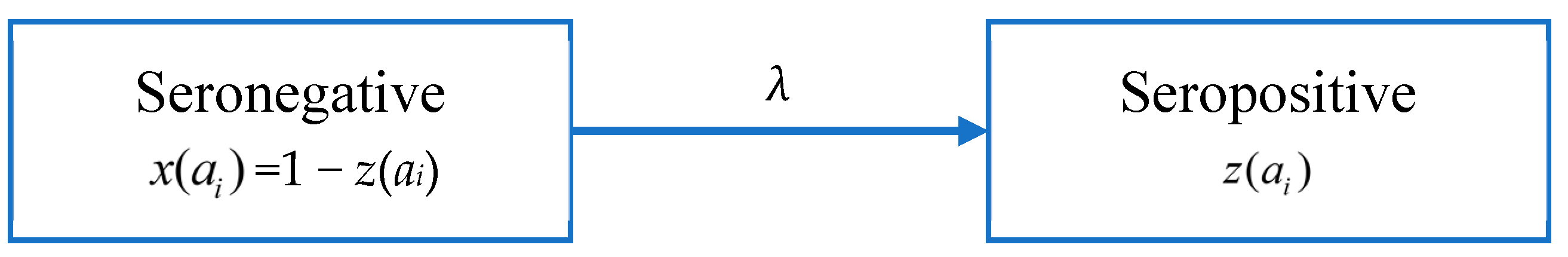Abstract
In recent decades, the global incidence of dengue has risen sharply, with more than 75% of infected people showing mild or no symptoms. Since the year 2000, dengue in China has spread quickly. At this stage, there is an urgent need to fully understand its transmission intensity and spread in China. Serological data provide reliable evidence for symptomatic and recessive infections. Through a literature search, we included 23 studies that collected age-specific serological dengue data released from 1980 to 2021 in China. Fitting four catalytic models to these data, we distinguished the transmission mechanisms by deviation information criterion and estimated force of infection and basic reproduction number (R0), important parameters for quantifying transmission intensity. We found that transmission intensity varies over age in most of the study populations, and attenuation of antibody protection is identified in some study populations; the R0 of dengue in China is between 1.04–2.33. Due to the scarceness of the data, the temporal trend cannot be identified, but data shows that transmission intensity weakened from coastal to inland areas and from southern to northern areas in China if assuming it remained temporally steady during the study period. The results should be useful for the effective control of dengue in China.
1. Introduction
1.1. Current Status of Dengue
Dengue is a mosquito-borne virus infectious disease mainly transmitted through the bites of female Aedes aegypti and Aedes albopictus. Dengue virus (DENV) has four different serotypes (DENV-1, 2, 3 and 4), and both infants and adults are susceptible. The incubation period is generally 4–10 days, and the infection period is 2–7 days [1]. Dengue is one of the 17 neglected tropical diseases in the Neglected Tropical Diseases (NTD) roadmap [2]. In recent decades, the incidence of dengue has risen dramatically. The World Health Organization (WHO) estimates 100 to 400 million cases of infection each year worldwide, and nearly half of the world’s population is at risk of the infection [3]. Moreover, since most cases of dengue manifest as asymptomatic infections (recessive infections), the actual number may exceed the reported number. According to Bhatt et al. [4], about 390 (95% CI: 284, 528) million dengue infections occur each year, of which only about 96 (95% CI: 67, 136) million express clinical symptoms. Dengue has caused a huge burden of disease globally. The year 2019 saw the highest number of dengue cases reported globally in recent memory. Many countries and regions have been affected, with the first recorded transmission of dengue in Afghanistan. The United States alone has reported 3.1 million cases, with more than 25,000 severe cases, while large numbers of cases have also been reported in Bangladesh (101,000), Malaysia (131,000), the Philippines (420,000) and Vietnam (320,000) in Asia [3].
China is in eastern Asia and is adjacent to some Southeast Asia countries that have high dengue incidence. Its southeast coast is a high-incidence area of dengue, especially in Guangdong province [5]. Dengue cases were reported almost every year, and a large outbreak of 45,217 cases occurred in 2014 in China [6]. In addition, in the northern and inland regions, from 2018 to 2020, there were successive outbreaks of dengue in Hebei province [7], Yunnan province [8], Hunan province [9], Hubei province [10], with a total of more than 300 reported cases. China is not the original area of dengue, and most dengue outbreaks in China were caused by imported cases from abroad. Because recessive dengue infections are not detected by symptom screening, it is very likely that a large number of those infections [4] become a source of infection and lead to a significant increase in the positivity rate [11,12,13,14]. The social and economic burdens caused by dengue are getting heavier. In the specific measures to achieve the three major goals of dengue control, the WHO clearly proposed to conduct research on the transmission kinetics of dengue and develop models to quantify the joint method of vaccine and vector control in transmission [15]. In this paper, we used catalytic models based on serological data to estimate dengue transmission intensity and review the changes in dengue transmission in China over the past years.
1.2. Dengue Data and Modelling
In the modeling study of infectious diseases, the important parameters used to characterize transmission intensity are the force of infection (FOI, defined as the instantaneous per capita infection rate at which susceptible individuals acquire infection [16]), and basic reproductive number (R0, defined as the average number of secondary cases caused by an infectious individual entering a susceptible population [17]). The role of FOI has greater significance than the widely used incidence rate because it can distinguish potential age-related changes in infection rates [16]. When an infectious disease first occurs, a patient must infect at least one individual; that is what keeps the epidemic going. However, not every susceptible person who comes into contact with an infected person will be infected, and the probability is determined by FOI. When the susceptible population within a region has got infected and then acquired immunity or died, the proportion of the susceptible population will decline; but due to the supplement of newborn babies and migrant susceptible people from external populations, the proportion of susceptible people may increase again. To characterize the spread potential of an infectious disease under the constantly changing susceptibility, the effective reproduction number, which is defined as a product of basic reproduction number and susceptibility (i.e., ), is an appropriate measure. When a population reaches a steady-state for an infectious disease, its effective reproduction is equal to 1, implying the total number of infections is neither increasing nor decreasing. Therefore, R0 determines not only the growth rate of the infectious disease but also the proportion (1/R0) of the steady-state population infected by the disease [18].
In China, we learned through a literature search that there were few studies using serological data for dengue modeling. Due to the lag in the infectious disease surveillance and reporting system and the differences in regional regulatory systems, there is a problem with using case notification data in that the number of reports does not match the reality. The spread of dengue shows a high degree of geographic heterogeneity [19] and even needs to be measured in a very fine spatial range [20]. Moreover, in many situations, dengue cases show mild or asymptomatic [4] or are only diagnosed as another viral infection in the clinic, which may cause dengue to be misclassified or difficult to diagnose, even the highly sensitive disease surveillance systems may also underestimate the incidence of dengue [21,22]. Therefore, the use of dengue case notification data for research may underestimate dengue transmission intensity.
Using serological data to estimate dengue transmission intensity has great advantages because it can detect past symptomatic and asymptomatic infection cases [19] and more accurately reflect transmission intensity. A literature review of dengue studies in China showed that most studies used IgM and IgG ELISA (Enzyme-Linked Immunosorbent Assay) data. Although PRNT (Plaque Reduction Neutralization Test) and PCR (Polymerase Chain Reaction) can identify different serotypes of dengue virus, their difficulty and cost are relatively high, while ELISA has the advantages of low cost and high efficiency. Imai [19] used IgG and IgM ELISA, IE (Inhibition ELISA) and PRNT data to estimate dengue transmission intensity, and they showed that the FOI estimated by the ELISA data was equal to the sum of the FOI estimated from the specific serological data. In this study, we used non-serotype-specific data to estimate FOI and R0.
2. Materials and Methods
2.1. Literature Search and Data
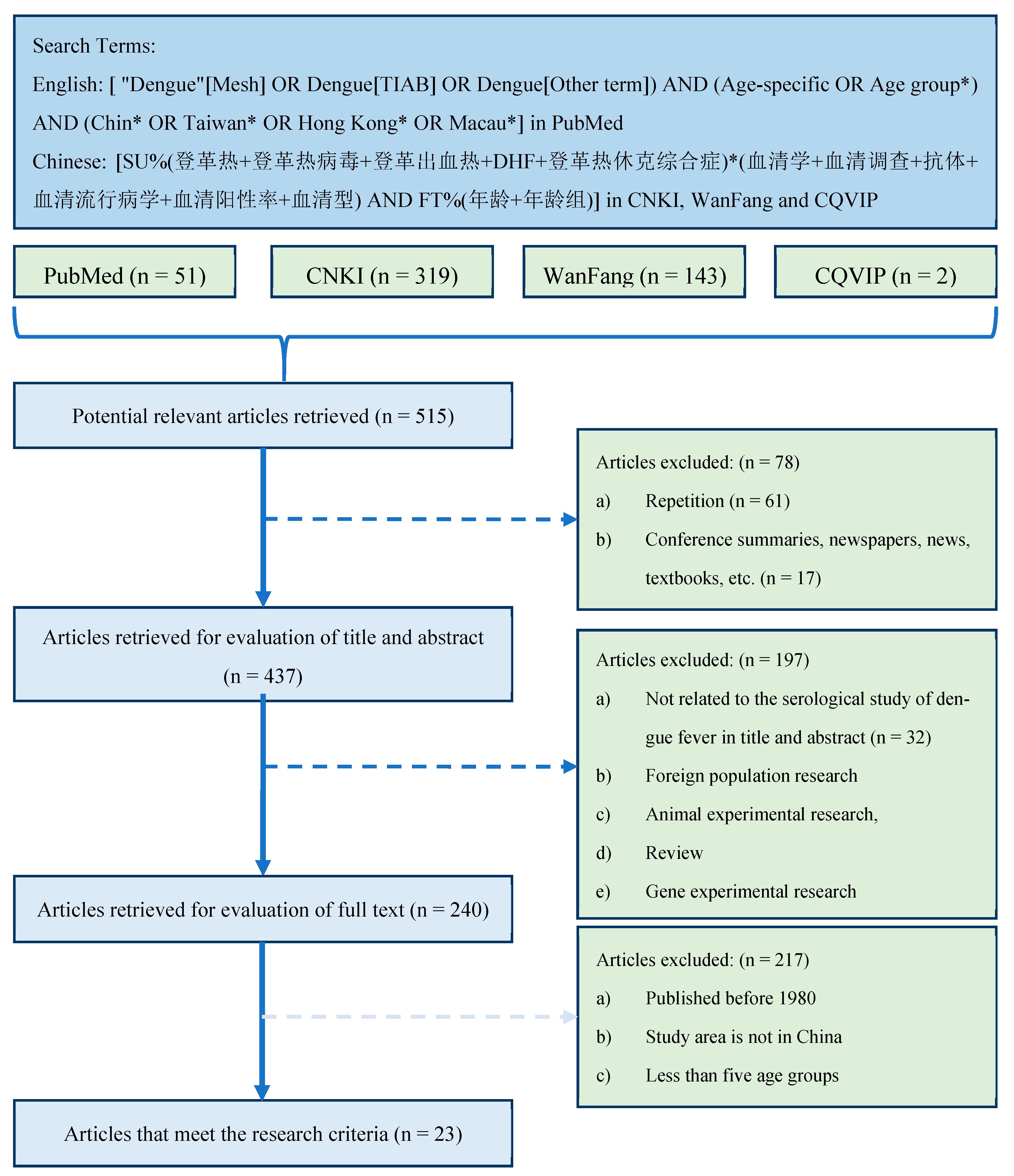
We searched multiple literature databases for potentially available studies related to dengue serology in China. Since we mainly studied the spread of dengue in China in recent years, articles published before 1980 and articles whose study areas are not in China were excluded. Since a wider age group may not accurately reflect the difference in the seropositivity rate of the age group [19], the studies that had at least five age groups were included. Based on these selection criteria (Figure 1), 23 studies [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45] were finally included (Supplementary File S1).
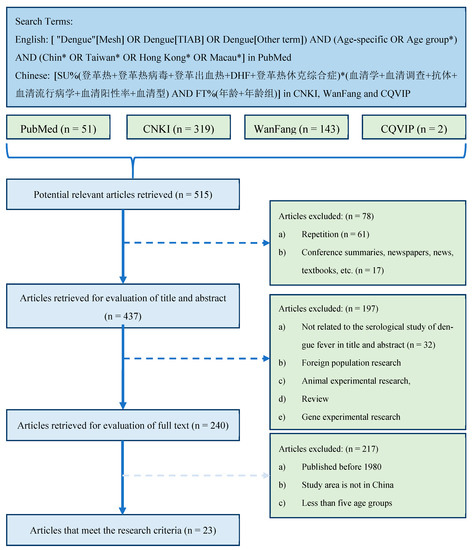
Figure 1.
Literature search and screening process. Notice: (1) In the search query [ "Dengue"[Mesh] OR Dengue[TIAB] OR Dengue[Other term]) AND (Age-specific OR Age group*) AND (Chin* OR Taiwan* OR Hong Kong* OR Macau*], *, an asterisk, is the truncation symbol in PubMed, which was used at the end of a word to search for all terms that begin with that basic word root. (2) The search query [SU%(登革热+登革热病毒+登革出血热+DHF+登革热休克综合症)*(血清学+血清调查+抗体+血清流行病学+血清阳性率+血清型) AND FT%(年龄+年龄组)] in CNKI database can be translated into [SU%(Dengue + Dengue virus + Dengue hemorrhagic fever +DHF+ Dengue shock syndrome)*(Serology + serum survey + antibody + seroepidemiology + Seropositive rate + Serotype) AND FT%(age + age group)], where SU stands for subject, or TITLE, ABSTRACT, and/or KEYWORD; % stands for INCLUDE; + stands for the boolean operator OR; * stands for the boolean operator AND; FT stands for FULL TEXT. (3) The search queries for other two Chinese literature databases are similar to the one used in CNKI database.
The 23 studies involved eight provinces and one region in China, namely Guangdong, Guangxi, Zhejiang, Hunan, Guizhou, Hainan, Yunnan, Taiwan and Hong Kong, with a total of 18 study regions and 31 data sets (Table 1). They are all located south of the Yangtze River in China. Among them, Guangdong, Guangxi, Zhejiang, Hainan, Taiwan and Hong Kong are all coastal provinces, while Hunan, Guizhou and Yunnan are inland provinces. In addition, Yunnan Province and Guangxi Province are adjacent to Southeast Asian countries. Most of the eight provinces or regions are located between 20° and 30° north latitude, with tropical or subtropical seasonal characteristics, high temperature and humid climate.

Table 1.
23 studies for estimation of dengue transmission intensity in China.
Those studies reported the survey data on the age-stratified non-serotype-specific prevalence of dengue from 1980 to 2019. In the last column of Table 1, we use “Herd” to indicate that its sample is from the healthy general population; Use “Hospital/CDC” to indicate that the data was collected at the hospital and/or Centers for Disease Control and Prevention (CDC); “Blood donation center” means that the sample population is healthy blood donors in the blood donation center. “Health Checkup Center” means the sample population is the health checkup population at the port health checkup center. In addition, single-year cross-sectional data from 2011 to 2013 can be extracted from the study [27] and from 2013 to 2015 from the study [45]. The study [43] was based on the phased data collected at different stages of a dengue outbreak in the study area. For the three studies, we fitted our models to the data of each year.
2.2. Dengue Models
Due to the presence of dengue immune antibodies, the seroprevalence rate of the population increases with age. This rate of change with age can be interpreted as a measure of the “strength” of the spread of dengue in the past. The significant change in the seroprevalence rate of each age group may be further due to a unique change in the risk of infection in a certain age window or caused by a change in unique risk factors that are not related to age, or a combination of the two [46]. Therefore, the seroprevalence rate provides information about the overall cumulative risk of infection experienced by the entire age group [47]. In addition, the individual’s dengue immune antibody level may also decrease with age (antibody protection decay effect). Here, we consider the impact of different infection mechanisms (Model A–D) on the dengue transmission intensity. For Model A, we assume that the FOI does not change with age; that is, the FOI is a constant. For Model B, since the seroprevalence rate of some data sets seems to decrease with age, we assume that antibody protection decays at a constant rate. For Models C and D, we consider that the seropositivity rate changed significantly at a certain age due to changes in the exposure levels or other reasons and introduced the concept of threshold age (Acrit). In view of that changes in population structure can greatly complicate mathematical models and require a large amount of longitudinal population data, we ignore demographic changes such as population mobility and natural birth/death rates. In the following, we give the details of the four models.
2.2.1. Model A: Constant Force of Infection
According to Muench’s catalytic model [48], people in age group i changes from a seronegative group to a seropositive group after infection at a rate λ, as shown in Figure 2. Here λ is used to denote FOI, and the proportions of seronegative and seropositive in the age group i are x(ai) and z(ai), respectively.
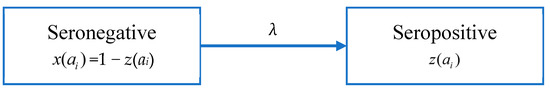
Figure 2.
Model A: the catalytic model that assumes constant FOI without antibody decay.
Since the data is not serotype-specific, we assume that the total FOI of the four serotypes is constant and that individuals receive lifelong immunity after infection. Assuming that λ is a constant, the proportion of the seropositive population in age group i, z(ai) is given by:
Here ai is the median age of the age group i.
2.2.2. Model B: Antibody Protection Decay
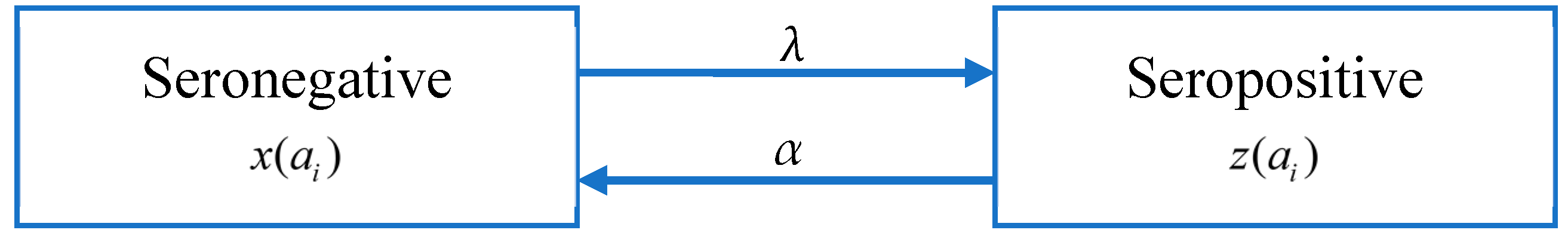
There are four serotypes of the dengue virus. A person who is infected with one serotype will have acquired immunity. Although there is serotype cross-immunity, the duration of cross-immunity is very different from person to person [49,50,51]. Studies have shown that in the first six months after primary infection with dengue, the neutralizing antibody (NAb) titers against all serotypes are the highest [52], but after that, NAb titers gradually weaken, which mainly depends on the intensity of dengue and the degree of exposure [53,54,55,56]. According to the data we have obtained, the seroprevalence rate of some data sets decreases with age, which means that there may be a phenomenon that antibody levels decrease with age. Following [19], it is assumed that the immunity level of a seropositive group decays at a rate of α, causing individuals to return to the seronegative group (Figure 3).
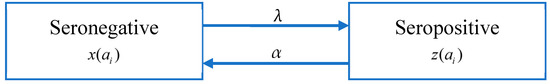
Figure 3.
Model B: The catalytic model that assumes constant FOI and antibody decay.
As shown in Figure 3, the population is divided into the seropositive population (z(ai)) and seronegative population (x(ai) = 1 − z(ai)); the antibody level of the seropositive population decays at a constant rate α, while seronegative population may be infected to become seropositive people. By extending Model A, the change in the proportion of the seropositive population in age group i, z(ai) is given by:
Assuming that both λ and α are constant, integrating the above formula gives,
2.2.3. Models That Include Threshold Age
For people of different ages (such as the young and old), their risk of getting dengue may be different due to differences in the immune system, lifestyle, and other factors, even if they are exposed to the same environmental conditions. Assuming that the population is homogeneously mixed, for example, young people may reach a wider range of people, while the range of activities of the elderly is limited due to reasons such as study and work. Their potential exposure patterns are also different. In view of these potential factors, we assume that the seropositivity rate may change significantly in a certain age window due to changes in the exposure level or other reasons. We assume that there is a critical age (Acrit) by which the population is divided into two different age groups. Within each age group, the FOI is still assumed to be constant but varies between age groups. Based on this, Model A and Model B are extended to Model C and Model D as follows.
Model C: constant FOI is broken by a critical age
Model D: antibody protection decay with constant FOI broken by a critical age
Here, λ1 and λ2 are the respective FOI when age a is less than or equal to Acrit or greater than Acrit, and the decay rate of antibody protection (α) remains constant throughout all the age groups.
2.3. Inference Method
To estimate FOI and R0, we fit the predicted seroprevalence rates of each age group to observed data, wherein the observed proportions of seropositivity in each age group are calculated based on the age-stratified seroprevalence survey data. In this study, we use Bayesian inference to estimate the model parameters [57]. The prior information on parameters is obtained through literature review and experience, and serological data are extracted from the literature as described in Section 2.1 (Supplementary File S1). Combining these with the likelihood function (see Section 2.4), the Markov Chain Monte Carlo (MCMC) method via normal random walk Metropolis-Hastings sampling method is used to generate the posterior distribution of model parameters [57], from which the median and its 95% credible interval (CrI) are obtained. The R statistical software (version 4.2.2 [58]) is used for calculations.
2.4. Negative Log-Likelihood (-LnL)
In Bayesian statistics, the likelihood function is calculated through the sample information (observation data). We assume that the probability of seropositive individuals in the age group i obeys the Beta Binomial Distribution:
Here, Ni is the total number of individuals in age group i, pi is the proportion of seropositive individuals observed in age group i, and γ represents the overdispersion parameter of the beta-binomial distribution.
Following Imai et al. [19], the total negative log-likelihood function for all age groups, −LnL(p), is given by:
where B[a,b] is the beta function with standard parameters a (predicted seropositive number) and b (predicted seronegative number), Xi is the number of seropositive individuals in age group i, mi is the predicted proportion of seropositive patients in the age group i.
2.5. Estimation of Basic Reproduction Number (R0)
Assuming that FOI is constant for a certain amount of time, R0 can be estimated from the formula [46]:
Here f(a) is the probability density function of the population age distribution, and z(a) is the predicted seroprevalence from the proposed models. We collected the age distribution data of the population in each study area from the National Bureau of Statistics [59] of China and the website [60] of the Red and Black Population Database to calculate f(a). In actual calculations, the age was divided into na groups, and Formula (7) sums over these age groups. For the age group i, ai is its median age, and f(ai) is approximated by f(ai) = ni/Total_N, with ni being the size in age group i and Total_N being the total number of the population.
2.6. Deviation Information Criterion (DIC) and Model Selection
It should be borne in mind that the infectious disease system can be modeled because epidemics involve relatively simple processes that occur within a large number of individuals [18]. In the modeling studies of infectious diseases, models are used to simplify the complex real world, and the performance of model fitting varies among models. To compare model performance, we use the deviation information criterion (DIC) proposed by Spiegelhalter et al. [61], which combines the fit and complexity of the model and can compare models of any structure. The model that has the smallest DIC is the best and will be chosen [57].
Burnham and Anderson [62] suggested models receiving the Akaike Information Criterion (AIC) within 1–2 of the “best” deserve consideration, and 3–7 have considerably less support. According to Spiegelhalter et al. [61], these rules of thumb appear to work reasonably well for DIC. Therefore, in this study, we chose the critical value 3 as the criterion for DIC to select the best model. In other words, when comparing models, we believe that when the DIC difference between different models exceeds 3, there will be a performance difference. Considering that a simple model is more beneficial for result interpretation, for the data set whose DIC difference between the two models is less than 3, the model with a relatively simple structure is selected as the best model.
3. Results
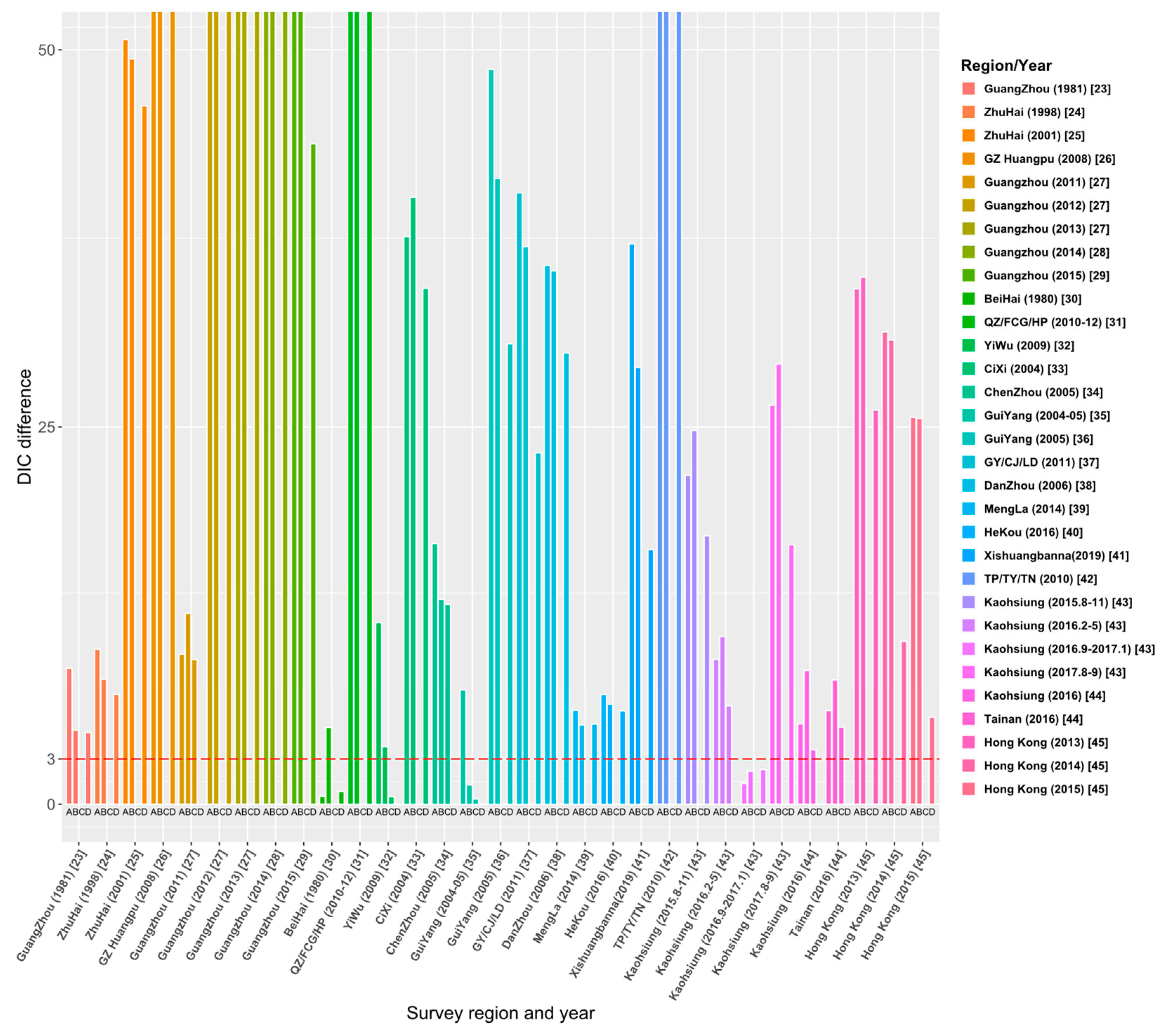
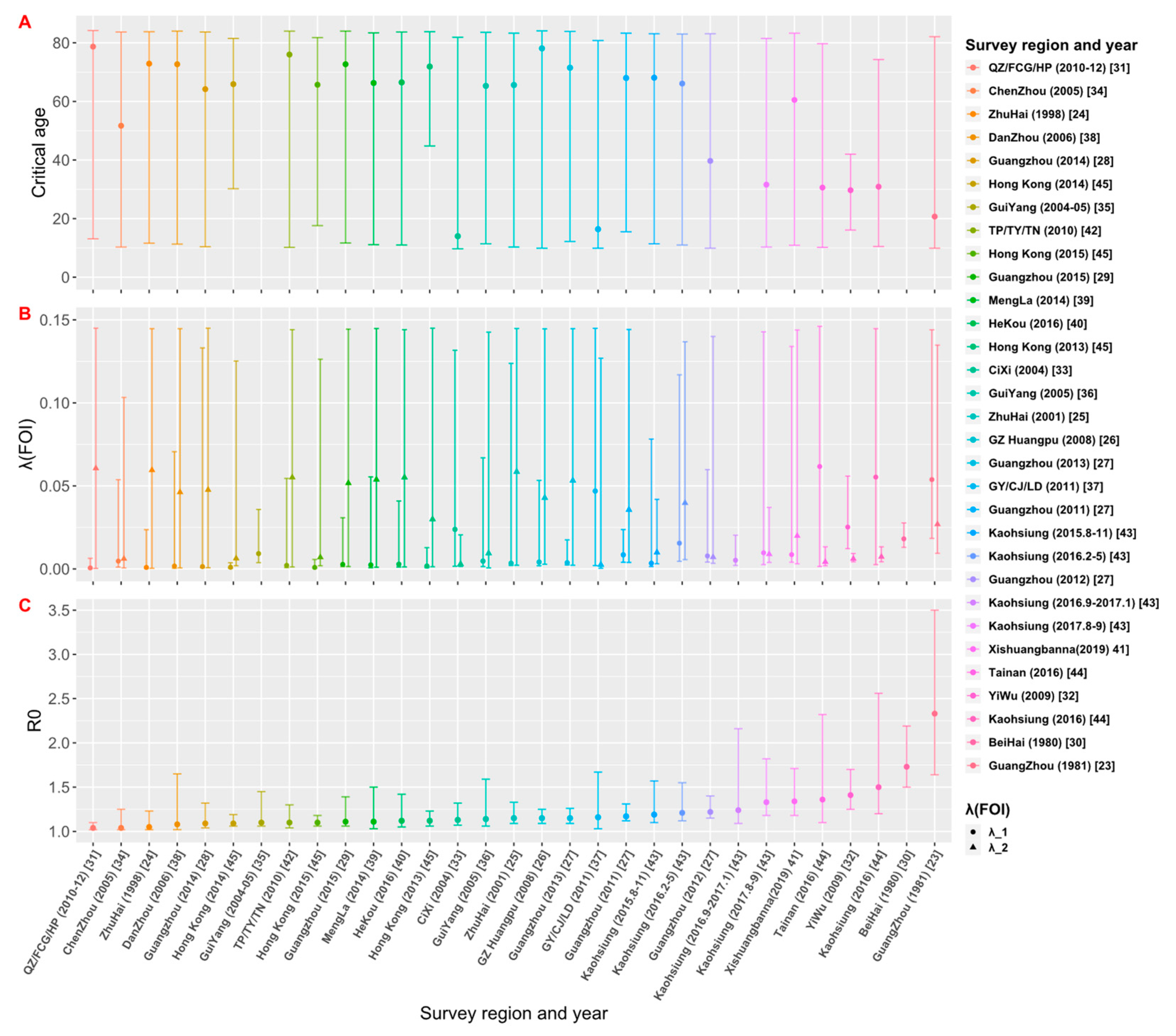
Through the literature review, we selected 23 studies from 515 articles, and the study areas included 8 provinces, 14 cities or regions in China. Those studies reported the survey data of dengue age-stratified serological prevalence from 1980 to 2019. For each data set, we estimated its FOI and R0 using the four models (the details are given in Tables S1–S4 in Supplementary File S2). The model fitting curves of the four models to 31 data sets are illustrated in Figures S1–S4 in Supplementary File S2. Figure 4 and Table S5 show the comparison results of DIC among the four models, and the final estimates (Table 2) are based on the best models that have the smallest values of DIC.
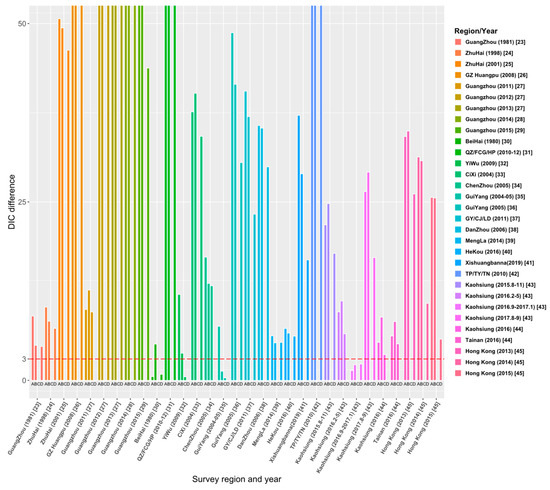
Figure 4.
Comparison of DIC values over four models for 31 data sets. The ordinate ‘DIC difference’ is the difference between the DIC and the minimum DIC obtained by fitting the four models to data sets.

Table 2.
Summary of estimation results from the best model fitting to 31 data sets.
3.1. Model Comparison and Selection
Figure 4 demonstrates the relative DIC values to the best model for the 31 data sets. The results show that the best model for data sets [23,24,25,26,28,29,32,33,36,37,38,39,40,41,42,44,45] is Model C. In addition, Model C is also applicable to the data sets of 2012 and 2013 in the Guangzhou study [27] and the data sets from August to November 2015 and from August to September 2017 in the Kaohsiung study [43]. The best model for 25 data sets among the 31 data sets is Model C: constant FOI is broken by a critical age. This indicates the age effect on the intensity of dengue transmission among these study areas. Model D (Constant FOI broken by a threshold age with antibody protection decay) was applied to the data sets of 2011–2013 in the study of Guangzhou [27], Chenzhou in 2005 [34], and Kaohsiung in 2016 from February to May [43]. Model A (constant FOI without antibody protection decay) applies to data sets from September 2016 to January 2017 in the study in Kaohsiung [43] and the 1980 study in Beihai City, Guangxi [30]. Only the study [35] in Guiyang in 2004–05 is applied to Model B, which assumes constant FOI and antibody protection decay of dengue infection. Overall, model fittings illustrate that age has a universal effect on the transmission of dengue fever, but the protective attenuation effect of antibodies was not absent in the transmission of dengue fever.
3.2. Estimates of FOI and R0
The estimates of the critical age (Acrit), FOI and R0 from the best models selected for each data set are shown in Table 2 and Figure 5. Among the 28 data sets that have model C or D as their best models, they can be divided into two groups: For the 19 data sets that have estimated critical ages older than 60 years, the FOI for ages younger than the critical age (λ1) is weaker than the FOI for ages older than the critical age (λ2); while for the 9 data sets that have Acrit < 60 years, λ1 is greater than λ2. Assuming that the spread of dengue in the study area is in a local potential endemic state, the estimates of R0 would be greater than 1 (Figure 5). The estimate of R0 obtained by the best model fittings of 31 data sets was between 1.04 and 2.33 (Table 2). The study [23] conducted in Guangzhou in 1981 had the largest estimate of R0 = 2.33 (95% CrI: 1.64, 3.50), and the study conducted by Zhou et al. [31] and Gao et al. [34] had the smallest estimate of R0 = 1.04 (95% CrI: 1.02, 1.10) and R0 = 1.04 (95% CrI: 1.02, 1.25), respectively. The graph of estimates of R0 versus the study year from 1980 to 2019 (see Supplementary File S2: Figure S5) showed that R0 was over 1.5 in 1980 and 1981; since then, it dropped and fluctuated below 1.5.
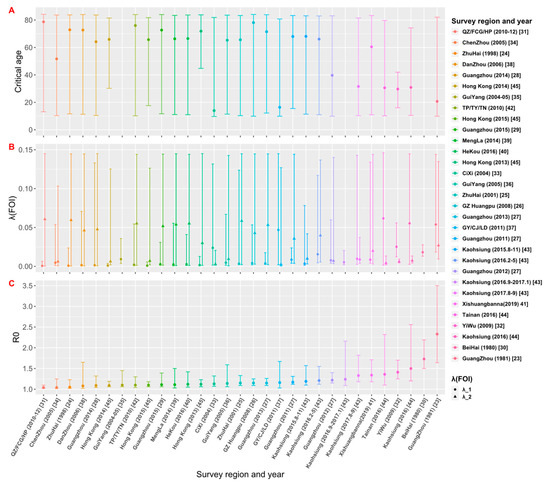
Figure 5.
Estimates of (A) critical age (Acrit), (B) force of infection (FOI), and (C) basic reproduction number (R0) based on the best model fittings to data sets. The estimates from 31 data sets are arranged in the ascending order of R0.
3.3. Time-Space Comparison
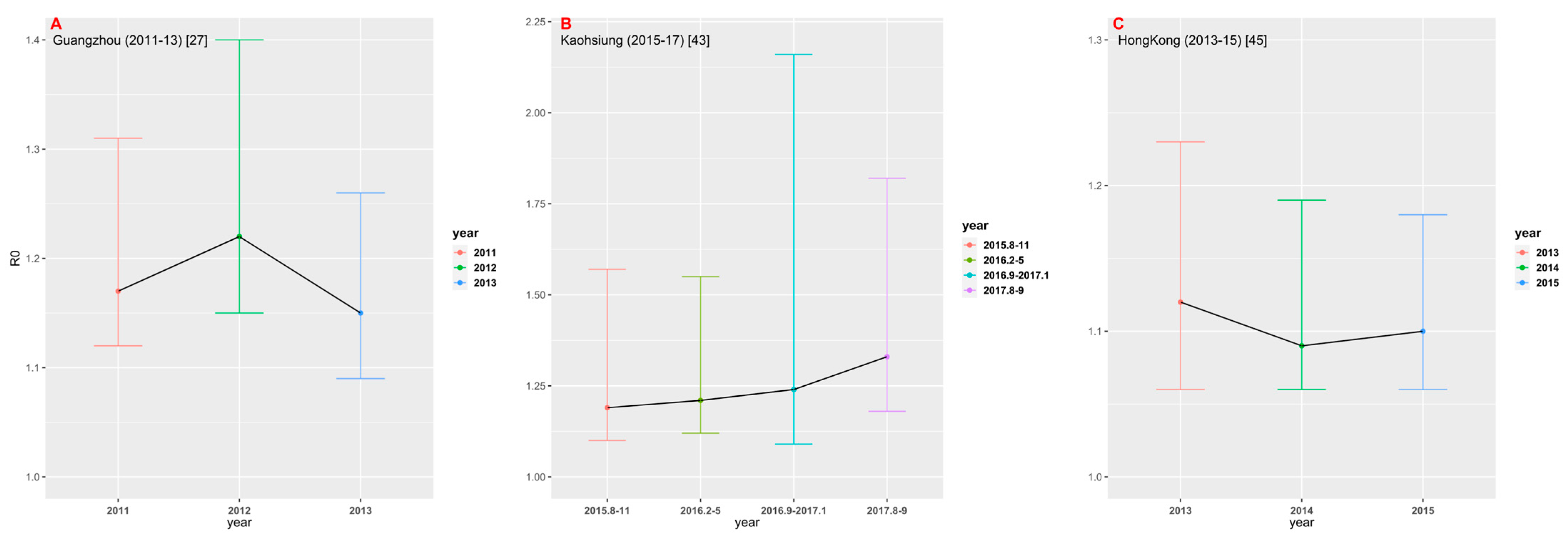
Among 23 studies selected for this analysis, only three studies provided multiple years of data. We estimated FOI and R0, respectively, by using their best models in different years (Figure 6). In the study conducted in Guangzhou [27] from 2011 to 2013, the estimate of R0 in 2012 was the largest (1.22, 95% CrI: 1.15, 1.40). In the study [43] conducted in Kaohsiung from 2015 to 2017, the estimate of R0 showed an upward trend, with R0 = 1.33 (95% CrI: 1.18, 1.82) for the data set collected in August-September 2017. In the study [45] conducted in Hong Kong between 2013 and 2015, R0 was estimated to be the largest in 2013 (1.12, 95% CrI: 1.06, 1.23). Figure 6 shows that there appears to be no significant time change trend in dengue transmission intensity in the three study areas.
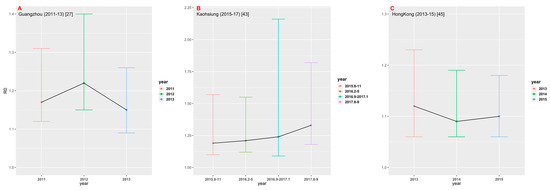
Figure 6.
Comparison of R0 in different time periods in the three regions: (A) Guangzhou; (B) Kaohsiung; and (C) Hong Kong.
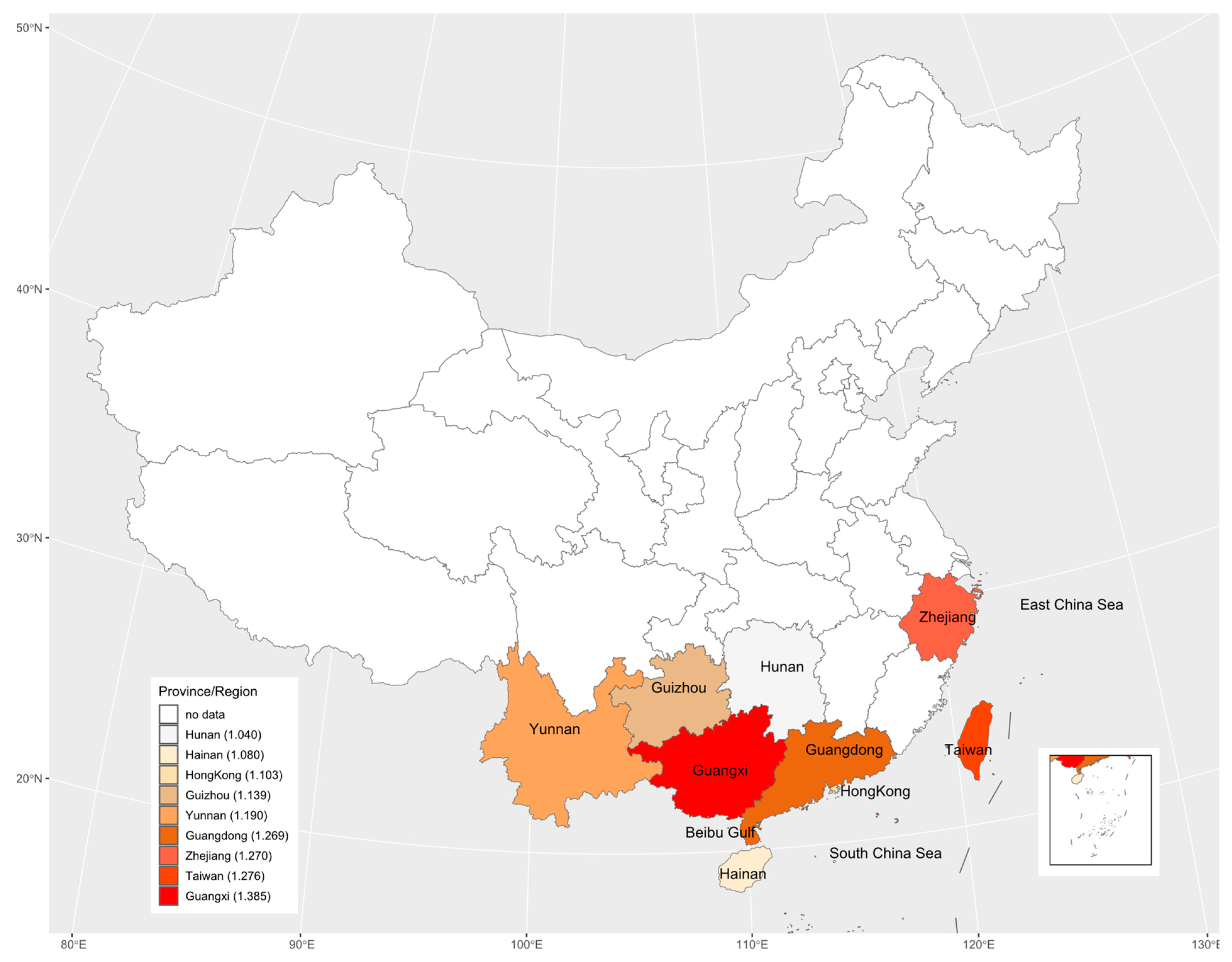
The study areas included 8 provinces and 1 region in China with 31 data sets. These nine regions are geographically illustrated with their respective mean estimate of R0 in Figure 7. The results suggested there may be an underlying spatial pattern of dengue spread in China: the intensity of dengue infection in coastal areas is generally higher than in inland areas, and the more it extends to the north, the lower the infection intensity.
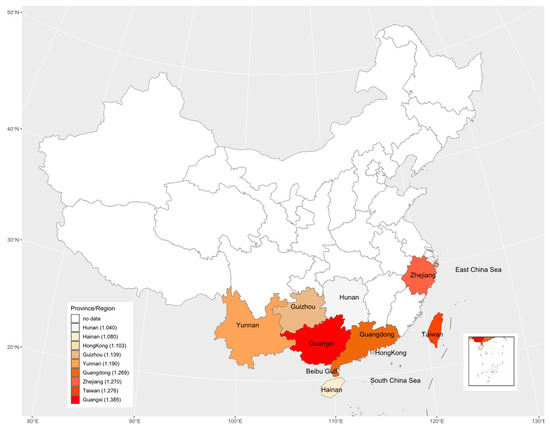
Figure 7.
The geographic distribution of the basic reproduction number across the nine study areas. The legend indicates that from top to bottom, the darker the color is, the greater the basic reproduction number is. The numbers in legend brackets are the average of the basic reproduction numbers for each province/region.
4. Discussion
Based on dengue serological age stratification data extracted from 23 studies in China, we used four catalytic models to distinguish the potential transmission mechanisms of dengue and estimated dengue transmission intensity in study areas. We found that dengue transmission intensity varies among different age groups in most of the study populations, and attenuation of antibody protection is identified in some study populations. Furthermore, we found that R0 of dengue in China was between 1.04–2.33, which agrees with that Imai’s estimate for China (1.15–2.88) [63] and is comparable with that in Singapore (1.24–1.48) and Vietnam (1.76–1.85), but lower than that in Thailand (1.96–3.96) and Brazil (2.07–2.60) [19]. Our estimate of R0 should provide useful information for the herd immunity threshold level and the effectiveness of vaccination or vector control measures required to control the spread of dengue in China [64].
Our study showed that there was a strong relationship between age and dengue transmission in the population. The model fitting indicated that the dengue transmission intensity changed at a critical age Acrit. Age could be used as a scale to quantify the exposure history in the past time, so we simply introduced Acrit to model the potential change in transmission intensity with age. The performance of Model C and Model D, which include a critical age in transmission intensity, was better in 28 of the 31 data sets from the 23 studies. This suggests that populations in those study areas have generally experienced changes in the risk of dengue transmission over a period of time. The critical age might be the time when the risk of dengue transmission changed in the study population. In addition, the existence and identification of the critical age provide a basis for the optimal formulation of dengue prevention and control measures. For example, for the study [41] conducted in 2019 in Xishuangbanna, Yunnan Province, Acrit was estimated to be 60.5 years (95% CrI: 10.9, 83.3), and the FOI was 0.0086 (95% CrI: 0.0040, 0.1340) and 0.0199 (95% CrI: 0.0031, 0.1439) for ages younger and older than the critical age, respectively; this indicates that the risk of dengue transmission was greater in the population aged older than 60.5 years, and the prevention and control of dengue fever should be focused on the elderly population. On the contrary, for the study [33] conducted in Cixi City, Zhejiang Province, in 2004, Acrit was estimated to be 14 years (95% CrI: 9.7, 81.9), and the FOI was 0.0238 (95% CrI: 0.0016, 0.1317) and 0.0029 (95% CrI: 0.0015, 0.0205) for ages younger and older than 14 years, respectively, suggesting that children and adolescents were at greater risk of dengue transmission, and should be the focus of dengue prevention and control.
Primary infection with one serotype is often able to provide life-long immunity against the reinfection of the same serotype. However, cases of homotypic reinfection confirmed by reverse transcriptase-polymerase chain reaction (RT-PCR) have recently been observed in Nicaragua [65]. Severe DENV-2 transmission has also appeared in the DENV-2 antibody population in Iquitos, Peru [66,67]. The increase in infection in the older age group may be due to a significant change in the risk of dengue infection in the study group 51 years ago, as well as a decrease in antibody levels in the older age group. This indicates that isotype immunity may not be able to obtain complete protection, especially when the specific virulence of the virus strain is high, the infectivity is high, or the quality of host immunity is poor [68]. In addition, because there are four types of DENV, specific immune antibodies acquired through infection with one serotype might provide only partial protection against the other three serotypes, and it is still possible to infect a different serotype virus for a second time in the future. Due to the lack of specific serological data on dengue, it is difficult to estimate the transmission intensity of each serotype in this study. However, the potential relationship between the attenuation of antibody protection and the transmission of dengue fever in the population could be identified through mathematical modeling. Our comparison of model performance based on the DIC value illustrates this: The study [34] in Chenzhou, Zhejiang Province in 2005, provided evidence of this phenomenon. The applicable model for the data set from this study is Model D, with the estimated critical age being 51.7 years (95% CrI: 10.3, 83.7) and the FOI being 0.0047 (95% CrI: 0.0011, 0.0537) and 0.0061 (95% CrI: 0.0006, 0.1033) for ages younger and older than the critical age, respectively; its antibody protection is estimated to decay at a rate of 0.08 (95% CrI: 0.02, 0.10) per year.
Although we only estimated the total FOI of all serotypes in the study areas based on non-serotype-specific data, these data were still sufficient to assess the heterogeneity of overall dengue transmission intensity between different regions and populations. Related studies have demonstrated that FOI estimated from non-serotype-specific data is consistent with the sum of FOI estimated from PRNT data [27]. Although the data sets collected cover the period from 1981 to 2019, these data sets were obtained from different geographical locations across China. Among 23 studies collected, only three studies [27,43,45] provided the serological surveys over multiple years in the same locations; within the short time gaps only over 3 years, the data sets from the three studies cannot show any clear temporal changes in transmission intensity (Figure 6). If seroprevalence survey data over a long period, say more than 5 years were available, the potential periodicity or another temporal trend in dengue transmission intensity could be inferred (e.g., [19]). In view of temporally steady transmission intensity [19], the average transmission intensity over different locations within the same province and different study years (if there were more than one study in one province) showed the geographical patterns: It weakened from coastal areas to inland areas, and from southern areas to northern areas (Figure 7). This might reflect that the large and dense populations, including many foreigners in the southeast coastal areas of China, provided certain beneficial conditions for the spread of dengue. Compared to other inland provinces, Yunnan province had a relatively high transmission intensity. This might reflect its specialty: located in the southwest of China and bordered by Myanmar, Laos and Vietnam, which had a high incidence of dengue [19,20] and frequent population movements, trade, and cultural exchanges. The highest mean estimate of basic reproduction number comes from Guangxi Zhuang Autonomous Region; this could be the consequence of the following factors of the region: bordering with Vietnam and being parts of the coastline so that the pressure for imported cases to spread locally is enormous, and the hot and humid climate that is good for mosquitos to live and grow and therefore is more conducive to the spread of dengue fever.
It was reported that the force of infection has declined while the average age of dengue hemorrhagic fever (DHF) cases in Thailand has increased from 8.1 to 24.3 years over the last four decays. This is mainly driven by the decreased birth and death rate [69,70]. The limited data we collected in this study cannot show any clear temporal change in transmission intensity in China, as shown in Figure 6 and Figure S5. Further, our data are age-stratified serological data and cannot directly be used for analyzing the age of dengue hemorrhagic fever (DHF) cases. With the similar demographic transition due to decreased birth and death rates, it is interesting to investigate whether a similar change pattern in the age of dengue cases also occurred in China. This information is important for the control and prevention of dengue in China and is surely a future topic of investigation.
The advantage of using serological data for inferring the burden and transmission intensity of dengue is that it is not affected by infectious disease surveillance systems and case reporting systems. With more than 75% of people infected with dengue having no clinical symptoms [4], serological data can more accurately estimate the actual number of cases. However, there are still some problems. The main problem lies in the differences in the methods used in the studies included. In the 23 studies, seroprevalence surveys sampled different populations and used serum samples collected for different purposes, which might not be representative of the total population in the study area. For example, the sample populations of the studies [24,25] were entry and exit personnel at Zhuhai Port; the sample populations of studies [28,41] were the blood donors in the blood donation center; the sample populations of studies [39,40] were entry-exit personnel at port health examination centers; the sample population of the study [45] was patients sent to the Prince of Wales Hospital in Hong Kong for diagnosis. The use of convenient serum samples could increase the amount of serological data, but the potential bias introduced by such sampling must be considered when analyzing such data.
There are some other limitations in our research. First, when using the models to estimate R0 through FOI, we assumed that dengue was in endemic balance, which means that the estimates of R0 for all data sets were greater than 1. However, this was obviously not applicable to areas where dengue was not endemic or the cases were mainly imported from outside. Secondly, the literature review for dengue showed that there were still few studies in China using serological data as a tool to monitor dengue transmission. Most model studies used case notification data, and its reliability largely depended on the quality of the infectious diseases surveillance system and reporting system [71]. A better understanding of changes in transmission intensity can not only improve estimates of the burden of disease caused by dengue but also help policymakers develop effective prevention and control plans. Therefore, we advocate the more extensive and routine use of serological surveys as a monitoring tool to provide valuable data for the study of infectious disease as dengue.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/tropicalmed8020116/s1, Figure S1: The fitting curves of Model A to 31 datasets; Figure S2: The fitting curves of Model B to 31 datasets; Figure S3: The fitting curves of Model C to 31 datasets; Figure S4: The fitting curves of Model D to 31 datasets; Figure S5: Distribution of R0 in different study years; Table S1: Summary of model parameter estimates for Model A; Table S2: Summary of model parameter estimates for Model B; Table S3: Summary of model parameter estimates for Model C; Table S4: Summary of model parameter estimates for Model D; Table S5: Comparison of DIC among four models.
Author Contributions
Conceptualization, N.L. and X.-S.Z.; data Curation, N.L. and T.W.; formal Analysis, N.L., H.L., Z.C. and X.-S.Z.; funding acquisition, W.L.; investigation, N.L., H.X. and Z.L.; methodology: N.L., H.L. and X.-S.Z.; project administration: W.L.; visualization: N.L.; writing—original draft preparation, N.L.; writing—review & editing, N.L. and X.-S.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The work was jointly supported by the National Nature Science Foundation of China (No. 81860607) and the Innovative Research Team of Yunnan Province (No. 2019(6)), China. The funders had no role in study design, data collection and analysis, the decision to publish, or the preparation of the manuscript.
Institutional Review Board Statement
Ethical review and approval were waived for this study since this is a theoretical study with personal identifications being removed from the survey data.
Informed Consent Statement
Patient consent was waived due to patients’ identification being removed from the survey data.
Data Availability Statement
Data from previously published studies are all listed in the references and provided in Supplementary File S1.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- World Health Organization (WHO). Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- World Health Organization (WHO). Accelerating Work to Overcome the Global Impact of Neglected Tropical Diseases; World Health Organization: Geneva, Switzerland, 2007; Volume 7, p. 596. [Google Scholar]
- World Health Organization. Dengue and Severe Dengue, 10 January 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 22 October 2022).
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Huang, Y.; Zhang, X.; Xiong, L.; Li, Y.; Zhang, R. Epidemiology and genotyping of dengue fever in Shenzhen City in 2018. Chin. J. Infect. Dis. 2020, 38, 342–347. [Google Scholar] [CrossRef]
- Wu, T.; Qin, N.; Zhang, J.; Wang, W.; Li, J. Dengue fever epidemic risk in Tianjin from 2009 to 2015. Mod. Prev. Med. 2016, 43, 1925–1927+1943. [Google Scholar]
- Cai, Y.; Liu, S.; Wei, Y.; Han, X.; Han, Z.; Zhang, Y.; Xu, Y.; Qi, S.; Li, Q. Epidemic characteristics and prevention and control Strategies of imported Dengue fever in Hebei province from 2011 to 2018. Pract. Prev. Med. 2022, 27, 798–801. [Google Scholar]
- Li, H.; Jiang, J.; Do, L.; Chen, Z.; Chen, S.; Li, J.; Liu, H. Emergency monitoring and analysis of the epidemiological characteristics and vectors of a dengue fever outbreak in Mengla County in 2018. J. Parasit. Biol. 2020, 15, 83–85+90. [Google Scholar] [CrossRef]
- Feng, S.; Guan, J.; Chen, J.; Rao, Q.; Sun, Q. Clinical and laboratory characteristics of 96 cases of dengue fever in Qiyang County, Hunan Province, China in 2018. Chin. J. Biol. 2020, 33, 423–428+433. [Google Scholar]
- Dai, B.; Wang, F.; Pan, J.; Han, C.; Huang, K. Epidemic characteristics and treatment effect of the first dengue fever outbreak in Hubei Province. J. Public Health Prev. Med. 2020, 31, 62–65. [Google Scholar] [CrossRef]
- Ning, D.; Sun, J.; Peng, Z.; Wu, D. The epidemiological situation and epidemiological characteristics of dengue fever in Guangdong Province. South China Prev. Med. 2017, 43, 368–372. [Google Scholar]
- Cai, W.; Jing, Q.; Liu, W.; Chen, C. Analysis of epidemiological characteristics of local dengue fever cases in Guangzhou from 2015 to 2019. South China Prev. Med. 2020, 46, 138–140. [Google Scholar]
- Yang, J.; Chen, M.; Wang, H.; Zhang., S. Analysis of epidemiological characteristics of dengue fever outbreak in Fuzhou in 2016. Chin. Trop. Med. 2017, 17, 795–797+805. [Google Scholar]
- Mai, G.; He, Y.; Chen, Z. Analysis of the epidemiological characteristics of dengue antibody positive in Gaoming District, Foshan City from 2015 to 2019. Public Health Prev. Med. 2020, 31, 122–124. [Google Scholar]
- World Health Organization (WHO). Global Strategy for Dengue Prevention and Control, 2012–2020; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Grenfell, B.T.; Anderson, R.M. The estimation of age-related rates of infection from case notifications and serological data. J. Hyg. 1985, 95, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; May, R.M. Infectious Diseases of Humans: Dynamics and Control (Oxford Science Publications); OUP Oxford: Oxford, UK, 1992. [Google Scholar]
- Ferguson, N.M. Mathematical prediction in infection. Medicine 2009, 37, 507–509. [Google Scholar] [CrossRef]
- Imai, N.; Dorigatti, I.; Cauchemez, S.; Ferguson, N.M. Estimating dengue transmission intensity from sero-prevalence surveys in multiple countries. PLoS Negl. Trop. Dis. 2015, 9, e0003719. [Google Scholar] [CrossRef]
- Thai, K.T.; Nagelkerke, N.; Phuong, H.L.; Nga, T.T.; Giao, P.T.; Hung, L.Q.; Binh, T.Q.; Nam, N.V.; De Vries, P.J. Geographical heterogeneity of dengue transmission in two villages in southern Vietnam. Epidemiol. Infect. 2010, 138, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.; Kuan, G.; Mercado, J.C.; Gresh, L.; Avilés, W.; Balmaseda, A.; Harris, E. The Nicaraguan pediatric dengue cohort study: Incidence of inapparent and symptomatic dengue virus infections, 2004–2010. PLoS Negl. Trop. Dis. 2013, 7, e2462. [Google Scholar] [CrossRef]
- Endy, T.P.; Chunsuttiwat, S.; Nisalak, A.; Libraty, D.H.; Green, S.; Rothman, A.L.; Vaughn, D.W.; Ennis, F.A. Epidemiology of inapparent and symptomatic acute dengue virus infection: A prospective study of primary school children in Kamphaeng Phet, Thailand. Am. J. Epidemiol. 2002, 156, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liang, J.; Lin, Z. Survey of dengue anti-antibody level in population after dengue fever epidemic in Guangzhou. Guangzhou Med. J. 1983, 20–25. [Google Scholar]
- Li, Z.; Zhang, J.; Ma, H. Surveillance of Dengue Fever Antibody Levels in People in Zhuhai Port Area. Chin. J. Front. Health Quar. 1999, 22, 141–143. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, H.; Lin, G.; Zeng, W.; Tu, C.; Ye, L.; Zhao, J.; Yao, R. Analysis of dengue antibody levels and related factors in different populations. Mod. Prev. Med. 2002, 29, 694–695. [Google Scholar] [CrossRef]
- Zheng, X.; Wu, Y.; Zhang, M.; He, A.; Li, Z.; Qu, Z.; Zhan, X. Sero-epidemiological Investigation of Dengue Fever in Guangzhou. J. Trop. Med. 2009, 9, 1397–1399+1404. [Google Scholar]
- Cao, Y.; Jiang, L.; Xu, Y.; Jing, Q.; Cao, Q.; Di, B.; Yang, Z. Monitoring and analysis of dengue fever serum antibody levels in Guangzhou from 2011 to 2013. South China J. Prev. Med. 2015, 41, 364–366. [Google Scholar] [CrossRef]
- Li, S.; Liao, Q.; Liang, Y.; Chen, J.; You, R.; Xiong, H.; Huang, K.; Rong, X. Study on the risk of dengue virus transmission by blood transfusion in Guangzhou area. Guangdong Med. J. 2017, 38, 1064–1067. [Google Scholar] [CrossRef]
- Jing, Q.; Li, Y.; Liu, J.; Jiang, L.; Chen, Z.; Su, W.; Birkhead, G.S.; Lu, J.; Yang, Z. Dengue Underestimation in Guangzhou, China: Evidence of Seroprevalence in Communities with No Reported Cases Before a Large Outbreak in 2014. Open Forum Infect. Dis. 2019, 6, ofz256. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Ai, C.; Li, C.; Wen, Y.; Liu, Y.; Xia, H. Epidemiological surveillance of dengue fever in Beihai City, Guangxi. J. Mil. Med. Sci. 1985, 38, 387–392. [Google Scholar]
- Zhou, K.; Chen, M.; Tan, Y.; Mo, Y.; Bi, Y. Serological surveillance of healthy population in Guangxi dengue surveillance sites. J. Appl. Prev. Med. 2013, 19, 236–237. [Google Scholar] [CrossRef]
- Sun, J.; Luo, S.; Lin, J.; Chen, J.; Hou, J.; Fu, T.; Lv, H.; Chen, Z.; Cong, L.; Ling, F.; et al. Inapparent infection during an outbreak of dengue fever in Southeastern China. Viral Immunol. 2012, 25, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Cen, D.; Wu, J.; Xu, Y. Application of Serum Bank in the Investigation and Analysis of Dengue Fever Antibody Level. Chin. Prev. Med. 2007, 8, 734–735. [Google Scholar] [CrossRef]
- Gao, L.; Xiao, J.; Zhang, H.; Duan, L.; Liu, F.; Dai, D.; Li, J.; Deng, Z. Sero-epidemiology investigation of dengue fever in Chenzhou. South China J. Prev. Med. 2007, 33, 34–35. [Google Scholar] [CrossRef]
- Gao, R.; Zhou, X.; Zhou, C.; Han, Y.; Luo, J.; Qin, J. Serological Epidemiological Research on Dengue Virus Antibodies among Personnel at Guiyang Port. Chin. J. Front. Health Quar. 2006, 29 (Suppl. S1), 57–59. [Google Scholar] [CrossRef]
- Tian, H.; Han, Y.; Dai, A.; Fu, D.; Zhou, Y.; Zhou, N.; Gao, R. The Investigation of the Population Infected with Dengue Virus at Guiyang Port and other Close Areas. J. Travel Med. Sci. 2007, 13, 28–29. [Google Scholar] [CrossRef]
- Jiang, W.; Zhou, J.; Tang, G.; Zhuang, Y.; Yun, C.; Fu, L. Investigation on Dengue Fever Infection among Healthy Population in Some Counties and Cities in Guizhou Province. Guizhou Med. J. 2013, 37, 164–165. [Google Scholar] [CrossRef]
- Jin, Y.; Sun, L.; Zeng, X.; Wu, W.; Ma, Y.; Su, X.; Lao, S.; Chen, Y.; Li, Z. Sero-epidemiological survey and analysis on dengue fever in Hainan Province. China Trop. Med. 2007, 7, 2007–2008. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Ji, R.; Luo, Z.; Zhang, Q.; Zhou, L.; Yang, X. Serological surveillance on arboviral diseases among exit-entry population at Sino-Laos port. Chin. J. Front. Health Quar. 2016, 39, 180–182. [Google Scholar] [CrossRef]
- Pu, L.; Chen, J.; Zhang, Q.; Zhang, C.; Li, Z.; Liu, W.; Wu, Z. Aedes surveillance and dengue fever serological survey at Sino-Vietnam Hekou-Laocai ports. Chin. J. Front. Health Quar. 2018, 41, 255–257+268. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.; Lu, S.; Dong, J.; Xu, H.; Zhang, Q.; Weng, R.; Yin, Y.; He, R.; Fang, P.; et al. Epidemiological survey and screening strategy for dengue virus in blood donors from Yunnan Province. BMC Infect. Dis. 2021, 21, 104. [Google Scholar] [CrossRef]
- Lee, Y.H.; Hsieh, Y.C.; Chen, C.J.; Lin, T.Y.; Huang, Y.C. Retrospective Seroepidemiology study of dengue virus infection in Taiwan. BMC Infect. Dis. 2021, 21, 96. [Google Scholar] [CrossRef]
- Tsai, J.J.; Liu, C.K.; Tsai, W.Y.; Liu, L.T.; Tyson, J.; Tsai, C.Y.; Lin, P.C.; Wang, W.K. Seroprevalence of dengue virus in two districts of Kaohsiung City after the largest dengue outbreak in Taiwan since World War II. PLoS Negl. Trop. Dis. 2018, 12, e0006879. [Google Scholar] [CrossRef]
- Pan, Y.H.; Liao, M.Y.; Chien, Y.W.; Ho, T.S.; Ko, H.Y.; Yang, C.R.; Chang, S.F.; Yu, C.Y.; Lin, S.Y.; Shih, P.W.; et al. Use of seroprevalence to guide dengue vaccination plans for older adults in a dengue non-endemic country. PLoS Negl. Trop. Dis. 2021, 15, e0009312. [Google Scholar] [CrossRef]
- Lee, P.; Yeung, A.C.M.; Chen, Z.; Chan, M.C.W.; Sze, K.H.; Chan, P.K.S. Age-specific seroprevalence of dengue infection in Hong Kong. J. Med. Virol. 2018, 90, 1427–1430. [Google Scholar] [CrossRef]
- Ferguson, N.M.; Donnelly, C.A.; Anderson, R.M. Transmission dynamics and epidemiology of dengue: Insights from age-stratified sero-prevalence surveys. Philos Trans. R. Soc. Lond. B Biol. Sci. 1999, 354, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Becker, N.G. Martingale methods for the analysis of epidemic data. Stat. Methods Med. Res. 1993, 2, 93–112. [Google Scholar] [CrossRef] [PubMed]
- Muench, H. Catalytic Models in Epidemiology; Harvard University Press: Cambridge, MA, USA; London, UK, 1959. [Google Scholar] [CrossRef]
- Salje, H.; Lessler, J.; Endy, T.P.; Curriero, F.C.; Gibbons, R.V.; Nisalak, A.; Nimmannitya, S.; Kalayanarooj, S.; Jarman, R.G.; Thomas, S.J.; et al. Revealing the microscale spatial signature of dengue transmission and immunity in an urban population. Proc. Natl. Acad. Sci. USA 2012, 109, 9535–9538. [Google Scholar] [CrossRef]
- Reich, N.G.; Shrestha, S.; King, A.A.; Rohani, P.; Lessler, J.; Kalayanarooj, S.; Yoon, I.K.; Gibbons, R.V.; Burke, D.S.; Cummings, D.A. Interactions between serotypes of dengue highlight epidemiological impact of cross-immunity. J. R. Soc. Interface 2013, 10, 20130414. [Google Scholar] [CrossRef] [PubMed]
- OhAinle, M.; Balmaseda, A.; Macalalad, A.R.; Tellez, Y.; Zody, M.C.; Saborío, S.; Nuñez, A.; Lennon, N.J.; Birren, B.W.; Gordon, A.; et al. Dynamics of dengue disease severity determined by the interplay between viral genetics and serotype-specific immunity. Sci. Transl. Med. 2011, 3, 114ra128. [Google Scholar] [CrossRef]
- Gibbons, R.V.; Kalanarooj, S.; Jarman, R.G.; Nisalak, A.; Vaughn, D.W.; Endy, T.P.; Mammen, M.P., Jr.; Srikiatkhachorn, A. Analysis of repeat hospital admissions for dengue to estimate the frequency of third or fourth dengue infections resulting in admissions and dengue hemorrhagic fever, and serotype sequences. Am. J. Trop. Med. Hyg. 2007, 77, 910–913. [Google Scholar] [CrossRef]
- Puschnik, A.; Lau, L.; Cromwell, E.A.; Balmaseda, A.; Zompi, S.; Harris, E. Correlation between dengue-specific neutralizing antibodies and serum avidity in primary and secondary dengue virus 3 natural infections in humans. PLoS Negl. Trop. Dis. 2013, 7, e2274. [Google Scholar] [CrossRef]
- Lai, C.Y.; Williams, K.L.; Wu, Y.C.; Knight, S.; Balmaseda, A.; Harris, E.; Wang, W.K. Analysis of cross-reactive antibodies recognizing the fusion loop of envelope protein and correlation with neutralizing antibody titers in Nicaraguan dengue cases. PLoS Negl. Trop. Dis. 2013, 7, e2451. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Montoya, M.; Gresh, L.; Balmaseda, A.; Harris, E. Neutralizing antibody titers against dengue virus correlate with protection from symptomatic infection in a longitudinal cohort. Proc. Natl. Acad. Sci. USA 2016, 113, 728–733. [Google Scholar] [CrossRef]
- Guzman, M.G.; Alvarez, M.; Rodriguez-Roche, R.; Bernardo, L.; Montes, T.; Vazquez, S.; Morier, L.; Alvarez, A.; Gould, E.A.; Kouri, G.; et al. Neutralizing antibodies after infection with dengue 1 virus. Emerg. Infect. Dis. 2007, 13, 282–286. [Google Scholar] [CrossRef]
- Zhang, X.S.; Xiong, H.; Chen, Z.; Liu, W. Importation, Local Transmission, and Model Selection in Estimating the Transmissibility of COVID-19: The Outbreak in Shaanxi Province of China as a Case Study. Trop. Med. Infect. Dis. 2022, 7, 227. [Google Scholar] [CrossRef]
- R Core Team. R: A language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 25 December 2022).
- National Bureau of Statistics of China. Census Data. Available online: http://www.stats.gov.cn/tjsj/pcsj/ (accessed on 25 December 2022).
- The Red and Black Population Database. Ranking of Each Province by Region. Available online: https://www.hongheiku.com/ (accessed on 25 December 2022).
- Spiegelhalter, D.J.; Best, N.G.; Carlin, B.; Linde, A. Bayesian measures of model complexity and fit. J. R. Stat. Soc. Ser. B Stat. Methodol. 2001, 64. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Inference; Springer: New York, NY, USA, 1998. [Google Scholar]
- Imai, N.; Dorigatti, I.; Cauchemez, S.; Ferguson, N.M. Estimating Dengue Transmission Intensity from Case-Notification Data from Multiple Countries. PLoS Negl. Trop. Dis. 2016, 10, e0004833. [Google Scholar] [CrossRef] [PubMed]
- Fine, P.; Eames, K.; Heymann, D.L. “Herd immunity”: A rough guide. Clin. Infect. Dis. 2011, 52, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Waggoner, J.J.; Balmaseda, A.; Gresh, L.; Sahoo, M.K.; Montoya, M.; Wang, C.; Abeynayake, J.; Kuan, G.; Pinsky, B.A.; Harris, E. Homotypic Dengue Virus Reinfections in Nicaraguan Children. J. Infect. Dis. 2016, 214, 986–993. [Google Scholar] [CrossRef]
- Williams, M.; Mayer, S.V.; Johnson, W.L.; Chen, R.; Volkova, E.; Vilcarromero, S.; Widen, S.G.; Wood, T.G.; Suarez-Ognio, L.; Long, K.C.; et al. Lineage II of Southeast Asian/American DENV-2 is associated with a severe dengue outbreak in the Peruvian Amazon. Am. J. Trop. Med. Hyg. 2014, 91, 611–620. [Google Scholar] [CrossRef]
- Forshey, B.M.; Reiner, R.C.; Olkowski, S.; Morrison, A.C.; Espinoza, A.; Long, K.C.; Vilcarromero, S.; Casanova, W.; Wearing, H.J.; Halsey, E.S.; et al. Incomplete Protection against Dengue Virus Type 2 Re-infection in Peru. PLoS Negl. Trop. Dis. 2016, 10, e0004398. [Google Scholar] [CrossRef] [PubMed]
- Rico-Hesse, R. Dengue virus virulence and transmission determinants. Curr. Top. Microbiol. Immunol. 2010, 338, 45–55. [Google Scholar] [CrossRef]
- Cummings, D.A.T.; Iamsirithaworn, S.; Lessler, J.T.; McDermott, A.; Prasanthong, R.; Nisalak, A.; Jarman, R.G.; Burke, D.S.; Gibbons, R.V. The Impact of the Demographic Transition on Dengue in Thailand: Insights from a Statistical Analysis and Mathematical Modeling. PLoS Med. 2009, 6, e1000139. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.T.; Takahashi, S.; Salje, H.; Garcia-Carreras, B.; Anderson, K.; Endy, T.; Thomas, S.; Rothman, A.L.; Klungthong, C.; Jones, A.R.; et al. Assessing the role of multiple mechanisms increasing the age of dengue cases in Thailand. Proc. Natl. Acad. Sci. USA 2022, 119, e2115790119. [Google Scholar] [CrossRef]
- Doherty, J.A. Final report and recommendations from the National Notifiable Diseases Working Group. Can. Commun. Dis. Rep. 2006, 32, 211–225, Erratum in Can. Commun. Dis. Rep. 2008, 34, 24–25(In English and French). [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).