Serum Cortisol as a Biomarker of Severe Dengue
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Population
2.3. Measurement of Serum Cortisol
2.4. Statistical Analysis
3. Results
3.1. Serum Cortisol Level
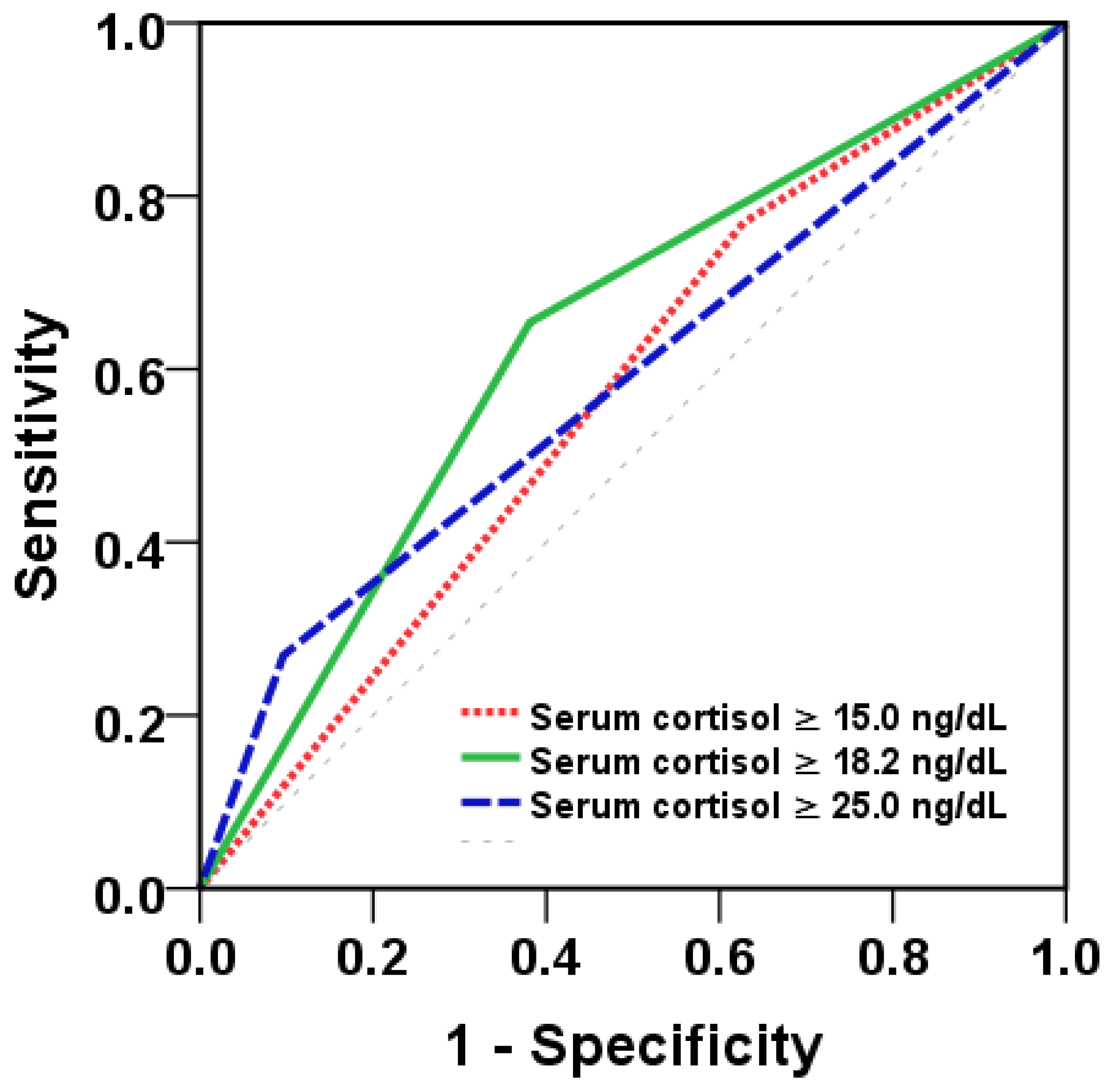
3.2. Serum Cortisol Levels as a Biomarker to Predict Severity of Dengue Infections
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Dengue and Severe Dengue 2021 [updated 19 May 2021]. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 25 September 2021).
- Zhang, F.; Kramer, C.V. Corticosteroids for dengue infection. Cochrane Database Syst. Rev. 2014, 7, Cd003488. [Google Scholar] [CrossRef] [PubMed]
- Thein, T.L.; Gan, V.C.; Lye, D.C.; Yung, C.F.; Leo, Y.S. Utilities and limitations of the World Health Organization 2009 warning signs for adult dengue severity. PLoS Negl. Trop. Dis. 2013, 7, e2023. [Google Scholar] [CrossRef] [PubMed]
- Myo-Khin, M.K.; Soe-Thein, S.T.; Thein-Thein-Myint, T.T.M.; Than-Nu-Swe, T.N.S.; Tin-Tin-Saw, T.T.S.; Muya-Than, M.T. Serum cortisol levels in children with dengue haemorrhagic fever. J. Trop. Pediatr. 1995, 41, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Wacharasindhu, S.; Bunjobpudsa, Y.; Tongmeesee, S.; Aroonparkmongkol, S.; Sahakitrungrueng, T.; Supornsilchai, V. Endocrine changes in children with dengue virus infection. Asian Biomed. 2009, 3, 557–561. [Google Scholar]
- Sura-Amornkul, S.; Pitabut, N.; Pholtawornkulchai, K.; Matangkasombut, P.; Sakuntabhai, A.; Singhasivanon, P. Association between circulating cortisol and ACTH and severity of dengue infection in adult patients. J. Med. Assoc. Thail. 2019, 102, 76–81. [Google Scholar]
- Klungthong, C.; Manasatienkij, W.; Phonpakobsin, T.; Chinnawirotpisan, P.; Rodpradit, P.; Hussem, K.; Thaisomboonsuk, B.; Ong-ajchaowlerd, P.; Nisalak, A.; Kalayanarooj, S.; et al. Monitoring and improving the sensitivity of dengue nested RT-PCR used in longitudinal surveillance in Thailand. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2015, 63, 25–31. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Dengue—Guidelines for Diagnosis, Treatment, Prevention and Control--New Edition; WHO Library Cataloguing-in-Publication Data: Geneva, Switzerland, 2009. [Google Scholar]
- Hillebrand, J.J.; Wickenhagen, W.V.; Heijboer, A.C. Improving Science by Overcoming Laboratory Pitfalls with Hormone Measurements. J. Clin. Endocrinol. Metab. 2021, 106, e1504–e1512. [Google Scholar] [CrossRef] [PubMed]
- Holub, M.; Džupová, O.; Růžková, M.; Stráníková, A.; Bartáková, E.; Máca, J.; Beneš, J.; Herwald, H.; Beran, O. Selected Biomarkers Correlate with the Origin and Severity of Sepsis. Mediat. Inflamm 2018, 2018, 7028267. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiţă, V.; Barbu, A.E.; Gheorghiu, M.L.; Căruntu, F.A. Endocrine dysfunction in sepsis: A beneficial or deleterious host response? Germs 2015, 5, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Phung, N.T.N.; Vo, D.M.; Le, T.N.; Doan, T.T. Total serum cortisol level is low in children with severe dengue shock syndrome. Trop. Biomed. 2021, 38, 396–402. [Google Scholar] [PubMed]
- Pal, R. COVID-19, hypothalamo-pituitary-adrenal axis and clinical implications. Endocrine 2020, 68, 251–252. [Google Scholar] [CrossRef] [PubMed]
- Wheatland, R. Molecular mimicry of ACTH in SARS—Implications for corticosteroid treatment and prophylaxis. Med. Hypotheses 2004, 63, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Russell, G.; Lightman, S. The human stress response. Nat. Rev. Endocrinol. 2019, 15, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.H.; Duval, C.W. Studies upon the etiology of dengue fever: I. experimental transmission to the lower animal. J. Exp. Med. 1924, 40, 817–833. [Google Scholar] [CrossRef] [PubMed]
- John, D.V.; Lin, Y.S.; Perng, G.C. Biomarkers of severe dengue disease—A review. J. Biomed. Sci. 2015, 22, 83. [Google Scholar] [CrossRef] [PubMed]
- Setrkraising, K.; Bongsebandhu-phubhakdi, C.; Voraphani, N.; Pancharoen, C.; Thisyakorn, U.; Thisyakorn, C. D-dimer as an indicator of dengue severity. Asian Biomed. 2007, 1, 53–57. [Google Scholar]
- Bongsebandhu-Phubhakdi, C.; Hemungkom, M.; Thisyakorn, U.; Thisyakom, C. Risk factors influencing severity in pediatric dengue infection. Asian Biomed. 2008, 2, 409–413. [Google Scholar]
- Pare, G.; Neupane, B.; Eskandarian, S.; Harris, E.; Halstead, S.; Gresh, L.; Kuan, G.; Balmaseda, A.; Villar, L.; Rojas, E.; et al. Genetic risk for dengue hemorrhagic fever and dengue fever in multiple ancestries. EBioMedicine 2020, 51, 102584. [Google Scholar] [CrossRef] [PubMed]
- Arayasongsak, U.; Naka, I.; Ohashi, J.; Patarapotikul, J.; Nuchnoi, P.; Kalambaheti, T.; Sa-Ngasang, A.; Chanama, S.; Chaorattanakawee, S. Genetic association study of interferon lambda 3, CD27, and human leukocyte antigen-DPB1 with dengue severity in Thailand. BMC Infect. Dis. 2020, 20, 948. [Google Scholar] [CrossRef] [PubMed]
- Hadinegoro, S.R. The revised WHO dengue case classification: Does the system need to be modified? Paediatr. Int. Child Health 2012, 32 (Suppl. S1), 33–38. [Google Scholar] [CrossRef] [PubMed]

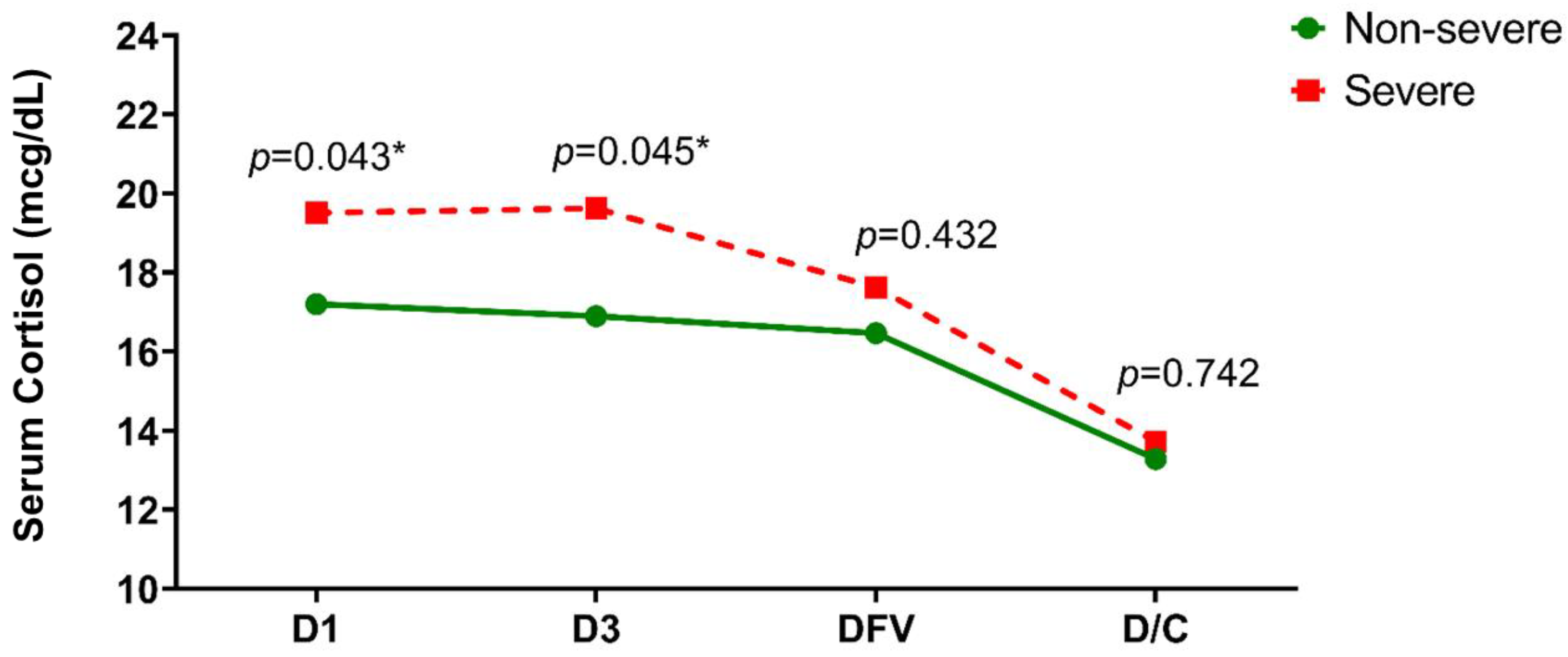
| Total n = 265 (100%) | Non-Severe n = 239 (90.2%) | Severe n = 26 (9.8%) | p-Value | |
|---|---|---|---|---|
| Male, n (%) | 120 (45.3%) | 107 (44.7%) | 13 (50%) | 0.681 |
| Age (years), median (IQR) | 17 (13,27.5) | 18 (14.0,28.0) | 13.50 (10.8,24.0) | 0.061 |
| Day of fever (day), median (IQR) * | 3 (2, 4) | 3 (2, 4) | 3 (1, 4) | 0.015 |
| Pertinent symptoms | ||||
| History of vomiting (>2 times) *, n (%) | 48 (18.1%) | 38 (15.9%) | 10 (38.5%) | 0.012 |
| Abdominal pain, n (%) | 45 (17%) | 40 (16.7%) | 5 (19.2%) | 0.783 |
| Drowsiness, n (%) | 4 (1.5%) | 3 (1.3%) | 1 (3.8%) | 0.340 |
| Mucosal bleeding/GI bleeding, n (%) | 23 (8.7%) | 20 (8.4%) | 3 (11.5%) | 0.481 |
| Physical Examination | ||||
| Body temperature (°C) median (IQR) * | 37.7 (36.9, 38.8) | 37.6 (36.8, 38.7) | 38.7 (37.7, 39.8) | 0.001 |
| Systolic blood pressure (mmHg), median (IQR) | 110 (100, 120) | 110 (100, 120) | 110 (100, 120) | 0.822 |
| Diastolic blood pressure (mmHg), median (IQR) | 70 (60, 70) | 70 (60, 70) | 70 (60, 80) | 0.140 |
| Pulse rate (/min), median (IQR) * | 94 (82, 105) | 92 (80, 104) | 110 (100, 115.50) | <0.0001 |
| Respiratory rate (/min), median (IQR) * | 20 (20, 24) | 20 (20, 24) | 24 (20, 28) | 0.001 |
| Laboratory findings | ||||
| WBC (103/cumm), median (IQR) | 3.20 (2.30, 4.65) | 3.22 (2.30, 4.69) | 3.11 (2.64, 4.45) | 0.298 |
| Neutrophil (%), mean (SD) * | 51.41 (18.40) | 50.25 (18.54) | 61.96 (13.16) | 0.002 |
| Hb (gm%), mean (SD) | 13.33 (1.81) | 13.36 (1.80) | 13.17 (1.95) | 0.627 |
| Hct (%), mean (SD) | 40.16 (5.00) | 40.24 (4.86) | 39.41 (6.15) | 0.420 |
| Platelet count 103/cu.mm, median (IQR) | 96.00 (64.00, 148.75) | 96.00 (64.00–146.25) | 105.00 (63.25, 156.75) | 0.285 |
| NS1 Ag: Positive, n (%) * | 186 (72.9%) | 162 (70.4%) | 24 (96%) | 0.004 |
| IgM: Positive, n (%) * | 148 (55.8%) | 142 (59.4%) | 6 (23.1%) | 0.001 |
| IgG: Positive, n (%) * | 158 (59.6%) | 148 (61.9%) | 10 (38.5%) | 0.034 |
| Cortisol Cutoff (mcg/dL) | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| 15.0 | 0.77 | 0.37 | 0.12 | 0.94 |
| 18.2 | 0.65 | 0.62 | 0.16 | 0.94 |
| 25.0 | 0.27 | 0.90 | 0.23 | 0.92 |
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Demographic profile | ||||||
| Age | 0.97 | 0.93–1.01 | 0.181 | |||
| Gender | 0.82 | 0.36–1.84 | 0.624 | |||
| Fever details | ||||||
| Day of fever | 0.70 | 0.53–0.93 | 0.015 * | 0.82 | 0.59–1.12 | 0.211 |
| Warning signs | ||||||
| Abdominal pain or tenderness | 1.18 | 0.42–3.33 | 0.748 | |||
| Persistent vomiting | 3.31 | 1.40–7.83 | 0.007 * | 3.68 | 1.42–9.56 | 0.008 * |
| Mucosal bleed | 1.43 | 0.39–5.18 | 0.587 | |||
| Lethargy, restlessness | 3.15 | 0.32–31.40 | 0.329 | |||
| Liver enlargement | 0.00 | 0.000 | - | |||
| Lab values on admission | ||||||
| White blood cell | 1.00 | 1.00–1.00 | 0.294 | |||
| Neutrophil | 1.04 | 1.01–1.06 | 0.003 * | 1.08 | 1.00–1.17 | 0.058 |
| Lymphocyte | 0.97 | 0.94–1.00 | 0.029 * | 1.07 | 0.97–1.17 | 0.191 |
| Platelets | 1.00 | 1.00–1.00 | 0.131 | |||
| Hemoglobin | 0.95 | 0.76–1.18 | 0.626 | |||
| Hematocrit | 0.97 | 0.89–1.05 | 0.418 | |||
| Aspartate transaminase (AST) | 1.00 | 1.00–1.00 | 0.102 | |||
| Alanine aminotransferase (ALT) | 1.00 | 1.00–1.01 | 0.422 | |||
| Serum cortisol level (<18.2 vs. ≥18.2 mcg/dL) | 3.13 | 1.34–7.31 | 0.009 * | 2.15 | 0.87–5.34 | 0.098 |
| Variable | AUC | p-Value |
|---|---|---|
| Cortisol | 0.62 | 0.038 * |
| Persistent vomiting | 0.61 | 0.055 |
| Day of fever | 0.36 | 0.017 * |
| Persistent vomiting + Day of fever | 0.69 | 0.010 * |
| Persistent vomiting + Cortisol | 0.71 | 0.001 * |
| Day of fever + Cortisol | 0.67 | 0.005 * |
| Day of fever + Persistent vomiting + Cortisol | 0.74 | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bongsebandhu-phubhakdi, C.; Supornsilchai, V.; Aroonparkmongkol, S.; Limothai, U.; Tachaboon, S.; Dinhuzen, J.; Chaisuriyong, W.; Trongkamolchai, S.; Wanpaisitkul, M.; Chulapornsiri, C.; et al. Serum Cortisol as a Biomarker of Severe Dengue. Trop. Med. Infect. Dis. 2023, 8, 146. https://doi.org/10.3390/tropicalmed8030146
Bongsebandhu-phubhakdi C, Supornsilchai V, Aroonparkmongkol S, Limothai U, Tachaboon S, Dinhuzen J, Chaisuriyong W, Trongkamolchai S, Wanpaisitkul M, Chulapornsiri C, et al. Serum Cortisol as a Biomarker of Severe Dengue. Tropical Medicine and Infectious Disease. 2023; 8(3):146. https://doi.org/10.3390/tropicalmed8030146
Chicago/Turabian StyleBongsebandhu-phubhakdi, Chansuda, Vichit Supornsilchai, Suphab Aroonparkmongkol, Umaporn Limothai, Sasipha Tachaboon, Janejira Dinhuzen, Watchadaporn Chaisuriyong, Supachoke Trongkamolchai, Mananya Wanpaisitkul, Chatchai Chulapornsiri, and et al. 2023. "Serum Cortisol as a Biomarker of Severe Dengue" Tropical Medicine and Infectious Disease 8, no. 3: 146. https://doi.org/10.3390/tropicalmed8030146
APA StyleBongsebandhu-phubhakdi, C., Supornsilchai, V., Aroonparkmongkol, S., Limothai, U., Tachaboon, S., Dinhuzen, J., Chaisuriyong, W., Trongkamolchai, S., Wanpaisitkul, M., Chulapornsiri, C., Tiawilai, A., Tiawilai, T., Tantawichien, T., Thisyakorn, U., & Srisawat, N. (2023). Serum Cortisol as a Biomarker of Severe Dengue. Tropical Medicine and Infectious Disease, 8(3), 146. https://doi.org/10.3390/tropicalmed8030146








