The Role of GeneXpert® for Tuberculosis Diagnostics in Brazil: An Examination from a Historical and Epidemiological Perspective
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting
2.3. GeneXpert MTB/RIF Equipment Data (RMT-TB Equipment)
2.4. Data Collection of the Historical Context
2.5. GeneXpert® System Quantitative Data Collection
2.6. Health Care Indicators
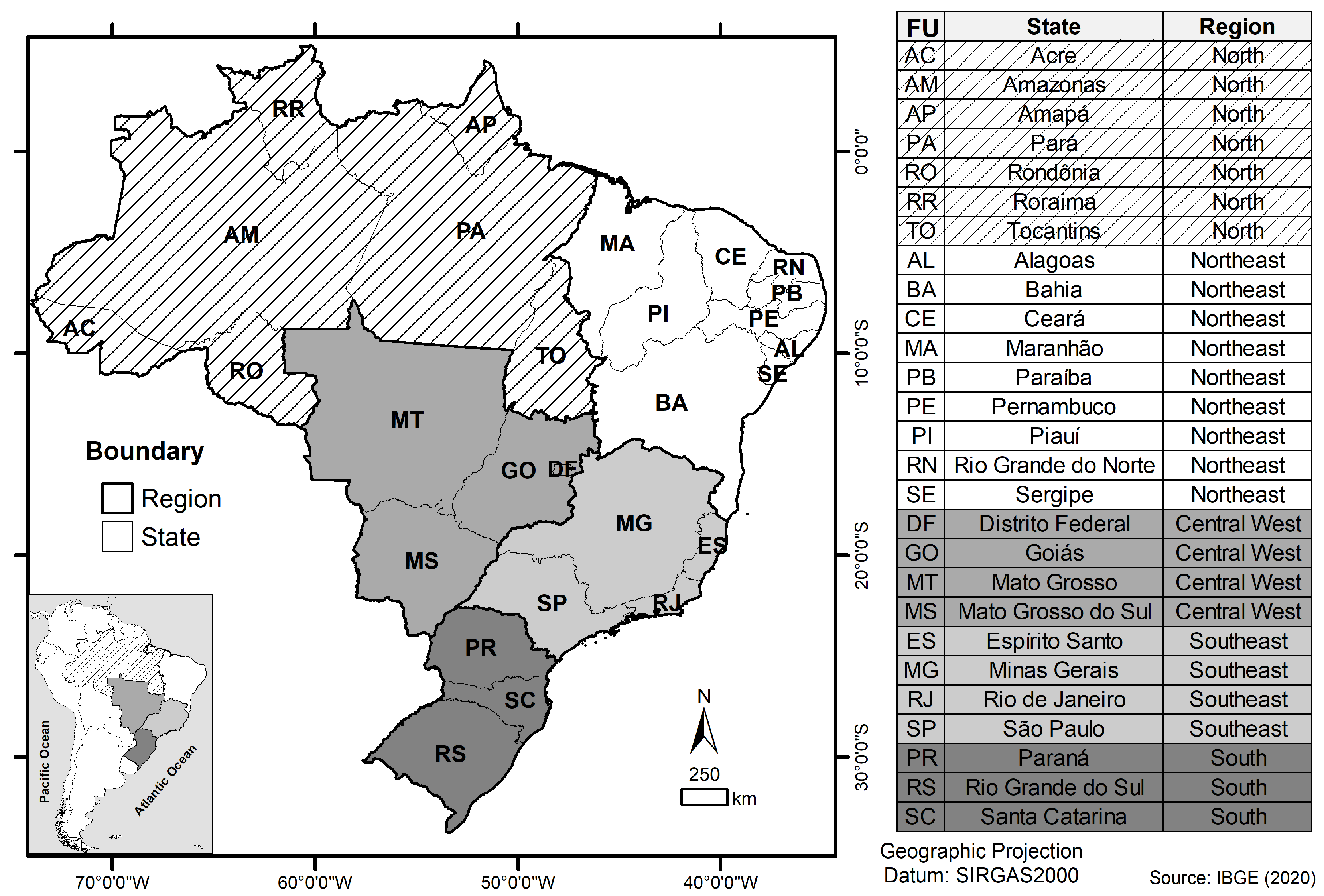
2.7. Geoprocessing
2.8. Statistics Analysis
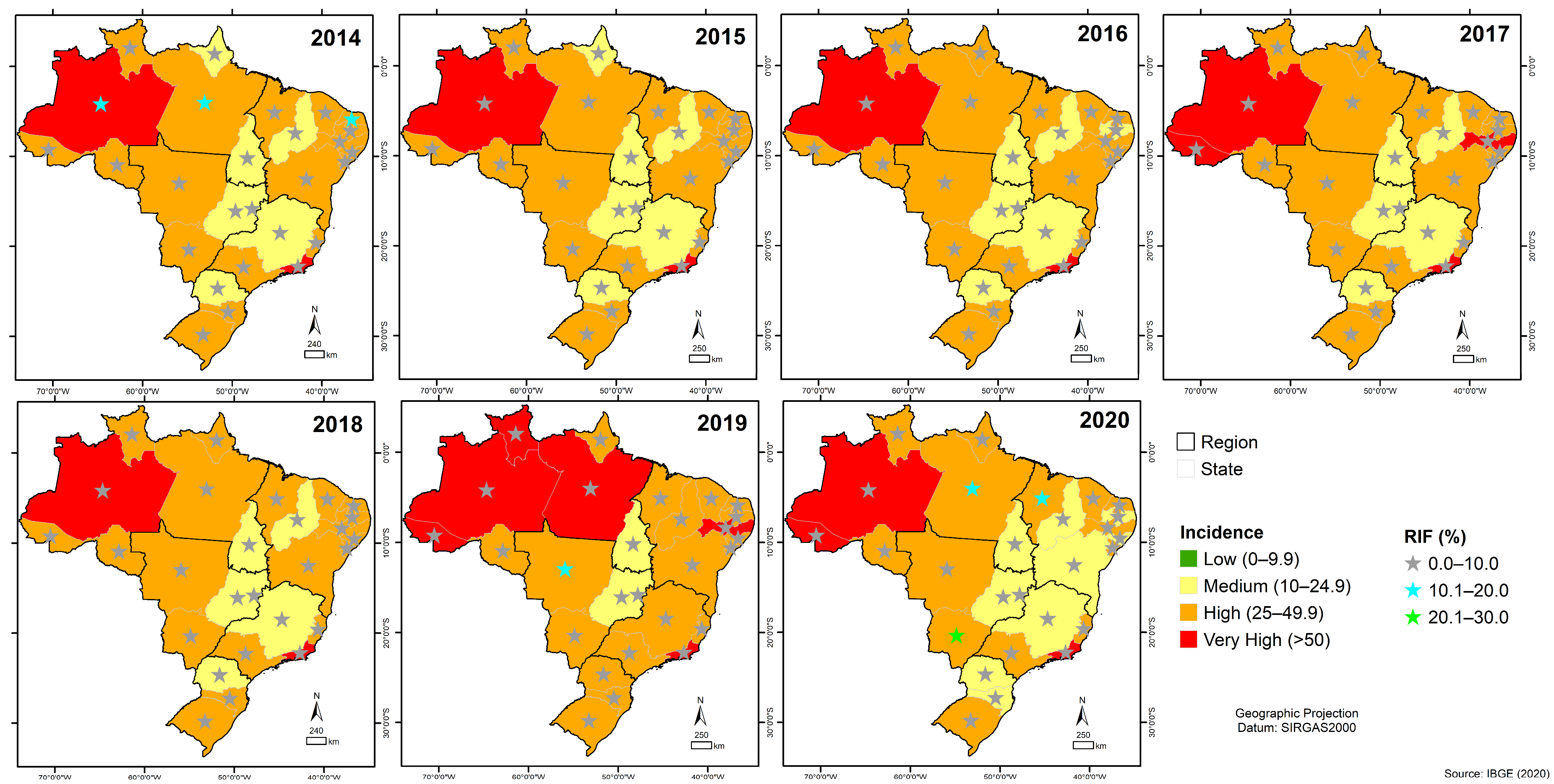
3. Results
3.1. Historical Background of GeneXpert® System in Brazil
3.2. The Interest of the Brazilian Ministry of Health in the Implantation of GeneXpert® System Equipment in SUS
3.3. Process for the Deployment of the GeneXpert® System Equipment in SUS
3.4. Authorization of the Use of GeneXpert® System Equipment by SUS
3.5. Start of Operation of GeneXpert® System Equipment in SUS
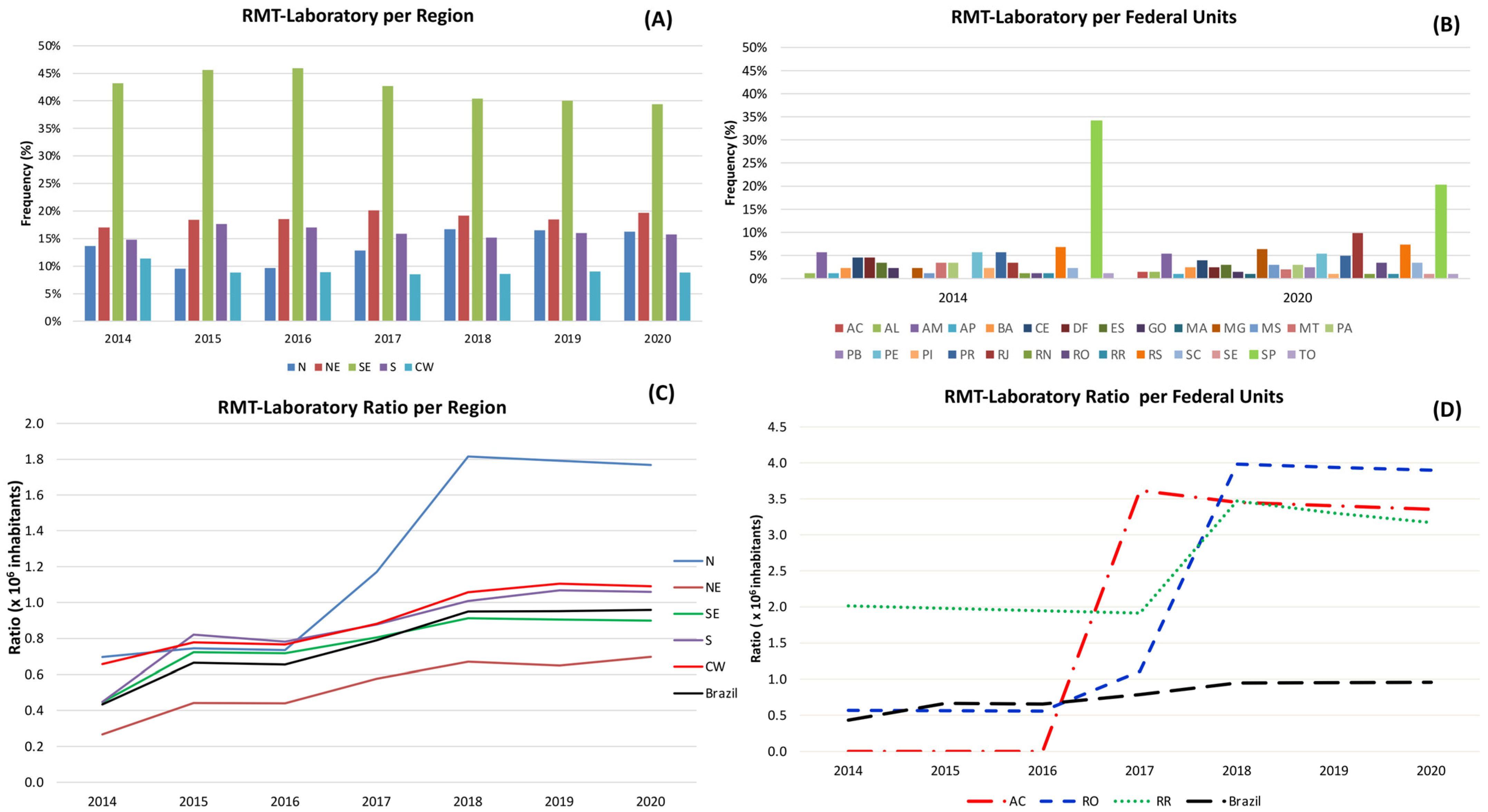
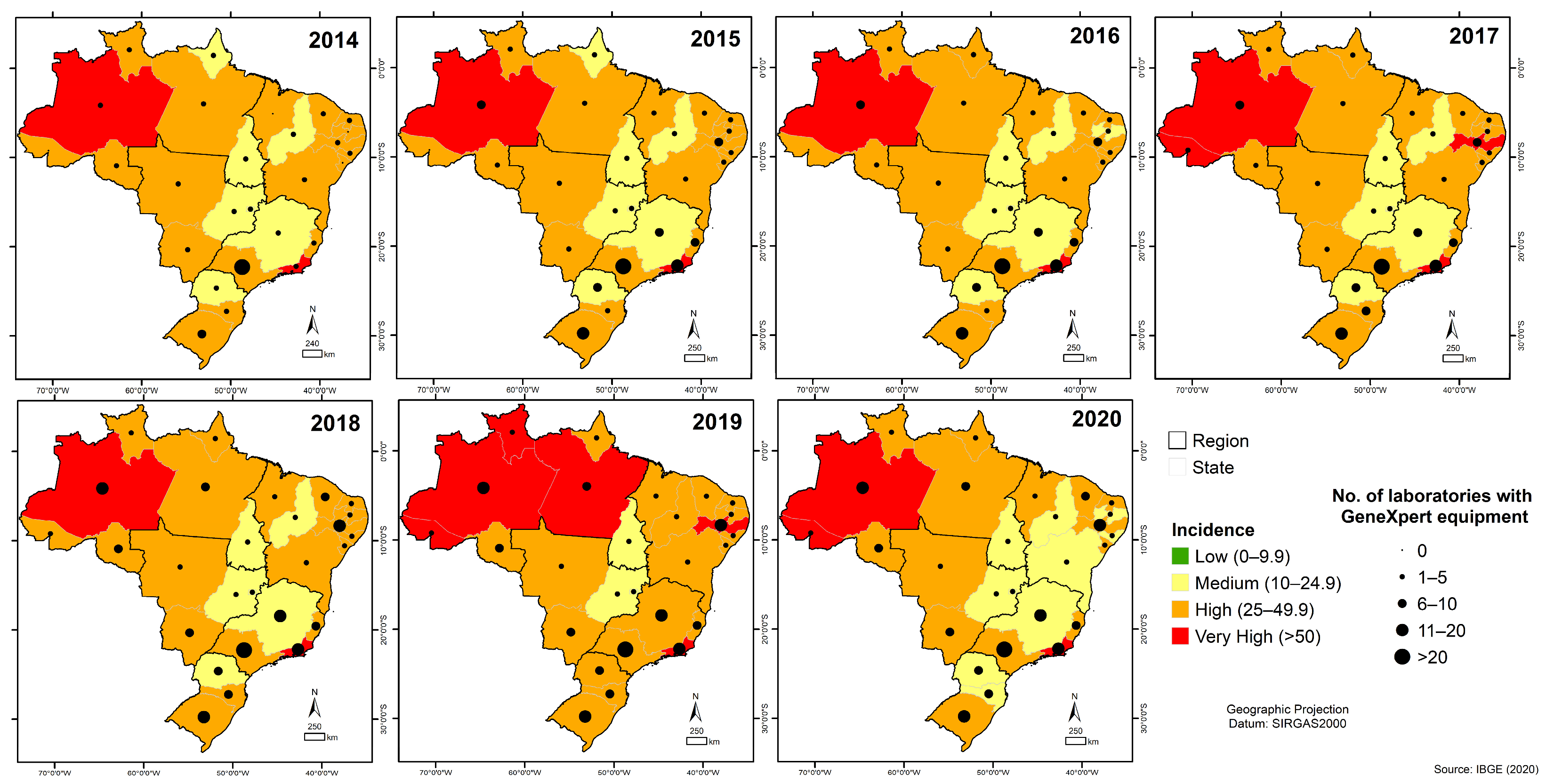
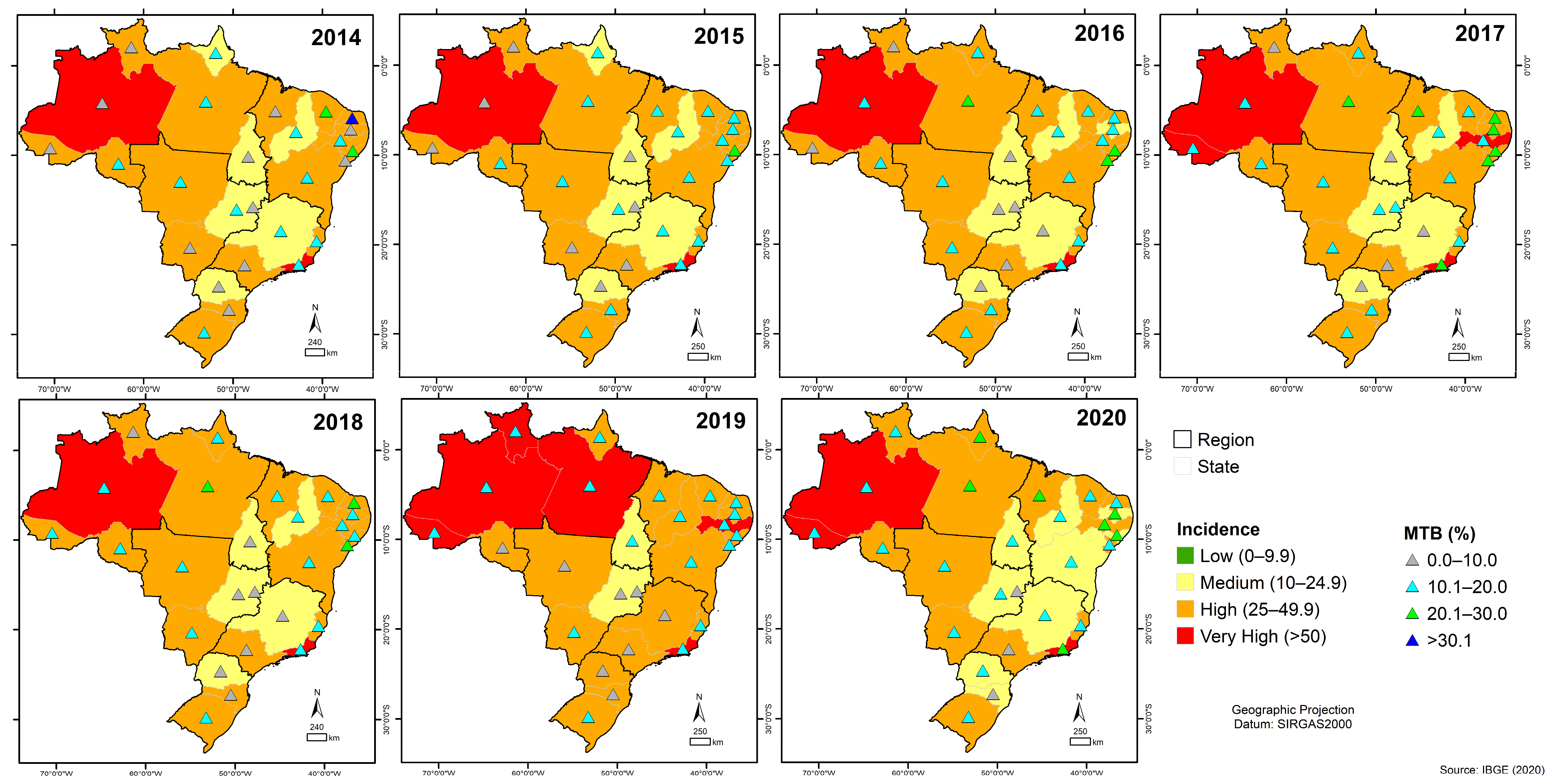
3.6. Quantitative Analysis of GeneXpert® System in Brazil
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, D.K.G.; Tandon, D.S.; Nagdeote, D.S.T.; Sharma, K.; Kumar, A.M.V. Role of CB-NAAT in Diagnosing Mycobacterial Tuberculosis and Rifampicin Resistance in Tubercular Peripheral Lymphadenopathy. Int. J. Med. Res. Rev. 2017, 5, 242–246. [Google Scholar] [CrossRef][Green Version]
- World Health Organization (WHO). Global Tuberculosis Report 2022; WHO: Geneva, Switzerland, 2023; p. 68. [Google Scholar]
- The Burden of Tuberculosis and Attributable Risk Factors in Brazil, 1990–2017: Results from the Global Burden of Disease Study 2017|Population Health Metrics|Full Text. Available online: https://pophealthmetrics.biomedcentral.com/articles/10.1186/s12963-020-00203-6 (accessed on 1 October 2023).
- Uplekar, M.; Weil, D.; Lonnroth, K.; Jaramillo, E.; Lienhardt, C.; Dias, H.M.; Falzon, D.; Floyd, K.; Gargioni, G.; Getahun, H.; et al. WHO’s New End TB Strategy; Elsevier: Amsterdam, The Netherlands, 2015; Volume 385, pp. 1799–1801. [Google Scholar]
- World Health Organization (WHO). Implementing the End TB Strategy: The Essentials; WHO: Geneva, Switzerland, 2015. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Rapid Communication: Molecular Assays as Initial Tests for the Diagnosis of Tuberculosis and Rifampicin Resistance; WHO: Geneva, Switzerland, 2020; p. 8. [Google Scholar]
- World Health Organization (WHO). Consolidated Guidelines on Tuberculosis—Module 2: Screening—Systematic Screening for Tuberculosis Disease—World; ReliefWeb; WHO: Geneva, Switzerland, 2021; Available online: https://reliefweb.int/report/world/who-consolidated-guidelines-tuberculosis-module-2-screening-systematic-screening (accessed on 1 October 2023).
- World Health Organization (WHO). Consolidated Guidelines on Tuberculosis: Module 3: Diagnosis—Tests for Tuberculosis Infection—World; ReliefWeb; WHO: Geneva, Switzerland, 2022; Available online: https://reliefweb.int/report/world/who-consolidated-guidelines-tuberculosis-module-3-diagnosis-tests-tuberculosis-infection (accessed on 1 October 2023).
- Brasil, Ministério Da Saúde. Rede de Teste Rápido para Tuberculose No Brasil: Primeiro Ano da Implantação; Ministério Da Saúde: Brasília, Brazil, 2015; ISBN 978-85-334-2342-8.
- Brasil, Ministério Da Saúde. OFÍCIO CIRCULAR No 7/2019/CGDR/.DCCI/SVS/MS—Atualização das Recomendações sobre o Diagnóstico Laboratorial da Tuberculose; Ministério da Saúde—Secretaria de Vigilância em Saúde: Brasília, Brazil, 2019; p. 9.
- De Almeida Ballestero, J.G.; Garcia, J.M.; Bollela, V.R.; Ruffino-Netto, A.; Dalcolmo, M.M.P.; Moncaio, A.C.S.; Miguel, N.S.; Rigolin, I.Z.; Palha, P.F. Management of Multidrug-Resistant Tuberculosis: Main Recommendations of the Brazilian Guidelines. J. Bras. Pneumol. 2020, 46, e20190290. [Google Scholar] [CrossRef] [PubMed]
- Brasil, Ministério da Saúde; Comissão Nacional de Incorporação de Tecnologias No SUS. Proposta de Incorporação Do Xpert Mtb/Rif como Teste Para Diagnóstico de Tuberculose e para Indicação de Resistência à Rifampicina; Ministério Da Saúde: Brasília, Brazil, 2013.
- Brasil, Ministério da Saúde. Boletim Epidemiológico da Tuberculose 2021; Ministério Da Saúde: Brasília, Brazil, 2021; p. 3:44.
- Durovni, B.; Saraceni, V.; van den Hof, S.; Trajman, A.; Cordeiro-Santos, M.; Cavalcante, S.; Menezes, A.; Cobelens, F. Impact of Replacing Smear Microscopy with Xpert MTB/RIF for Diagnosing Tuberculosis in Brazil: A Stepped-Wedge Cluster-Randomized Trial. PLoS Med. 2014, 11, e1001766. [Google Scholar] [CrossRef] [PubMed]
- Vassall, A.; van Kampen, S.; Sohn, H.; Michael, J.S.; John, K.R.; den Boon, S.; Davis, J.L.; Whitelaw, A.; Nicol, M.P.; Gler, M.T.; et al. Rapid Diagnosis of Tuberculosis with the Xpert MTB/RIF Assay in High Burden Countries: A Cost-Effectiveness Analysis. PLOS Med. 2011, 8, e1001120. [Google Scholar] [CrossRef]
- Pinto, M.; Entringer, A.P.; Steffen, R.; Trajman, A.; Pinto, M.; Entringer, A.P.; Steffen, R.; Trajman, A. Cost Analysis of Nucleic Acid Amplification for Diagnosing Pulmonary Tuberculosis, within the Context of the Brazilian Unified Health Care System. J. Bras. De Pneumol. 2015, 41, 536–538. [Google Scholar] [CrossRef]
- Durovni, B.; Saraceni, V.; Cordeiro-Santos, M.; Cavalcante, S.; Soares, E.; Lourenço, C.; Menezes, A.; van den Hof, S.; Cobelens, F.; Trajman, A. Operational Lessons Drawn from Pilot Implementation of Xpert MTB/Rif in Brazil. Bull. World Health Organ. 2014, 92, 613–617. [Google Scholar] [CrossRef]
- Dorman, S.E.; Schumacher, S.G.; Alland, D.; Nabeta, P.; Armstrong, D.T.; King, B.; Hall, S.L.; Chakravorty, S.; Cirillo, D.M.; Tukvadze, N.; et al. Xpert MTB/RIF Ultra for Detection of Mycobacterium Tuberculosis and Rifampicin Resistance: A Prospective Multicentre Diagnostic Accuracy Study. Lancet Infect. Dis. 2018, 18, 76–84. [Google Scholar] [CrossRef]
- Brasil, Ministério da Saúde. Teste Rápido Molecular para a Tuberculose Amplia Rede de Diagnóstico; Ministério Da Saúde: Brasília, Brazil, 2018.
- De Almeida, S.M.; Kussen, G.M.B.; Cogo, L.; Carvalho, J.H.; Nogueira, K. Diagnostic Characteristics of Xpert MTB/RIF Assay for the Diagnosis of Tuberculous Meningitis and Rifampicin Resistance in Southern Brazil. Arq. Neuropsiquiatr. 2020, 78, 700–707. [Google Scholar] [CrossRef]
- Aurilio, R.B.; Ferreira, S.; Parente, A.A.A.I.; Sant’Anna, M.D.F.P.; Pereira, C.S.; Malaquias, T.D.S.S.; Kritski, A.L.; Sant’Anna, C.C. Gene-Xpert Ultra for the Diagnosis of Extrapulmonary Tuberculosis in Children and Adolescents. Rev. Inst. Med. Trop. Sao Paulo 2022, 64, e12. [Google Scholar] [CrossRef]
- Oliveira, M.C.B.; Sant’Anna, C.C.; Luiz, R.R.; Soares, E.C.C.; Kritski, A.L. Contribution of Xpert MTB/RIF to Clinical Diagnosis in Adolescents with Tuberculosis in Rio de Janeiro, Brazil. Int. J. Tuberc. Lung Dis. 2019, 23, 1115–1121. [Google Scholar] [CrossRef]
- Azevedo, R.G.; Dinallo, F.S.; de Laurentis, L.S.; Boulware, D.R.; Vidal, J.E. Xpert MTB/RIF® Assay for the Diagnosis of HIV-Related Tuberculous Meningitis in São Paulo, Brazil. Int. J. Tuberc. Lung Dis. 2018, 22, 706–707. [Google Scholar] [CrossRef] [PubMed]
- Badal-Faesen, S.; Firnhaber, C.; Kendall, M.A.; Wu, X.; Grinsztejn, B.; da Silva Escada Escada, R.O.; Fernandez, M.; Hogg, E.; Sanne, I.; Johnson, P.; et al. Impact of Larger Sputum Volume on Xpert® MTB/RIF Assay Detection of Mycobacterium tuberculosis in Smear-Negative Individuals with Suspected Tuberculosis. J. Clin. Med. 2017, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- García-Basteiro, A.L.; DiNardo, A.; Saavedra, B.; Silva, D.R.; Palmero, D.; Gegia, M.; Migliori, G.B.; Duarte, R.; Mambuque, E.; Centis, R.; et al. Point of Care Diagnostics for Tuberculosis. Pulmonology 2018, 24, 73–85. [Google Scholar] [CrossRef] [PubMed]
- De Brito, G.M.X.; Mafort, T.T.; Ribeiro-Alves, M.; Reis, L.V.T.D.; Leung, J.; Leão, R.S.; Rufino, R.; Rodrigues, L.S. Diagnostic Performance of the Xpert MTB/RIF Assay in BAL Fluid Samples from Patients under Clinical Suspicion of Pulmonary Tuberculosis: A Tertiary Care Experience in a High-Tuberculosis-Burden Area. J. Bras. Pneumol. 2021, 47, e20200581. [Google Scholar] [CrossRef]
- De Camargo, K.R.; Guedes, C.R.; Caetano, R.; Menezes, A.; Trajman, A. The Adoption of a New Diagnostic Technology for Tuberculosis in Two Brazilian Cities from the Perspective of Patients and Healthcare Workers: A Qualitative Study. BMC Health Serv. Res. 2015, 15, 275. [Google Scholar] [CrossRef]
- De Castro, A.Z.; Moreira, A.R.; Oliveira, J.; Costa, P.A.; Graça, C.L.A.L.D.; Pérez, M.A.; Kritski, A.; Vater, M.C. Clinical Impact and Cost Analysis of the Use of Either the Xpert MTB Rif Test or Sputum Smear Microscopy in the Diagnosis of Pulmonary Tuberculosis in Rio de Janeiro, Brazil. Rev. Soc. Bras. Med. Trop. 2018, 51, 631–637. [Google Scholar] [CrossRef]
- Feliciano, C.S.; Menon, L.J.B.; Anselmo, L.M.P.; Dippenaar, A.; Warren, R.M.; Silva, W.A.; Bollela, V.R. Xpert MTB/RIF Performance to Diagnose Tuberculosis and Rifampicin Resistance in a Reference Centre in Southern Brazil. ERJ Open Res. 2019, 5, 00043-2019. [Google Scholar] [CrossRef]
- Hernandez, A.V.; de Laurentis, L.; Souza, I.; Pessanha, M.; Thota, P.; Roman, Y.M.; Barboza-Meca, J.; Boulware, D.R.; Vidal, J.E. Diagnostic Accuracy of Xpert MTB/RIF for Tuberculous Meningitis: Systematic Review and Meta-Analysis. Trop. Med. Int. Health 2021, 26, 122–132. [Google Scholar] [CrossRef]
- Iem, V.; Bimba, J.S.; Santos, V.S.; Dominguez, J.; Creswell, J.; Somphavong, S.; Wingfield, T.; Khan, J.A.M.; Cuevas, L.E. Pooling Sputum Testing to Diagnose Tuberculosis Using Xpert MTB/RIF and Xpert Ultra: A Cost-Effectiveness Analysis. BMC Infect. Dis. 2023, 23, 341. [Google Scholar] [CrossRef]
- Kritski, A.; Oliveira, M.M.; de Almeida, I.N.; Ramalho, D.; Andrade, M.K.N.; Carvalho, M.; Miranda, P.F.C.; Dalcolmo, M.P.; Braga, J.U.; Brígido, T.; et al. Clinical Impact of the Line Probe Assay and Xpert® MTB/RIF Assay in the Presumptive Diagnosis of Drug-Resistant Tuberculosis in Brazil: A Pragmatic Clinical Trial. Rev. Soc. Bras. Med. Trop. 2022, 55, e0191. [Google Scholar] [CrossRef]
- Lima, F.; Santos, A.S.; Oliveira, R.D.; Silva, C.C.R.; Gonçalves, C.C.M.; Andrews, J.R.; Croda, J. Oral Swab Testing by Xpert® MTB/RIF Ultra for Mass Tuberculosis Screening in Prisons. J. Clin. Tuberc. Other Mycobact. Dis. 2020, 19, 100148. [Google Scholar] [CrossRef] [PubMed]
- Luetkemeyer, A.F.; Firnhaber, C.; Kendall, M.A.; Wu, X.; Mazurek, G.H.; Benator, D.A.; Arduino, R.; Fernandez, M.; Guy, E.; Johnson, P.; et al. Evaluation of Xpert MTB/RIF Versus AFB Smear and Culture to Identify Pulmonary Tuberculosis in Patients with Suspected Tuberculosis From Low and Higher Prevalence Settings. Clin. Infect. Dis. 2016, 62, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Madico, G.; Mpeirwe, M.; White, L.; Vinhas, S.; Orr, B.; Orikiriza, P.; Miller, N.S.; Gaeddert, M.; Mwanga-Amumpaire, J.; Palaci, M.; et al. Detection and Quantification of Mycobacterium Tuberculosis in the Sputum of Culture-Negative HIV-Infected Pulmonary Tuberculosis Suspects: A Proof-of-Concept Study. PLoS ONE 2016, 11, e0158371. [Google Scholar] [CrossRef] [PubMed]
- Ocheretina, O.; Brandao, A.P.; Pang, Y.; Rodrigues, C.; Banu, S.; Ssengooba, W.; Dolinger, D.L.; Salfinger, M.; Ngabonziza, J.C.S.; Köser, C.U. Impact of the Bacillary Load on the Accuracy of Rifampicin Resistance Results by Xpert® MTB/RIF. Int. J. Tuberc. Lung Dis. 2021, 25, 881–885. [Google Scholar] [CrossRef]
- Pantoja, A.; Fitzpatrick, C.; Vassall, A.; Weyer, K.; Floyd, K. Xpert MTB/RIF for Diagnosis of Tuberculosis and Drug-Resistant Tuberculosis: A Cost and Affordability Analysis. Eur. Respir. J. 2013, 42, 708–720. [Google Scholar] [CrossRef]
- Pelissari, D.M.; Kuhleis, D.C.; Bartholomay, P.; Barreira, D.; Oliveira, C.L.P.; de Jesus, R.S.; Possa, L.A.; Jarczewski, C.A.; Nemeth, L.T.; de Araujo, N.D.; et al. Prevalence and Screening of Active Tuberculosis in a Prison in the South of Brazil. Int. J. Tuberc. Lung Dis. 2018, 22, 1166–1171. [Google Scholar] [CrossRef]
- Pereira, G.R.; Barbosa, M.S.; Dias, N.J.D.; de Almeida, C.P.B.; Silva, D.R. Impact of Introduction of Xpert MTB/RIF Test on Tuberculosis (TB) Diagnosis in a City with High TB Incidence in Brazil. PLoS ONE 2018, 13, e0193988. [Google Scholar] [CrossRef]
- De Matto Pires, M.; Pereira, G.R.; Barbosa, M.S.; Dias, N.J.D.; Secchi, C.; Hoff, J.S.; Silva, D.R. Association of Xpert MTB/RIF Cycle Threshold Values with Tuberculosis Treatment Outcomes. Lung 2020, 198, 985–989. [Google Scholar] [CrossRef]
- Pereira, G.R.; Barbosa, M.S.; Dias, N.J.D.; de Fraga dos Santos, F.; Rauber, K.A.; Silva, D.R. Evaluation of Xpert MTB/RIF Ultra Performance for Pulmonary Tuberculosis (TB) Diagnosis in a City with High TB Incidence in Brazil. Respir. Med. 2020, 162, 105876. [Google Scholar] [CrossRef]
- Pinto, M.; Steffen, R.E.; Cobelens, F.; van den Hof, S.; Entringer, A.; Trajman, A. Cost-Effectiveness of the Xpert® MTB/RIF Assay for Tuberculosis Diagnosis in Brazil. Int. J. Tuberc. Lung Dis. 2016, 20, 611–618. [Google Scholar] [CrossRef]
- Resende, M.R. How We Can Utilize the Xpert MTB/RIF Assay to Decide on Airborne Infection Isolation of Inpatients with Tuberculosis Suspicion in Brazil: A Brief Review of the Current Data. Braz. J. Infect. Dis. 2016, 20, 105–106. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Santos, A.; de Oliveira, R.D.; Lemos, E.F.; Lima, F.; Cohen, T.; Cords, O.; Martinez, L.; Gonçalves, C.; Ko, A.; Andrews, J.R.; et al. Yield, Efficiency, and Costs of Mass Screening Algorithms for Tuberculosis in Brazilian Prisons. Clin. Infect. Dis. 2021, 72, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.S.; Allgayer, M.F.; Kontogianni, K.; Rocha, J.E.; Pimentel, B.J.; Amorim, M.T.P.; Duarte, M.V.S.C.; de Melo Ferreira, P.; Moura, L.C.L.; de Lima, V.P.S.; et al. Pooling of Sputum Samples to Increase Tuberculosis Diagnostic Capacity in Brazil during the COVID-19 Pandemic. Int. J. Infect. Dis. 2023, 129, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Sieiro, T.L.D.A.; Aurílio, R.B.; Soares, E.C.C.; Chiang, S.S.; Sant Anna, C.C. The Role of the Xpert MTB/RIF Assay among Adolescents Suspected of Pulmonary Tuberculosis in Rio de Janeiro, Brazil. Rev. Soc. Bras. Med. Trop. 2018, 51, 234–236. [Google Scholar] [CrossRef]
- Silva, D.R.; Sotgiu, G.; D’Ambrosio, L.; Pereira, G.R.; Barbosa, M.S.; Dias, N.J.D.; Saderi, L.; Centis, R.; Migliori, G.B. Diagnostic performances of the Xpert MTB/RIF in Brazil. Respir. Med. 2018, 134, 12–15. [Google Scholar] [CrossRef]
- Da Silva, T.M.; Soares, V.M.; Ramos, M.G.; Santos, A.D. Accuracy of a Rapid Molecular Test for Tuberculosis in Sputum Samples, Bronchoalveolar Lavage Fluid, and Tracheal Aspirate Obtained from Patients with Suspected Pulmonary Tuberculosis at a Tertiary Referral Hospital. J. Bras. Pneumol. 2019, 45, e20170451. [Google Scholar] [CrossRef]
- Di Tanna, G.L.; Khaki, A.R.; Theron, G.; McCarthy, K.; Cox, H.; Mupfumi, L.; Trajman, A.; Zijenah, L.S.; Mason, P.; Bandason, T.; et al. Effect of Xpert MTB/RIF on Clinical Outcomes in Routine Care Settings: Individual Patient Data Meta-Analysis. Lancet Glob. Health 2019, 7, e191–e199. [Google Scholar] [CrossRef]
- De Oliveira Tomaz, A.P.; Raboni, S.M.; Kussen, G.M.B.; da Silva Nogueira, K.; Lopes Ribeiro, C.E.; Costa, L.M.D. The Xpert® MTB/RIF Diagnostic Test for Pulmonary and Extrapulmonary Tuberculosis in Immunocompetent and Immunocompromised Patients: Benefits and Experiences over 2 Years in Different Clinical Contexts. PLoS ONE 2021, 16, e0247185. [Google Scholar] [CrossRef]
- Trajman, A.; Durovni, B.; Saraceni, V.; Cordeiro-Santos, M.; Cobelens, F.; van den Hof, S. High Positive Predictive Value of Xpert in a Low Rifampicin Resistance Prevalence Setting. Eur. Respir. J. 2014, 44, 1711–1713. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Meeting Report of a Technical Expert Consultation: Non-Inferiority Analysis of Xpert MTB/RIF Ultra Compared to Xpert MTB/RIF. Available online: https://www.who.int/publications-detail-redirect/WHO-HTM-TB-2017.04 (accessed on 1 October 2023).
- Bonnet, M. Xpert MTB/RIF Ultra: What Is the Real Impact? Lancet 2020, 8, 325–326. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Use of Xpert MTB/RIF and Xpert MTB/RIF Ultra on GeneXpert 10-Colour Instruments—WHO Policy Statement. Available online: https://www.who.int/news/item/03-12-2021-use-of-xpert-mtb-rif-and-xpert-mtb-rif-ultra-on-genexpert-10-colour-instruments-who-policy-statement (accessed on 15 October 2023).
- Pillay, S.; Steingart, K.R.; Davies, G.R.; Chaplin, M.; De Vos, M.; Schumacher, S.G.; Warren, R.; Theron, G. Xpert MTB/XDR for Detection of Pulmonary Tuberculosis and Resistance to Isoniazid, Fluoroquinolones, Ethionamide, and Amikacin. Cochrane Database Syst. Rev. 2022, 5, CD014841. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). 2.1 TB Incidence. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022/tb-disease-burden/2-1-tb-incidence (accessed on 12 October 2023).
- Iúdice, T.N.S.; Conceição, M.L.; Brito, A.C.; Souza, N.M.; Mesquita, C.R.; Guimarães, R.J.P.S.; Furlaneto, I.P.; Saboia, A.S.; Lourenço, M.C.S.; Lima, K.V.B.; et al. Evaluation of GeneXpert® in Brazil Through a Historical and Epidemiological Approach. Res. Sq. 2022. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iúdice, T.N.d.S.; da Conceição, M.L.; de Brito, A.C.; de Souza, N.M.; Mesquita, C.R.; Guimarães, R.J.d.P.S.e.; Furlaneto, I.P.; Saboia, A.d.S.; Lourenço, M.C.d.S.; Lima, K.V.B.; et al. The Role of GeneXpert® for Tuberculosis Diagnostics in Brazil: An Examination from a Historical and Epidemiological Perspective. Trop. Med. Infect. Dis. 2023, 8, 483. https://doi.org/10.3390/tropicalmed8110483
Iúdice TNdS, da Conceição ML, de Brito AC, de Souza NM, Mesquita CR, Guimarães RJdPSe, Furlaneto IP, Saboia AdS, Lourenço MCdS, Lima KVB, et al. The Role of GeneXpert® for Tuberculosis Diagnostics in Brazil: An Examination from a Historical and Epidemiological Perspective. Tropical Medicine and Infectious Disease. 2023; 8(11):483. https://doi.org/10.3390/tropicalmed8110483
Chicago/Turabian StyleIúdice, Tirça Naiara da Silva, Marília Lima da Conceição, Artemir Coelho de Brito, Nicole Menezes de Souza, Cristal Ribeiro Mesquita, Ricardo José de Paula Souza e Guimarães, Ismari Perini Furlaneto, Alessandra de Souza Saboia, Maria Cristina da Silva Lourenço, Karla Valéria Batista Lima, and et al. 2023. "The Role of GeneXpert® for Tuberculosis Diagnostics in Brazil: An Examination from a Historical and Epidemiological Perspective" Tropical Medicine and Infectious Disease 8, no. 11: 483. https://doi.org/10.3390/tropicalmed8110483
APA StyleIúdice, T. N. d. S., da Conceição, M. L., de Brito, A. C., de Souza, N. M., Mesquita, C. R., Guimarães, R. J. d. P. S. e., Furlaneto, I. P., Saboia, A. d. S., Lourenço, M. C. d. S., Lima, K. V. B., & Conceição, E. C. (2023). The Role of GeneXpert® for Tuberculosis Diagnostics in Brazil: An Examination from a Historical and Epidemiological Perspective. Tropical Medicine and Infectious Disease, 8(11), 483. https://doi.org/10.3390/tropicalmed8110483






