Development and Utility of Practical Indicators of Critical Outcomes in Dengue Patients Presenting to Hospital: A Retrospective Cross-Sectional Study
Abstract
1. Introduction
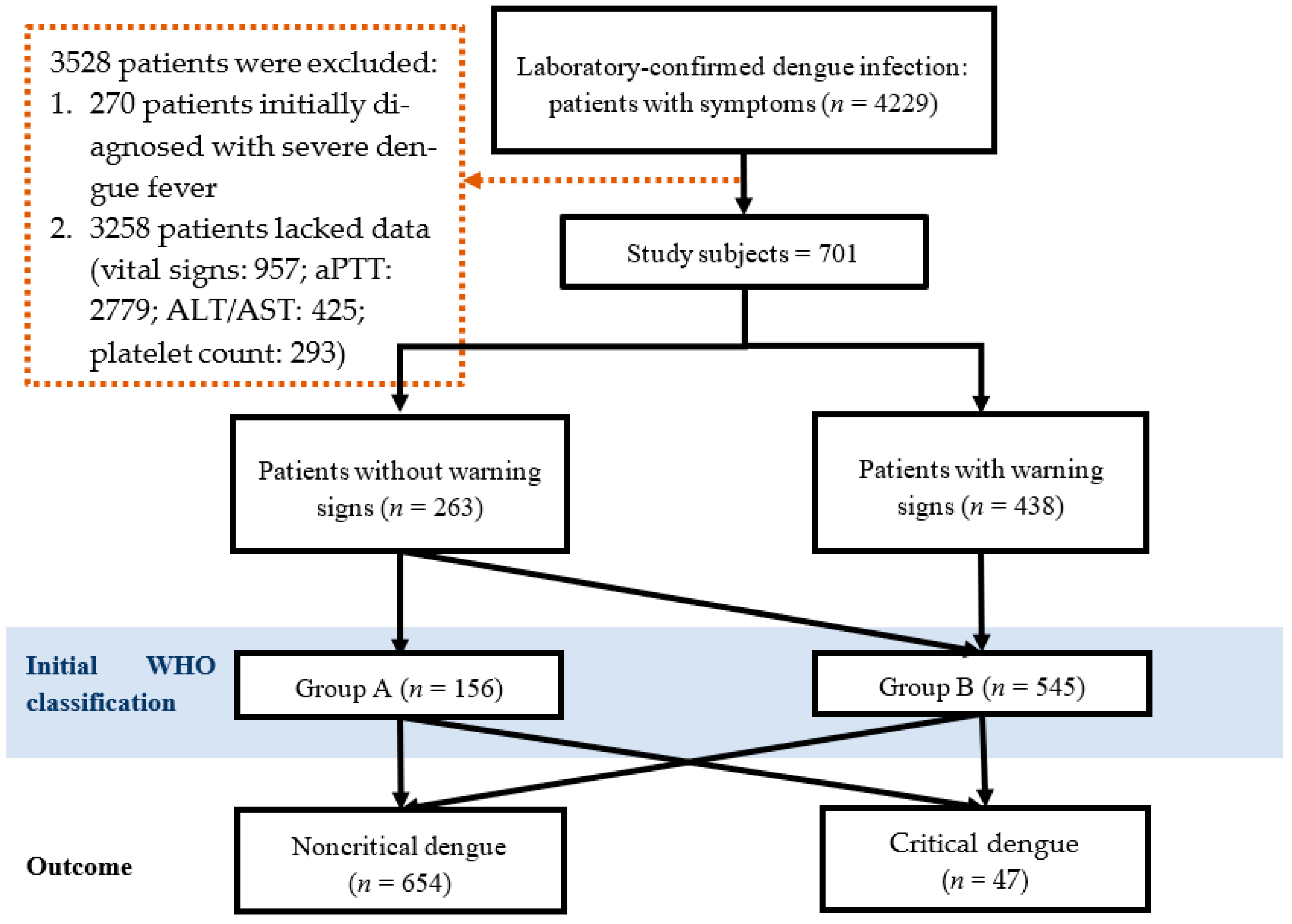
2. Materials and Methods
2.1. Study Design and Setting
2.2. Definition of Cases and Variables
2.3. Model Analysis for the Dengue Severity Score
2.4. Data Analysis
2.5. Ethics Statement
3. Results
3.1. Major Parameters for Predicting Critical Outcomes in Dengue Patients
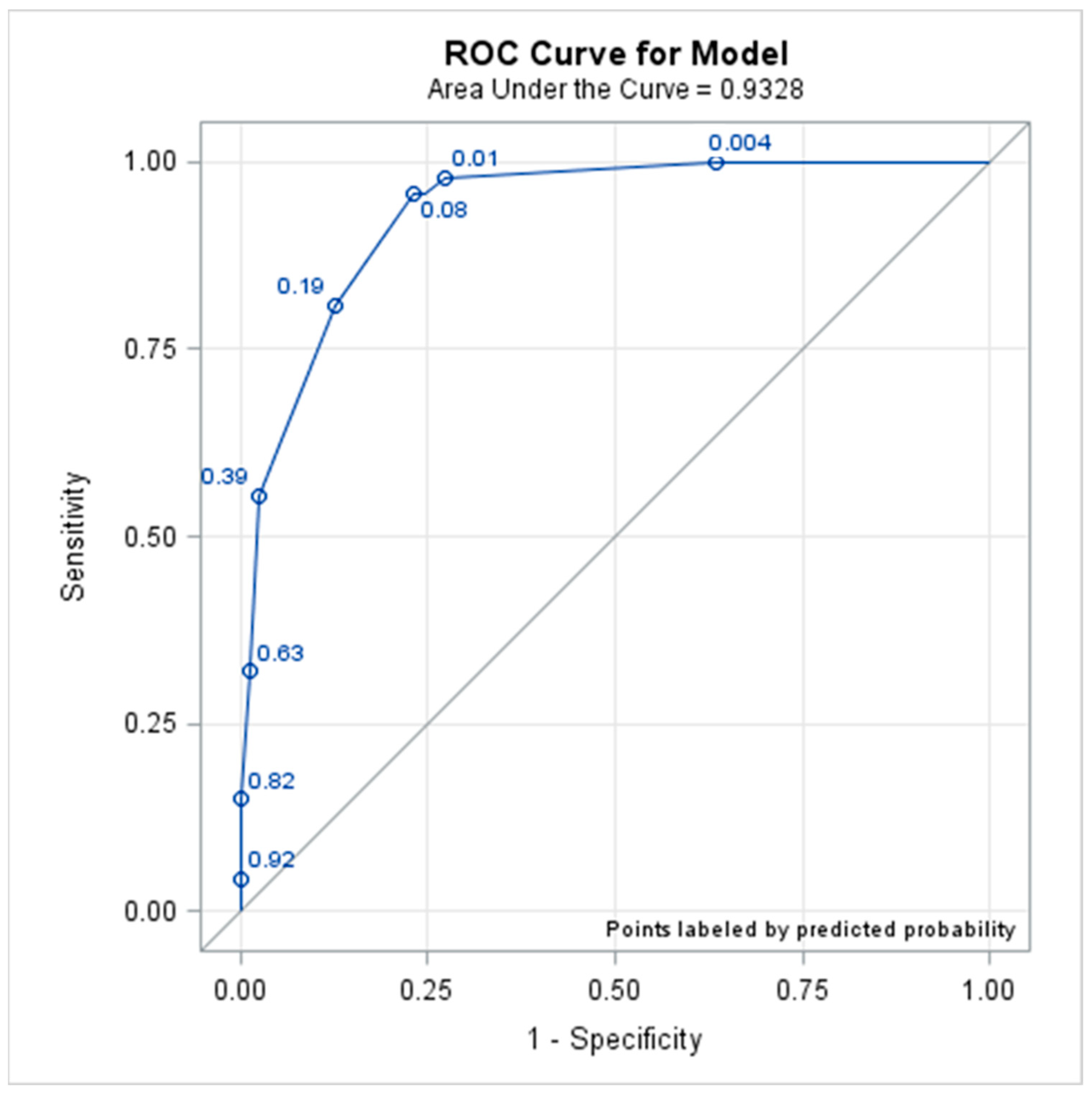
3.2. Utility Evaluation of the Indicators from the External Data
4. Discussion Estimation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brady, O.J.; Gething, P.W.; Bhatt, S.; Messina, J.P.; Brownstein, J.S.; Hoen, A.G.; Moyes, C.L.; Farlow, A.W.; Scott, T.W.; Hay, S.I. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl. Trop. Dis. 2012, 6, e1760. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Halstead, S.B.; Artsob, H.; Buchy, P.; Farrar, J.; Gubler, D.J.; Hunsperger, E.; Kroeger, A.; Margolis, H.S.; Martinez, E.; et al. Dengue: A continuing global threat. Nat. Rev. Microbiol. 2010, 8, S7–S16. [Google Scholar] [CrossRef]
- Lee, M.S.; Hwang, K.P.; Chen, T.C.; Lu, P.L.; Chen, T.P. Clinical characteristics of dengue and dengue hemorrhagic fever in a medical center of southern Taiwan during the 2002 epidemic. J. Microbiol. Immunol. Infect. 2006, 39, 121–129. [Google Scholar] [PubMed]
- CDC Annual Report 2016. Available online: https://www.cdc.gov.tw/InfectionReport/Info/MBykt7DHDDcSbrcfxoLRoQ?infoId=HCzh6FgAgovrYiZVrpJrbw (accessed on 15 August 2020).
- Hsu, J.C.; Hsieh, C.L.; Lu, C.Y. Trend and geographic analysis of the prevalence of dengue in Taiwan, 2010-2015. Int. J. Infect. Dis. 2017, 54, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Mohd-Zaki, A.H.; Brett, J.; Ismail, E.; L’Azou, M. Epidemiology of dengue disease in Malaysia (2000–2012): A systematic literature review. PLoS Negl. Trop. Dis. 2014, 8, e3159. [Google Scholar] [CrossRef]
- Bravo, L.; Roque, V.G.; Brett, J.; Dizon, R.; L’Azou, M. Epidemiology of dengue disease in the Philippines (2000–2011): A systematic literature review. PLoS Negl. Trop. Dis. 2014, 8, e3027. [Google Scholar] [CrossRef]
- Anders, K.L.; Nguyet, N.M.; Chau, N.V.; Hung, N.T.; Thuy, T.T.; Lien Le, B.; Farrar, J.; Wills, B.; Hien, T.T.; Simmons, C.P. Epidemiological factors associated with dengue shock syndrome and mortality in hospitalized dengue patients in Ho Chi Minh City, Vietnam. Am. J. Trop. Med. Hyg. 2011, 84, 127–134. [Google Scholar] [CrossRef]
- Rigau-Perez, J.G.; Clark, G.G.; Gubler, D.J.; Reiter, P.; Sanders, E.J.; Vorndam, A.V. Dengue and dengue haemorrhagic fever. Lancet 1998, 352, 971–977. [Google Scholar] [CrossRef]
- World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23762963 (accessed on 15 August 2020).
- Pang, J.; Thein, T.L.; Leo, Y.S.; Lye, D.C. Early clinical and laboratory risk factors of intensive care unit requirement during 2004-2008 dengue epidemics in Singapore: A matched case-control study. BMC Infect. Dis. 2014, 14, 649. [Google Scholar] [CrossRef]
- Low, J.G.; Ong, A.; Tan, L.K.; Chaterji, S.; Chow, A.; Lim, W.Y.; Lee, K.W.; Chua, R.; Chua, C.R.; Tan, S.W.; et al. The early clinical features of dengue in adults: Challenges for early clinical diagnosis. PLoS Negl. Trop. Dis. 2011, 5, e1191. [Google Scholar] [CrossRef]
- Pang, J.; Leo, Y.S.; Lye, D.C. Critical care for dengue in adult patients: An overview of current knowledge and future challenges. Curr. Opin. Crit. Care 2016, 22, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.M.; Khoo, E.M.; Ho, B.K.; Lee, Y.K.; Omar, M.; Ayadurai, V.; Mohamed Yusoff, F.; Suli, Z.; Mudin, R.N.; Goh, P.P.; et al. Dengue in Malaysia: Factors Associated with Dengue Mortality from a National Registry. PLoS ONE 2016, 11, e0157631. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.C.; Castro, D.B.; Albuquerque, B.C.; Sampaio Vde, S.; Passos, R.A.; Costa, C.F.; Sadahiro, M.; Braga, J.U. Mortality Predictors in Patients with Severe Dengue in the State of Amazonas, Brazil. PLoS ONE 2016, 11, e0161884. [Google Scholar] [CrossRef] [PubMed]
- Thein, T.L.; Leo, Y.S.; Fisher, D.A.; Low, J.G.; Oh, H.M.; Gan, V.C.; Wong, J.G.; Lye, D.C. Risk factors for fatality among confirmed adult dengue inpatients in Singapore: A matched case-control study. PLoS ONE 2013, 8, e81060. [Google Scholar] [CrossRef]
- Gubler, D.J. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 1998, 11, 480–496. [Google Scholar] [CrossRef]
- Guzman, M.G.; Kouri, G. Dengue: An update. Lancet. Infect. Dis. 2002, 2, 33–42. [Google Scholar] [CrossRef]
- Chien, Y.W.; Wang, C.C.; Wang, Y.P.; Lee, C.Y.; Perng, G.C. Risk of Leukemia after Dengue Virus Infection: A Population-Based Cohort Study. Cancer Epidemiol. Biomark. Prev. 2020, 29, 558–564. [Google Scholar] [CrossRef]
- Yeh, C.Y.; Chen, P.L.; Chuang, K.T.; Shu, Y.C.; Chien, Y.W.; Perng, G.C.; Ko, W.C.; Ko, N.Y. Symptoms associated with adverse dengue fever prognoses at the time of reporting in the 2015 dengue outbreak in Taiwan. PLoS Negl. Trop. Dis. 2017, 11, e0006091. [Google Scholar] [CrossRef]
- Diagnostics, S. SD BIOLINE Dengue Duo. Available online: https://www.globalpointofcare.abbott/en/product-details/sd-bioline-dengue-duo-ns1-ag---ab-combo.html (accessed on 20 July 2016).
- Hsieh, C.C.; Cia, C.T.; Lee, J.C.; Sung, J.M.; Lee, N.Y.; Chen, P.L.; Kuo, T.H.; Chao, J.Y.; Ko, W.C. A Cohort Study of Adult Patients with Severe Dengue in Taiwanese Intensive Care Units: The Elderly and APTT Prolongation Matter for Prognosis. PLoS Negl. Trop. Dis. 2017, 11, e0005270. [Google Scholar] [CrossRef]
- Pongpan, S.; Wisitwong, A.; Tawichasri, C.; Patumanond, J.; Namwongprom, S. Development of dengue infection severity score. ISRN Pediatr. 2013, 2013, 845876. [Google Scholar] [CrossRef]
- Vijayaraghavan; Thay Wee, Y.; Foong Shing, W.; Hafsa, P. Predictors of Dengue Shock Syndrome: APTT Elevation as a Risk Factor in Children with Dengue Fever. J. Infect. Dis. Epidemiol. 2020, 6, 111. [Google Scholar] [CrossRef]
- Hamsa, B.T.; Srinivasa, S.V.; Prabhakar, K.; Raveesha, A.; Manoj, A.G. Significance of APTT as early predictor of bleeding in comparison to thrombocytopenia in dengue virus infection. Int. J. Res. Med. Sci. 2019, 7, 67–70. [Google Scholar] [CrossRef]
- Kuo, H.J.; Lee, I.K.; Liu, J.W. Analyses of clinical and laboratory characteristics of dengue adults at their hospital presentations based on the World Health Organization clinical-phase framework: Emphasizing risk of severe dengue in the elderly. J. Microbiol. Immunol. Infect. 2018, 51, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Werneck, G.L.; Macias, A.E.; Mascarenas, C.; Coudeville, L.; Morley, D.; Recamier, V.; Guergova-Kuras, M.; Puentes-Rosas, E.; Baurin, N.; Toh, M.L. Comorbidities increase in-hospital mortality in dengue patients in Brazil. Mem. Do Inst. Oswaldo Cruz 2018, 113, e180082. [Google Scholar] [CrossRef]
- Sangkaew, S.; Ming, D.; Boonyasiri, A.; Honeyford, K.; Kalayanarooj, S.; Yacoub, S.; Dorigatti, I.; Holmes, A. Risk predictors of progression to severe disease during the febrile phase of dengue: A systematic review and meta-analysis. Lancet. Infect. Dis. 2021, 21, 1014–1026. [Google Scholar] [CrossRef]
- Gönen, M. A Review of: “ROC Curves for Continuous Data, by W. J. Krzanowski and D. J. Hand”. J. Biopharm. Stat. 2010, 20, 485–487. [Google Scholar] [CrossRef]
- Chien, Y.W.; Chuang, H.N.; Wang, Y.P.; Perng, G.C.; Chi, C.Y.; Shih, H.I. Short-term, medium-term, and long-term risks of nonvariceal upper gastrointestinal bleeding after dengue virus infection. PLoS Negl. Trop. Dis. 2022, 16, e0010039. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.W.; Huang, H.M.; Ho, T.C.; Tseng, F.C.; Ko, N.Y.; Ko, W.C.; Perng, G.C. Seroepidemiology of dengue virus infection among adults during the ending phase of a severe dengue epidemic in southern Taiwan, 2015. BMC Infect. Dis. 2019, 19, 338. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.F.; Wong, L.P.; AbuBakar, S. Diagnosis of severe dengue: Challenges, needs and opportunities. J. Infect. Public Health 2020, 13, 193–198. [Google Scholar] [CrossRef]
- Diaz-Quijano, F.A.; Figueiredo, G.M.; Waldman, E.A.; Figueiredo, W.M.; Cardoso, M.R.A.; Campos, S.R.C.; Costa, A.A.; Pannuti, C.S.; Luna, E.J.A. Comparison of clinical tools for dengue diagnosis in a pediatric population-based cohort. Trans. R Soc. Trop. Med. Hyg. 2019, 113, 212–220. [Google Scholar] [CrossRef]
- Wang, S.F.; Chang, K.; Loh, E.W.; Wang, W.H.; Tseng, S.P.; Lu, P.L.; Chen, Y.H.; Chen, Y.A. Consecutive large dengue outbreaks in Taiwan in 2014–2015. Emerg. Microbes. Infect. 2016, 5, e123. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | ED (n = 669) | Outpatient Clinic (n = 32) | p Value |
|---|---|---|---|
| Age (years) | 0.7124 a | ||
| Mean (SD) | 54.1 (19.2) | 55 (19) | |
| Median (Q1, Q3) | 56 (37, 71) | 60 (39.5, 72) | |
| Temperature (°C) | 0.0004 a | ||
| Mean (SD) | 38.3 (1) | 37.6 (1) | |
| Median (Q1, Q3) | 38.4 (37.5, 39) | 37.2 (36.8, 38.3) | |
| Respiration rate (/min) | 0.896 a | ||
| Mean (SD) | 19.7 (3.4) | 19.8 (2.9) | |
| Median (Q1, Q3) | 20 (18, 20) | 20 (18, 20) | |
| Pulse (/min) | 0.0018 a | ||
| Mean (SD) | 97.7 (19.3) | 86.6 (18) | |
| Median (Q1, Q3) | 97 (84, 111) | 86 (71.5, 99.5) | |
| DBP (mmHg) | 0.0006 a | ||
| Mean (SD) | 83 (15.9) | 72.7 (14.9) | |
| Median (Q1, Q3) | 82 (73, 91) | 75 (61.5, 82) | |
| ALT (IU/L) | <0.0001 a | ||
| Mean (SD) | 51.4 (251.6) | 183.7 (384.9) | |
| Median (Q1, Q3) | 21 (12, 42) | 41 (28, 102) | |
| AST (IU/L) | 0.0036 a | ||
| Mean (SD) | 113.7 (609) | 408 (1234.8) | |
| Median (Q1, Q3) | 43 (30, 77) | 82 (41, 141) | |
| Platelet count (103/µL) | 0.0001 a | ||
| Mean (SD) | 141.6 (69.8) | 94.1 (858.3) | |
| Median (Q1, Q3) | 140 (101, 184) | 77 (26, 131.5) | |
| aPTT (sec) | 0.0252 a | ||
| Mean (SD) | 39 (7) | 44.8 (13.8) | |
| Median (Q1, Q3) | 37.9 (34.8, 41.5) | 41.8 (34.4, 52.6) | |
| Advanced age, n (%) | 0.8889 b | ||
| ≥65 years | 222 (33.2) | 11 (34.4) | |
| <65 years | 447 (66.8) | 21 (65.6) | |
| Sex, n (%) | 0.5695 b | ||
| Male | 348 (52.0) | 15 (46.9) | |
| Female | 321 (48.0) | 17 (53.1) | |
| Comorbidities, n (%) | |||
| DM | 83 (12.4) | 6 (18.8) | 0.2788 c |
| CKD/ESRD | 42 (6.4) | 5 (15.6) | 0.0601 c |
| Liver cirrhosis | 4 (0.6) | 0 (0) | 1.0000 c |
| Neoplasm | 50 (7.5) | 3 (9.3) | 0.0005 c |
| COPD | 21 (3.1) | 0 (0) | 0.1944 c |
| WHO classification | 0.0006 b | ||
| Group A | 141 (21.1) | 15 (46.9) | |
| Group B | 528 (78.9) | 17 (53.1) |
| Parameters | Critical Outcomes (n = 47) | Non-Critical Outcomes (n = 654) | OR (95% CI) | p Value |
|---|---|---|---|---|
| Group B * | 45 (95.7) | 500 (76.5) | 76.92 (18.45–322.58) | <0.0001 a |
| Male | 23 (48.9) | 340 (52) | 0.89 (0.49–1.60) | 0.6859 a |
| Temperature ≥ 38.5 °C | 10 (21.3) | 329 (50.3) | 0.27 (0.55–0.13) | 0.0001 a |
| Tachypnea ≥ 20/min | 35 (74.5) | 408 (62.4) | 1.76 (0.90–3.45) | 0.0971 a |
| Tachycardia ≥ 120/min | 6 (12.8) | 85 (13) | 0.98 (0.40–2.38) | 0.9637 a |
| DBP < 60 mmHg | 8 (17) | 23 (3.5) | 5.63 (2.36–13.39) | 0.0005 b |
| Platelet count < 100,000/µL | 29 (61.7) | 154 (23.6) | 5.23 (2.83–9.68) | <0.0001 a |
| aPTT ≥ 50 s | 17 (36.2) | 24 (3.7) | 14.88 (7.23–30.58) | <0.0001 b |
| ALT or AST ≥ 200 U/L | 12 (25.5) | 29 (4.4) | 7.39 (3.5–15.7) | <0.0001 b |
| Characteristic | Odds Ratio * (95% CI) | p | Coefficient † | Score |
|---|---|---|---|---|
| Group B | 60.23 (14.11–257.05) | <0.0001 | 2.05 | 4 |
| Temperature < 38.5 °C | 2.68 (1.18–6.08) | 0.0183 | 0.49 | 1 |
| Lower DBP | 3.14 (1.01–9.76) | 0.0479 | 0.57 | 1 |
| Prolonged aPTT | 5.76 (2.30–14.42) | 0.0002 | 0.88 | 2 |
| Elevated liver enzyme | 3.16 (1.10–9.07) | 0.0327 | 0.57 | 1 |
| Criterion | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | LR+ | LR− |
|---|---|---|---|---|
| ≥1 | 100.00 (92.45–100.00) | 36.39 (32.70–40.21) | 1.57 (1.48–1.67) | — |
| ≥2 | 97.87 (88.71–99.95) | 72.63 (69.04–76.01) | 3.58 (3.13–4.08) | 0.03 (0–0.20) |
| ≥3 | 95.74 (85.46–99.48) | 75.38 (71.89–78.64) | 3.89 (3.36–4.51) | 0.06 (0.01–0.22) |
| ≥4 | 95.74 (85.4–99.48) | 76.76 (73.33–79.95) | 4.12 (3.54–4.79) | 0.06 (0.01–0.22) |
| ≥5 | 80.85 (66.74–90.85) | 87.46 (84.68–89.90) | 6.45 (5.04–8.24) | 0.22 (0.12–0.39) |
| ≥6 | 55.32 (40.12–69.83) | 97.40 (95.87–98.48) | 21.28 (12.47–36.33) | 0.46 (0.33–0.63) |
| ≥7 | 31.91 (19.09–47.12) | 98.62 (97.40–99.37) | 23.19 (10.72–50.17) | 0.69 (0.57–0.84) |
| ≥8 | 14.89 (6.20–28.31) | 99.85 (99.15–100.00) | 97.40 (12.24–775.25) | 0.85 (0.76–0.96) |
| ≥9 | 4.26 (0.52–14.54) | 100.00 (99.44–100.00) | — | 0.96 (0.90–1.02) |
| Score Range | Critical Outcomes (n = 65) | Non-Critical Outcomes (n = 722) | Estimation | ||
|---|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | Accuracy * | |||
| Mean (SD) | 5.2 (1) | 3.1 (1.7) | |||
| Median (Q1, Q3) | 5 (5, 6) | 2 (2, 5) | |||
| Score ≥ 4 | 65 | 222 | 100.00 (94.48–100.00) | 69.25 (65.74–72.60) | 71.79 (68.51–74.91) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, C.-Y.; Sung, T.-C.; Chang, K.; Chien, Y.-W.; Hsu, H.-C.; Tu, Y.-F.; Huang, Y.-T.; Shih, H.-I. Development and Utility of Practical Indicators of Critical Outcomes in Dengue Patients Presenting to Hospital: A Retrospective Cross-Sectional Study. Trop. Med. Infect. Dis. 2023, 8, 188. https://doi.org/10.3390/tropicalmed8040188
Chi C-Y, Sung T-C, Chang K, Chien Y-W, Hsu H-C, Tu Y-F, Huang Y-T, Shih H-I. Development and Utility of Practical Indicators of Critical Outcomes in Dengue Patients Presenting to Hospital: A Retrospective Cross-Sectional Study. Tropical Medicine and Infectious Disease. 2023; 8(4):188. https://doi.org/10.3390/tropicalmed8040188
Chicago/Turabian StyleChi, Chia-Yu, Tzu-Ching Sung, Ko Chang, Yu-Wen Chien, Hsiang-Chin Hsu, Yi-Fang Tu, Yi-Ting Huang, and Hsin-I Shih. 2023. "Development and Utility of Practical Indicators of Critical Outcomes in Dengue Patients Presenting to Hospital: A Retrospective Cross-Sectional Study" Tropical Medicine and Infectious Disease 8, no. 4: 188. https://doi.org/10.3390/tropicalmed8040188
APA StyleChi, C.-Y., Sung, T.-C., Chang, K., Chien, Y.-W., Hsu, H.-C., Tu, Y.-F., Huang, Y.-T., & Shih, H.-I. (2023). Development and Utility of Practical Indicators of Critical Outcomes in Dengue Patients Presenting to Hospital: A Retrospective Cross-Sectional Study. Tropical Medicine and Infectious Disease, 8(4), 188. https://doi.org/10.3390/tropicalmed8040188








