossRef]
- Groen, J.; Koraka, P.; Velzing, J.; Copra, C.; Osterhaus, A.D.M.E. Evaluation of Six Immunoassays for Detection of Dengue Virus-Specific Immunoglobulin M and G Antibodies. Clin. Diagn. Lab. Immunol. 2000, 7, 867–871. [Google Scholar] [CrossRef] [PubMed]
| Category | Frequency | Percentage |
|---|---|---|
| Virus | ||
| DENV | 114 | 55.88% |
| JEV | 6 | 2.94% |
| WNV | 39 | 19.12% |
| ZIKV | 23 | 11.27% |
| Multiple viruses | 22 | 10.78% |
| DENV and JEV | 1 | 0.49% |
| DENV and WNV | 9 | 4.41% |
| DENV and ZIKV | 10 | 4.90% |
| DENV, WNV, and ZIKV | 1 | 0.49% |
| DENV, JEV, WNV, and ZIKV | 1 | 0.49% |
| ELISA Type | ||
| Commercial | 156 | 76.47% |
| In-house | 45 | 22.06% |
| Commercial and in-house | 3 | 1.47% |
| ELISA Format | ||
| Indirect | 143 | 70.10% |
| Antibody capture | 16 | 7.84% |
| Antigen capture | 15 | 7.35% |
| Others | 6 | 2.94% |
| Unspecified | 10 | 4.90% |
| Multiple formats | 14 | 6.86% |
| Indirect and antibody capture | 6 | 2.94% |
| Indirect and antigen capture | 1 | 0.49% |
| Indirect and others | 3 | 1.47% |
| Indirect and unspecified | 4 | 1.96% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vista, F.E.S.; Tantengco, O.A.G.; Dispo, M.D.; Opiso, D.M.S.; Badua, C.L.D.C.; Gerardo, J.P.Z.; Perez, J.R.M.; Baldo, K.A.T.; Chao, D.-Y.; Dalmacio, L.M.M. Trends in ELISA-Based Flavivirus IgG Serosurveys: A Systematic Review. Trop. Med. Infect. Dis. 2023, 8, 224. https://doi.org/10.3390/tropicalmed8040224
Vista FES, Tantengco OAG, Dispo MD, Opiso DMS, Badua CLDC, Gerardo JPZ, Perez JRM, Baldo KAT, Chao D-Y, Dalmacio LMM. Trends in ELISA-Based Flavivirus IgG Serosurveys: A Systematic Review. Tropical Medicine and Infectious Disease. 2023; 8(4):224. https://doi.org/10.3390/tropicalmed8040224
Chicago/Turabian StyleVista, Fatima Ericka S., Ourlad Alzeus G. Tantengco, Micah D. Dispo, Danna Mae S. Opiso, Christian Luke D. C. Badua, John Patrick Z. Gerardo, Juan Raphael M. Perez, Karol Ann T. Baldo, Day-Yu Chao, and Leslie Michelle M. Dalmacio. 2023. "Trends in ELISA-Based Flavivirus IgG Serosurveys: A Systematic Review" Tropical Medicine and Infectious Disease 8, no. 4: 224. https://doi.org/10.3390/tropicalmed8040224
APA StyleVista, F. E. S., Tantengco, O. A. G., Dispo, M. D., Opiso, D. M. S., Badua, C. L. D. C., Gerardo, J. P. Z., Perez, J. R. M., Baldo, K. A. T., Chao, D.-Y., & Dalmacio, L. M. M. (2023). Trends in ELISA-Based Flavivirus IgG Serosurveys: A Systematic Review. Tropical Medicine and Infectious Disease, 8(4), 224. https://doi.org/10.3390/tropicalmed8040224











