Cordylobia anthropophaga Myiasis Mimicking Hyperproliferative Skin Disorder in Traveler Returning from Sub-Saharan Africa
Abstract
1. Introduction
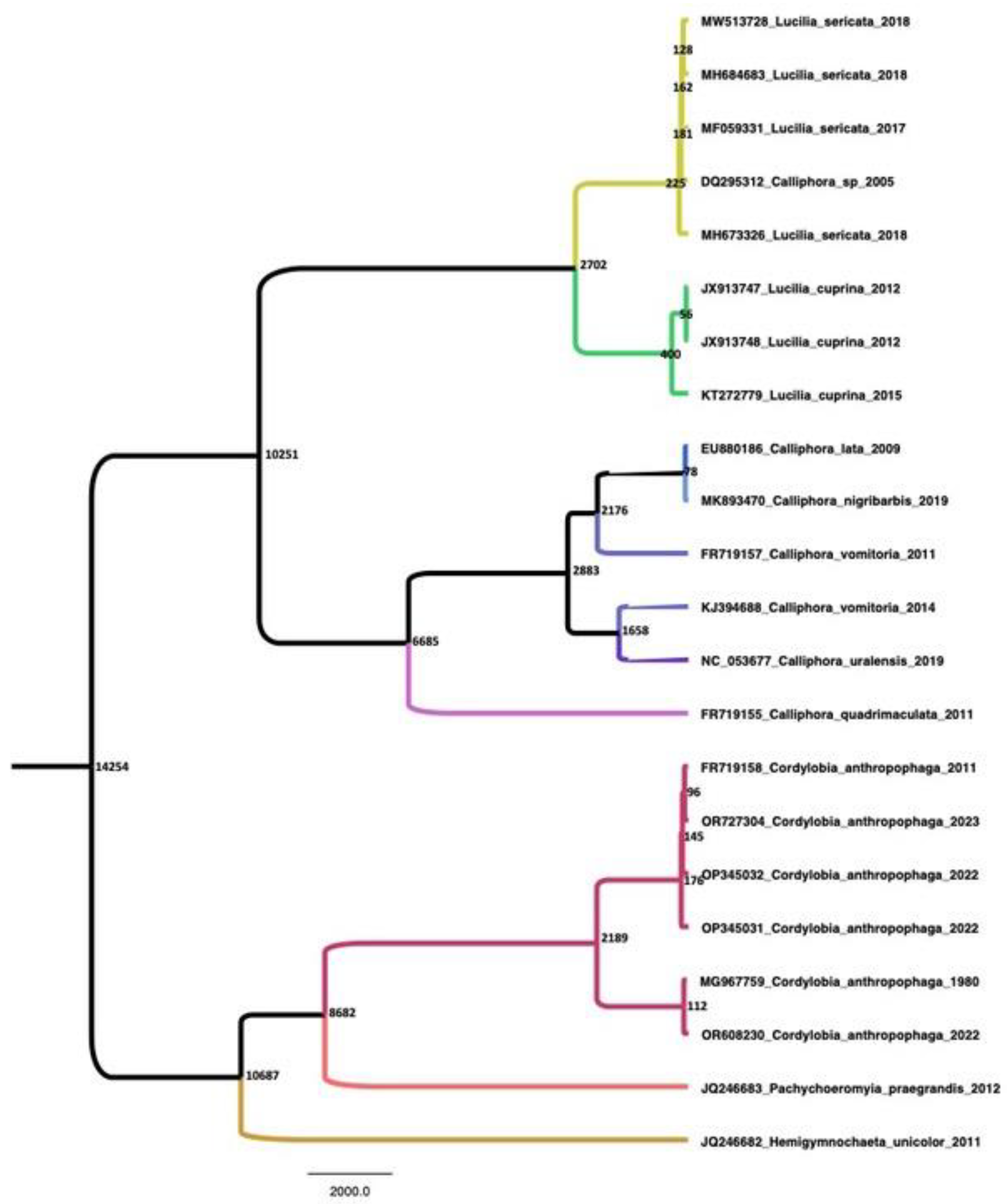
2. Materials and Methods
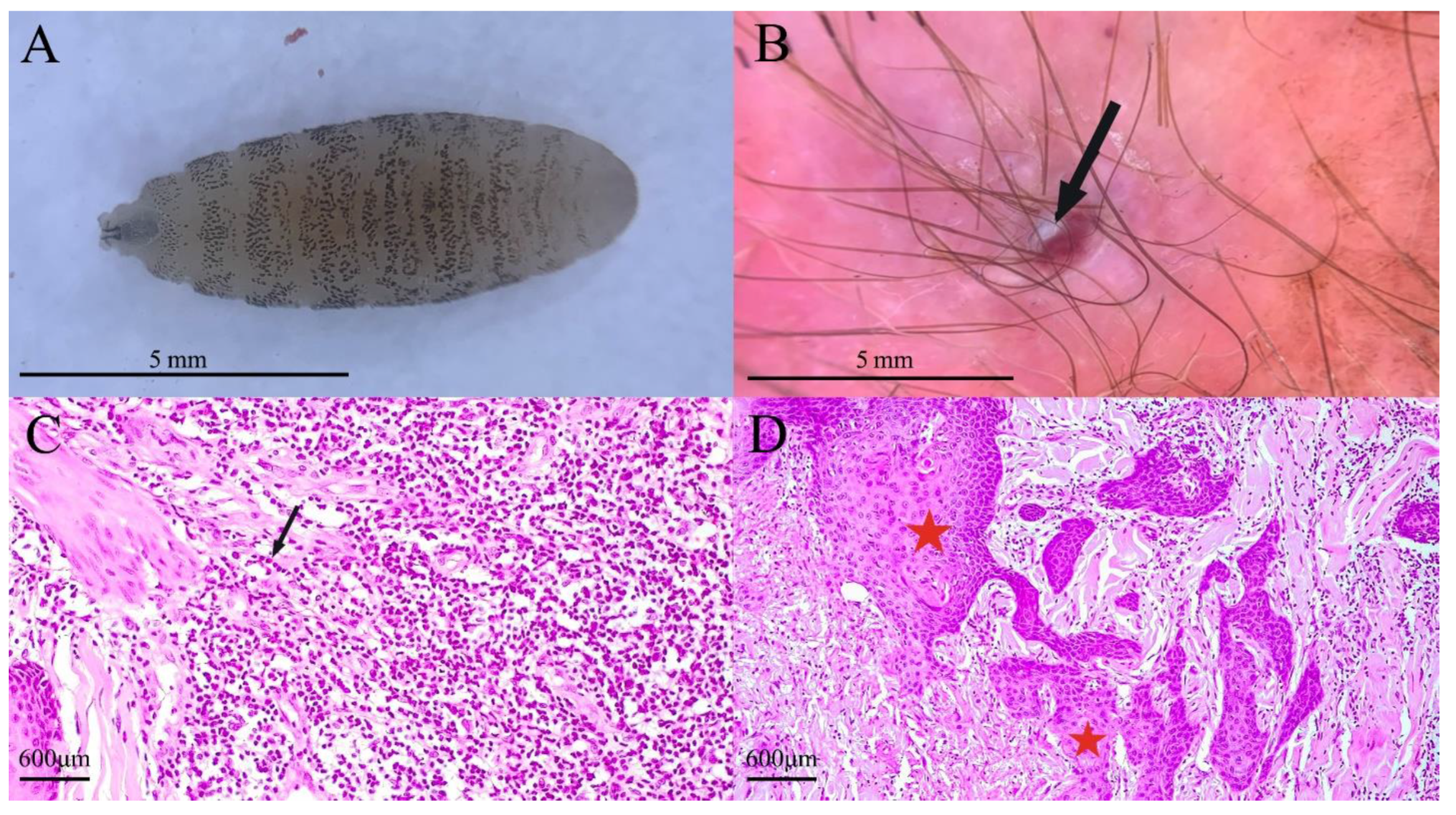
3. Case Presentation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bernhardt, V.; Finkelmeier, F.; Verhoff, M.A.; Amendt, J. Myiasis in Humans-a Global Case Report Evaluation and Literature Analysis. Parasitol. Res. 2019, 118, 389–397. [Google Scholar] [CrossRef]
- Dires, A.; Kebede, A.; Gedamu, S.; Dires, T. Case of Multiple Furuncular Myiasis in Northeast Ethiopia. Clin. Case Rep. 2022, 10, e6015. [Google Scholar] [CrossRef]
- Boggild, A.K.; Keystone, J.S.; Kain, K.C. Furuncular Myiasis: A Simple and Rapid Method for Extraction of Intact Dermatobia hominis Larvae. Clin. Infect. Dis. 2002, 35, 336–338. [Google Scholar] [CrossRef] [PubMed]
- Pathania, V.; Kashif, A.W.; Aggarwal, R.N. Cutaneous Myiasis: Think beyond Furunculosis. Med. J. Armed Forces India 2018, 74, 268–272. [Google Scholar] [CrossRef]
- Hannam, P.; Khairnar, K.; Downey, J.; Powis, J.; Ralevski, F.; Pillai, D.R. Cutaneous Myiasis in Traveler Returning from Ethiopia. Emerg. Infect. Dis. 2011, 17, 2385–2386. [Google Scholar] [CrossRef]
- MacFadden, D.R.; Waller, B.; Wizen, G.; Boggild, A.K. Imported and Locally Acquired Human Myiasis in Canada: A Report of Two Cases. CMAJ 2015, 187, 272–275. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zumpt, F. Myiasis in Man and Animals in the Old World: A Textbook for Physicians, Veterinarians, and Zoologists; Butterworths: London, UK, 1965. [Google Scholar]
- Lowe, P.; Naseem, S.; Bailey, C. Cordylobia anthropophaga: A Rare Surgical Emergency in the UK. BMJ Case Rep. 2013, 2013, bcr2012008659. [Google Scholar] [CrossRef]
- Laurence, B.R.; Herman, F.G. Letter: Tumbu Fly (Cordylobia) Infection Outside Africa. Trans. R. Soc. Trop. Med. Hyg. 1973, 67, 888. [Google Scholar] [CrossRef]
- Curtis, S.J.; Edwards, C.; Athulathmuda, C.; Paul, J. Case of the Month: Cutaneous Myiasis in a Returning Traveller from the Algarve: First Report of Tumbu Maggots, Cordylobia anthropophaga, Acquired in Portugal. Emerg. Med. J. 2006, 23, 236–237. [Google Scholar] [CrossRef] [PubMed]
- Dehecq, É.; Nzungu, P.N.; Cailliez, J.-C.; Guevart, É.; Delhaes, L.; Dei-Cas, E.; Bourel, B. Cordylobia anthropophaga (Diptera: Calliphoridae) Outside Africa: A Case of Furuncular Myiasis in a Child Returning from Congo. J. Med. Entomol. 2005, 42, 187–192. [Google Scholar] [CrossRef]
- Singh, A.; Singh, Z. Incidence of Myiasis among Humans—A Review. Parasitol. Res. 2015, 114, 3183–3199. [Google Scholar] [CrossRef] [PubMed]
- Song, S.-M.; Kim, S.-W.; Goo, Y.-K.; Hong, Y.; Ock, M.; Cha, H.-J.; Chung, D.-I. A Case of Furuncular Myiasis Due to Cordylobia anthropophaga in a Korean Traveler Returning from Uganda. Korean J. Parasitol. 2017, 55, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Cuestas, D.; Pedraza, J.; Herrera, H.; Motta, A.; Cuestas, A.; Forero, Y.; Porras, R.; Urrea, F.; Galvis, D.; Galvis, I.; et al. Cutaneous Myiasis in Skin Cancer and Malignant Wounds: A Systematic Review. Int. J. Dermatol. 2021, 60, 1529–1546. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Rannala, B.; Yang, Z. Probability distribution of molecular evolutionary trees: A new method of phylogenetic inference. J. Mol. Evol. 1996, 43, 304–311. [Google Scholar] [CrossRef]
- Drummond, A.J.; Ho, S.Y.W.; Phillips, M.J.; Rambaut, A. Relaxed phylogenetics and dating with confidence. PLoS Biol. 2006, 4, e88. [Google Scholar] [CrossRef]
- Drummond, A.J.; Suchard, M.A.; Xie, D.; Rambaut, A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012, 29, 1969–1973. [Google Scholar] [CrossRef]
- Oliva, E.; Bargiggia, G.; Quinzan, G.; Lanza, P.; Farina, C. Furuncular Myiasis in Italian Traveler Returning from Kenya. J. Infect. Dev. Ctries 2020, 14, 114–116. [Google Scholar] [CrossRef]
- Francesconi, F.; Lupi, O. Myiasis. Clin. Microbiol. Rev. 2012, 25, 79–105. [Google Scholar] [CrossRef]
- Kalezic, T.; Stojkovic, M.; Vukovic, I.; Spasic, R.; Andjelkovic, M.; Stanojlovic, S.; Bozic, M.; Dzamic, A. Human External Ophthalmomyiasis Caused by Lucilia sericata Meigen (Diptera: Calliphoridae)—A Green Bottle Fly. J. Infect. Dev. Ctries 2014, 8, 925–928. [Google Scholar] [CrossRef][Green Version]
- Dolinaj, V.; Grujić, J.; Križanović, D.; Potkonjak, A.; Pape, T.; Banović, P. The Price of Hospital Reshaping: Nasal Myiasis Caused by Flesh Fly (Diptera: Sarcophagidae) in Reallocated COVID-19 Intensive Care Unit. Healthcare 2023, 11, 1533. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, J.R.; North, D.; Santo, A. Furuncular Myiasis of the Foot Caused by the Tumbu Fly, Cordylobia anthropophaga: Report in a Medical Student Returning from a Medical Mission Trip to Tanzania. Int. Med. Case Rep. J. 2013, 6, 25–28. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Suárez, J.A.; Ying, A.; Orillac, L.A.; Cedeño, I.; Sosa, N. First Case of Furuncular Myiasis Due to Cordylobia anthropophaga in a Latin American Resident Returning from Central African Republic. Braz. J. Infect. Dis. 2018, 22, 70–73. [Google Scholar] [CrossRef]
- Whitehorn, J.S.; Whitehorn, C.; Thakrar, N.A.; Hall, M.J.R.; Godfrey-Faussett, P.; Bailey, R. The Dangers of an Adventurous Partner: Cordylobia anthropophaga Infestation in London. Trans. R. Soc. Trop. Med. Hyg. 2010, 104, 374–375. [Google Scholar] [CrossRef]
- Schechter, E.; Lazar, J.; Nix, M.E.; Mallon, W.K.; Moore, C.L. Identification of Subcutaneous Myiasis Using Bedside Emergency Physician Performed Ultrasound. J. Emerg. Med. 2011, 40, e1–e3. [Google Scholar] [CrossRef] [PubMed]
- Grogan, T.M.; Payne, C.M.; Payne, T.B.; Spier, C.; Cromey, D.W.; Rangel, C.; Richter, L. Cutaneous Myiasis. Immunohistologic and Ultrastructural Morphometric Features of a Human Botfly Lesion. Am. J. Dermatopathol. 1987, 9, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Sunny, B.; Sulthana, L.; James, A.; Sivakumar, T. Maggot Infestation: Various Treatment Modalities. J. Am. Coll. Clin. Wound Spec. 2016, 8, 51–53. [Google Scholar] [CrossRef]
- Caissie, R.; Beaulieu, F.; Giroux, M.; Berthod, F.; Landry, P.-E. Cutaneous Myiasis: Diagnosis, Treatment, and Prevention. J. Oral. Maxillofac. Surg. 2008, 66, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Puente, S.; Otranto, D.; Panadero, R.; Herrero, M.D.; Rivas, P.; Ramírez-Olivencia, G.; Mariscal, C., Jr.; Perteguer, M.J.; Díez-Baños, P.; Gárate, T. First Diagnosis of an Imported Human Myiasis Caused by Hypoderma sinense (Diptera: Oestridae), Detected in a European Traveler Returning from India. J. Travel Med. 2010, 17, 419–423. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popović Dragonjić, L.; Jović, A.; Janković, I.; Miladinović, J.; Ranković, A.; Cvetanović, M.; Beck, R.; Novosel, D.; Pape, T.; Banović, P. Cordylobia anthropophaga Myiasis Mimicking Hyperproliferative Skin Disorder in Traveler Returning from Sub-Saharan Africa. Trop. Med. Infect. Dis. 2023, 8, 505. https://doi.org/10.3390/tropicalmed8110505
Popović Dragonjić L, Jović A, Janković I, Miladinović J, Ranković A, Cvetanović M, Beck R, Novosel D, Pape T, Banović P. Cordylobia anthropophaga Myiasis Mimicking Hyperproliferative Skin Disorder in Traveler Returning from Sub-Saharan Africa. Tropical Medicine and Infectious Disease. 2023; 8(11):505. https://doi.org/10.3390/tropicalmed8110505
Chicago/Turabian StylePopović Dragonjić, Lidija, Andrija Jović, Irena Janković, Jelena Miladinović, Aleksandar Ranković, Maja Cvetanović, Relja Beck, Dinko Novosel, Thomas Pape, and Pavle Banović. 2023. "Cordylobia anthropophaga Myiasis Mimicking Hyperproliferative Skin Disorder in Traveler Returning from Sub-Saharan Africa" Tropical Medicine and Infectious Disease 8, no. 11: 505. https://doi.org/10.3390/tropicalmed8110505
APA StylePopović Dragonjić, L., Jović, A., Janković, I., Miladinović, J., Ranković, A., Cvetanović, M., Beck, R., Novosel, D., Pape, T., & Banović, P. (2023). Cordylobia anthropophaga Myiasis Mimicking Hyperproliferative Skin Disorder in Traveler Returning from Sub-Saharan Africa. Tropical Medicine and Infectious Disease, 8(11), 505. https://doi.org/10.3390/tropicalmed8110505








