Spatio-Temporal Determinants of Dengue Epidemics in the Central Region of Burkina Faso
Abstract
:1. Introduction
2. Materials and Methods
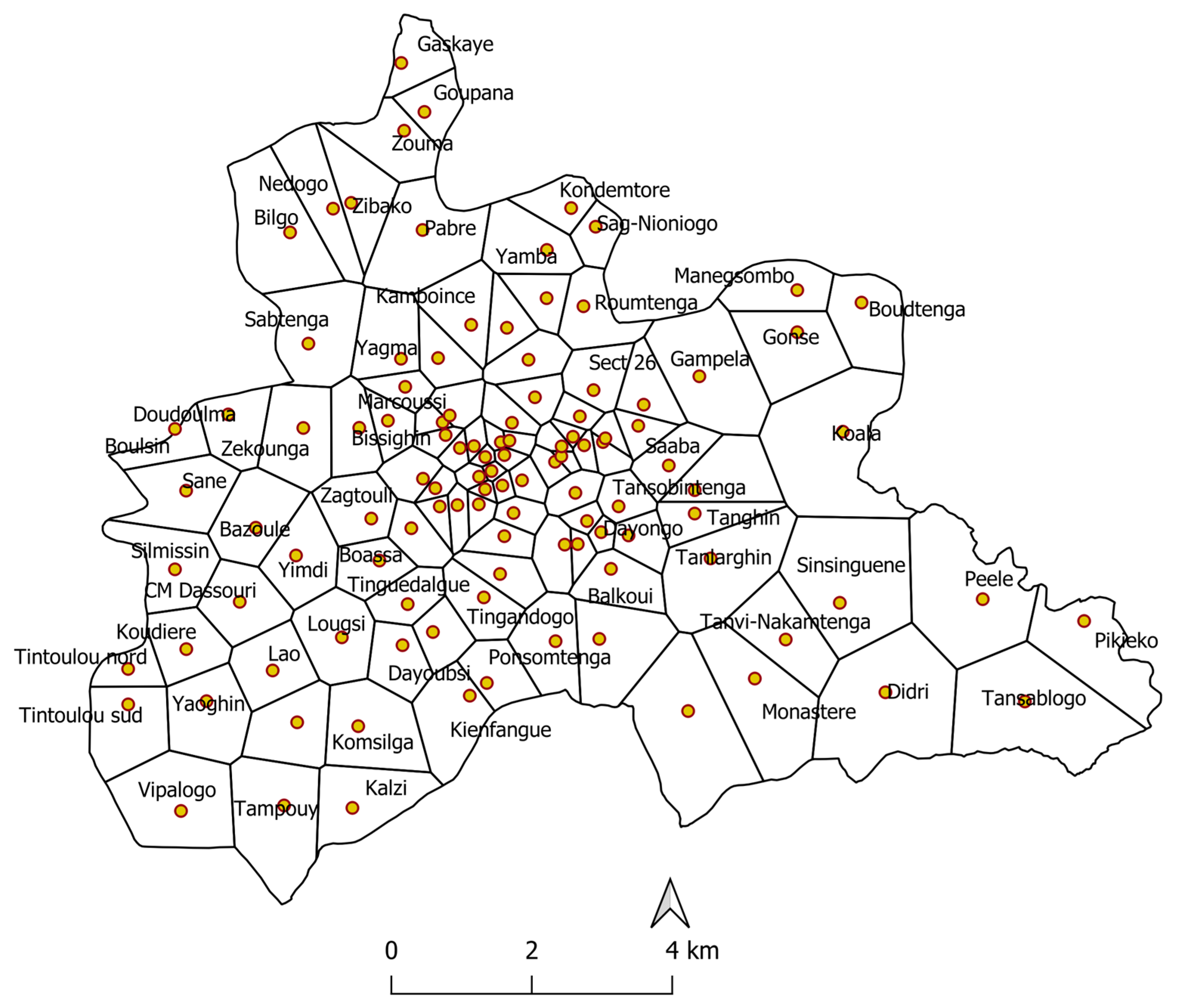
2.1. Study Site
2.2. Description of Data
- -
- Weekly cumulative rainfall (mm);
- -
- Weekly averages of daily minimum and maximum temperatures (°C);
- -
- Weekly averages of daily minimum and maximum relative humidity (%);
- -
- Monthly Normalized Difference Vegetation Index (NDVI);
- -
- Weekly averages of daily wind speed (m/s).
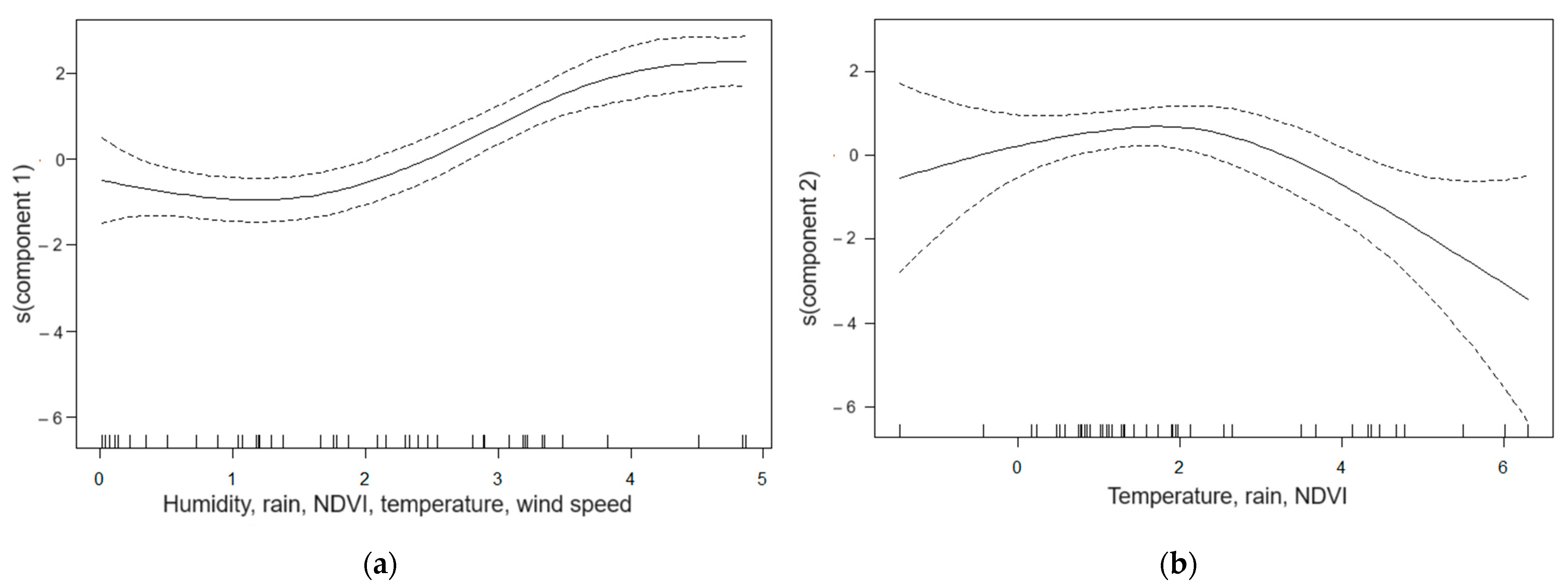
2.3. Data Analysis
3. Results
3.1. Incidence of Dengue Fever
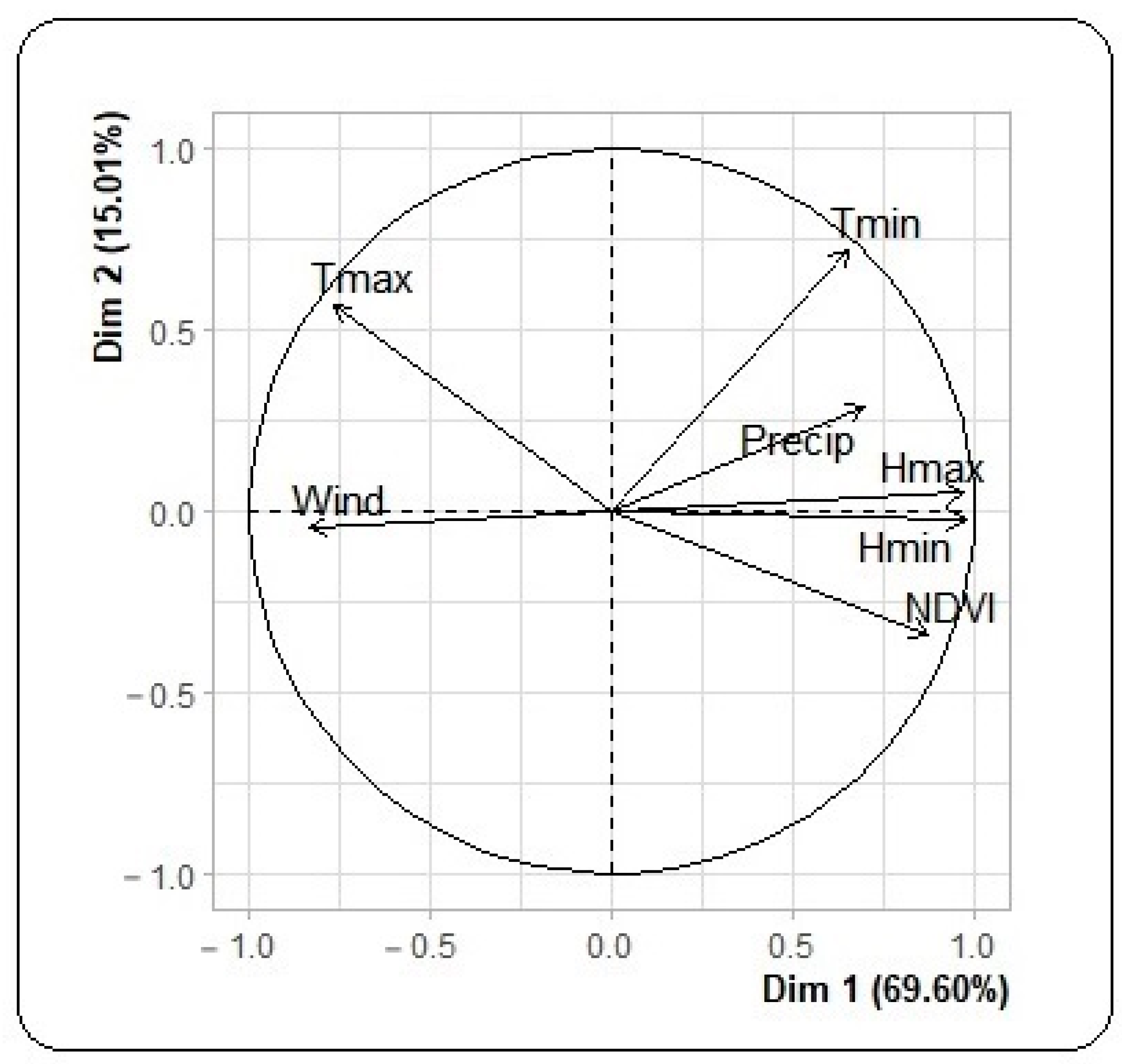
3.2. Meteorological, Environmental and Dengue Incidence Data
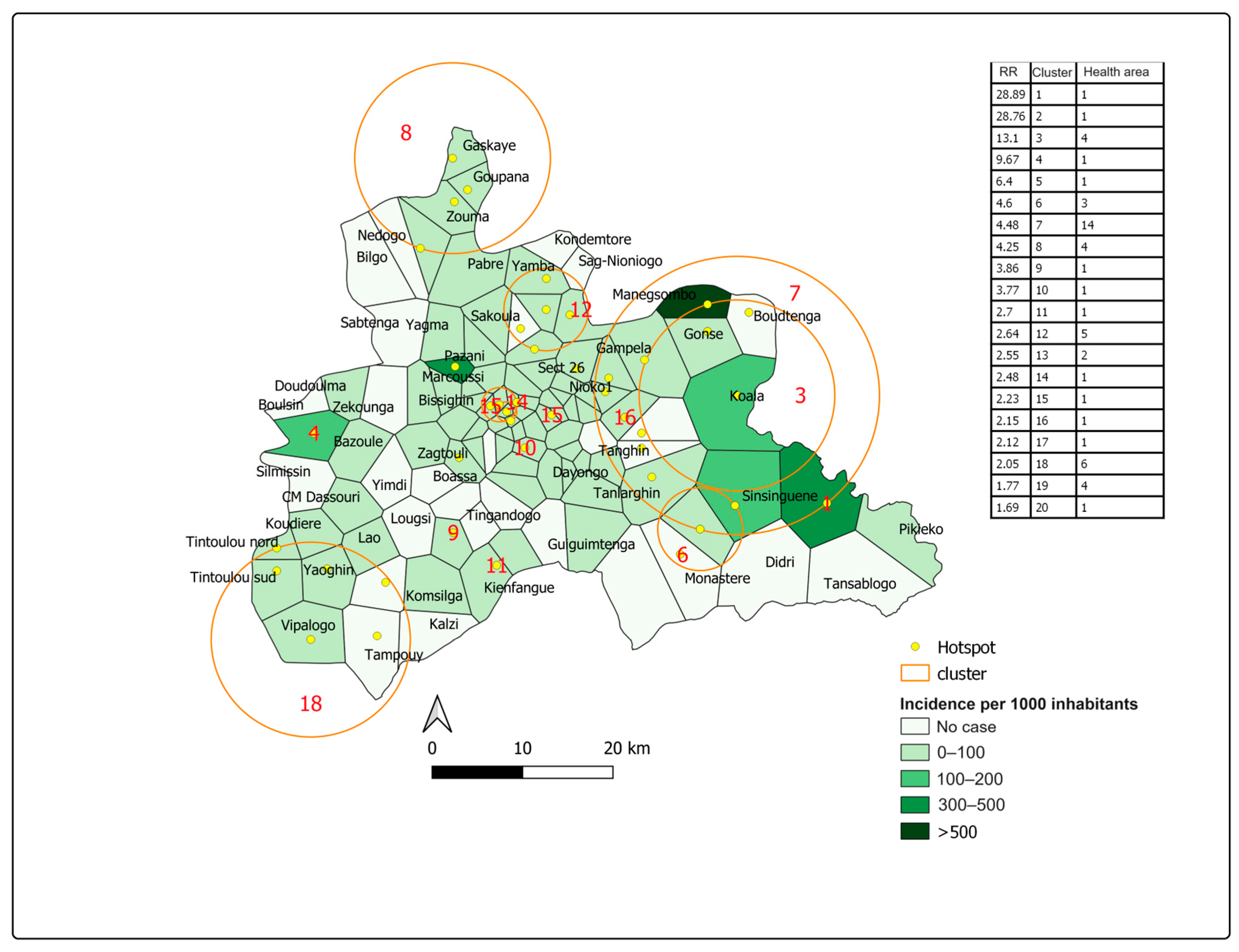
3.3. Spatial Distribution
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Dengue and Severe Dengue. Available online: https://www.who.int/fr/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 21 November 2019).
- World Health Organization. Emergency Preparations and Responses. The Dengue. Available online: https://www.who.int/csr/don/archive/disease/dengue_fever/fr/ (accessed on 21 November 2019).
- Naish, S.; Dale, P.; Mackenzie, J.S.; McBride, J.; Mengersen, K.; Tong, S. Climate change and dengue: A critical and systematic review of quantitative modelling approaches. BMC Infect. Dis. 2014, 14, 167. [Google Scholar] [CrossRef] [PubMed]
- European Center for Disease Prevention and Control. Aedes aegypti. Available online: https://www.ecdc.europa.eu/en/disease-vectors/facts/mosquito-factsheets/aedes-aegypti (accessed on 21 November 2019).
- Phanitchat, T.; Zhao, B.; Haque, U.; Pientong, C.; Ekalaksananan, T.; Aromseree, S.; Thaewnongiew, K.; Fustec, B.; Bangs, M.J.; Alexander, N. Spatial and temporal patterns of dengue incidence in northeastern Thailand 2006–2016. BMC Infect. Dis. 2019, 19, 743. [Google Scholar] [CrossRef] [PubMed]
- Béatrice Menard. Fondements Géographiques de la Dengue, décomposition et Recomposition Spatiales. Available online: https://www.persee.fr/doc/caoum_03735834_2001_num_54_215_3817 (accessed on 21 November 2019).
- Pierre Carnevale et Vincent Robert. Les Anophèles. Available online: https://books.openedition.org/irdeditions/10386?lang=fr (accessed on 21 November 2019).
- Abreu, F.V.S.; Morais, M.M.; Ribeiro, S.P.; Eiras, A.E. Influence of breeding site availability on the oviposition behavior of Aedes aegypti. Mem. Inst. Oswaldo Cruz. 2015, 110, 669–676. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The Fight against Vector. Available online: https://apps.who.int/iris/bitstream/handle/10665/42211/9242544949_fr.pdf?sequence=1&isAllowed=y (accessed on 21 November 2019).
- West African Health Organization. Annual Report 2018. Available online: https://www.wahooas.org/web-ooas/fr/publications-et-recherches/rapports-annuels (accessed on 21 November 2019).
- West African Health Organization. Dengue in Burkina Faso. Available online: https://www.who.int/csr/don/18-november-2016-dengue-burkina-faso/fr/ (accessed on 21 November 2019).
- Ministry of Health. Outbreak of Dengue Cases in Burkina Faso, Situation Report No. 26 of 26 October 2017. Available online: https://reliefweb.int/sites/reliefweb.int/files/resources/sitrep_dengue_ndeg26_2017.pdf (accessed on 21 November 2019).
- Ministry of Territorial Administration and Decentralization. Statistical Directory of Decentralization 2016. Available online: http://cns.bf/IMG/pdf/annuaire_statistique_decentralisation-2016_vf2.pdf (accessed on 21 November 2019).
- Jere, S.; Moyo, E. Modelling epidemiological data using Box–Jenkins procedure. Open J. Stat. 2016, 6, 295–302. [Google Scholar] [CrossRef]
- Metcalfe, A.V.R.; Cowpertwait, P.S.P. (Eds.) Introductory Time Series; Springer: New York, NY, USA, 2009. [Google Scholar]
- Shumway, R.H.; Stoffer David, S. Time Series Analysis and Its Applications; Springer: New York, NY, USA, 2011. [Google Scholar]
- Wood, S.R. (Ed.) Generalized Additive Models; Chapman & Hall/CRC: Boca Raton, FL, USA, 2006. [Google Scholar]
- Gaudart, J.; Giorgi, R.; Poudiougou, B.; Touré, O.; Ranque, S.; Doumbo, O.; et Demonjot, J. Détection de clusters spatiaux sans point source prédéfini: Utilisation de cinq méthodes et comparaison de leurs résultats. Rev. Epidemiol. Santé Publique. 2007, 55, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Bambrick, H.; Yakob, L.; Devine, G.; Lu, J.; Frentiu, F.D.; Yang, W.; Williams, G.; Hu, W. Spatiotemporal patterns and climatic drivers of severe dengue in Thailand. Sci. Total Environ. 2019, 656, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Tam, T.Y.T.; Chern, Y.R.; Lung, S.C.; Chen, N.T.; Wu, C.D. Spatial clustering of dengue fever incidence and its association with surrounding greenness. Int. J. Environ. Res. Public Health 2018, 15, 1869. [Google Scholar] [CrossRef] [PubMed]
- Earnest, A.; Tan, S.B.; Wilder-Smith, A. Meteorological factors and El Nino Southern Oscillation are independently associated with dengue infections. Epidemiol. Infect. 2012, 140, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Hii, Y.L.; Rocklov, J.; Ng, N.; Tang, C.S.; Pang, F.Y.; Sauerborn, R. Climate variability and increase in intensity and magnitude of dengue incidence in Singapore. Glob. Health Action 2009, 2, 2036. [Google Scholar] [CrossRef] [PubMed]
- Focks, D.A.; Haile, D.G.; Daniels, E.; Mount, G.A. Dynamic life table model for Aedes aegypti (Diptera: Culicidae): Analysis of the literature and model development. J. Med. Entomol. 1993, 30, 1003–1017. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, L.; Paaijmans, K.P.; Fansiri, T.; Carrington, L.B.; Kramer, L.D.; Thomas, M.B.; Scott, T.W. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc. Natl. Acad. Sci. USA 2011, 108, 7460–7465. [Google Scholar] [CrossRef] [PubMed]
- Watts, D.M.; Burke, D.S.; Harrison, B.A.; Whitmire, R.E.; Nisalak, A. Effect of temperature on the vector efficiency of Aedes aegypti for dengue 2 virus. Am. J. Trop. Med. Hyg. 1987, 36, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Carrington, L.B.; Armijos, M.V.; Lambrechts, L.; Scott, T.W. Fluctuations at a low mean temperature accelerate dengue virus transmission by Aedes aegypti. PLoS Negl. Trop. Dis. 2013, 7, 2190. [Google Scholar] [CrossRef] [PubMed]
- Junxiong, P.; Yee-Sin, L. Clustering, climate and dengue transmission. Expert Rev. Anti Infect. Ther. 2015, 1, 10. [Google Scholar] [CrossRef]
- Zheng, L.; Ren, H.Y.; Shi, R.H.; Lu, L. Spatiotemporal characteristics and primary influencing factors of typical dengue fever epidemics in China. Infect. Dis. Poverty 2019, 8, 24. [Google Scholar] [CrossRef]
- Ouattara, C.A.; Traore, S.; Sangare, I.; Traore, T.I.; Meda, Z.C.; Savadogo, L.G.B. Spatiotemporal analysis of dengue fever in Burkina Faso from 2016 to 2019. BMC Public Health 2022, 22, 462. [Google Scholar] [CrossRef] [PubMed]
- Ouattara, C.A.; Traore, T.I.; Traore, S.; Sangare, I.; Meda, C.Z.; Savadogo, L.G.B. Climate factors and dengue fever in Burkina Faso from 2017 to 2019. J. Public Health Afr. 2022, 13, 2145. [Google Scholar] [CrossRef] [PubMed]


| Data | Temporal Unit | Spatial Unit | Source |
|---|---|---|---|
| Dengue case | Week | Health area | Ministry of Health |
| Population | Year | Health area | Ministry of Health |
| Geographic coordinates and area | Year | Health area | Ministry of Health |
| Average wind speed | Week | Central region | NASA 1 https://giovanni.gsfc.nasa.gov/ (accessed on 24 June 2020) |
| Cumulative rainfall | Week | Central region | |
| Average minimum and maximum temperature | Week | Central region | |
| Average minimum and maximum relative humidity | Week | Central region | |
| Normalized Difference Vegetation Index | Month | Central region | |
| Population density (inhabitants/km2) | Year | Health area | |
| Road density (km/km2) | Year | Health area | OpenStreetMap (http://download.geofabrik.de/ (accessed on 24 June 2020)) |
| Density of vegetation cover (km2/km2) | Year | Health area |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouattara, C.A.; Traore, T.I.; Ouedraogo, B.; Sylla, B.; Traore, S.; Meda, C.Z.; Sangare, I.; Savadogo, L.B.G. Spatio-Temporal Determinants of Dengue Epidemics in the Central Region of Burkina Faso. Trop. Med. Infect. Dis. 2023, 8, 482. https://doi.org/10.3390/tropicalmed8110482
Ouattara CA, Traore TI, Ouedraogo B, Sylla B, Traore S, Meda CZ, Sangare I, Savadogo LBG. Spatio-Temporal Determinants of Dengue Epidemics in the Central Region of Burkina Faso. Tropical Medicine and Infectious Disease. 2023; 8(11):482. https://doi.org/10.3390/tropicalmed8110482
Chicago/Turabian StyleOuattara, Cheick Ahmed, Tiandiogo Isidore Traore, Boukary Ouedraogo, Bry Sylla, Seydou Traore, Clement Ziemle Meda, Ibrahim Sangare, and Leon Blaise G. Savadogo. 2023. "Spatio-Temporal Determinants of Dengue Epidemics in the Central Region of Burkina Faso" Tropical Medicine and Infectious Disease 8, no. 11: 482. https://doi.org/10.3390/tropicalmed8110482
APA StyleOuattara, C. A., Traore, T. I., Ouedraogo, B., Sylla, B., Traore, S., Meda, C. Z., Sangare, I., & Savadogo, L. B. G. (2023). Spatio-Temporal Determinants of Dengue Epidemics in the Central Region of Burkina Faso. Tropical Medicine and Infectious Disease, 8(11), 482. https://doi.org/10.3390/tropicalmed8110482






