Abstract
The objective of this study was to evaluate ecological aspects of Mansonia species before the construction of hydroelectric plants on the Madeira River, and thus enable the assessment of the impact of these projects on mosquitoes. A total of 199 samplings were carried out between November 2003 and August 2004, using the technique of attraction with protection. Temporal distribution was evaluated from monthly incidence values obtained from the bite index per man/hour. Relative abundance was subsequently calculated to evaluate the spatial distribution of species, according to land use and municipal districts; furthermore, the pattern of hematophagous activity was evaluated from 12-h and 4-h samplings. The data were analyzed according to the negative binomial distribution and generalized linear models to estimate the influence of environmental factors on the presence and abundance of Mansonia. A total of 1479 specimens were collected, distributed among four species—Mansonia titillans (87%), Mansonia humeralis (6.3%), Mansonia amazonensis (6%), and Mansonia indubitans (0.5%), and spatial distribution analysis showed Ma. titillans to be dominant. Hematophagous activity had peaks between 6:00 p.m. and 8:00 p.m. and species incidence was higher during the rainy season and in areas where domestic animals are raised. Therefore, the region studied presented characteristics favorable to the reproduction of Mansonia even before the construction of the hydroelectric plants and after construction, these conditions were enhanced, due to the increase in the availability of breeding sites for immatures and blood sources for females, as a consequence of changes in the environment.
1. Introduction
Mosquitoes (Culicidae) transmit the pathogens that cause malaria, yellow fever, dengue, Zika, and chikungunya, among others, resulting in a high number of human deaths and hospitalizations in different regions of the world [1]. The mosquito genus Mansonia of the subfamily Culicinae has two subgenera: Mansonioides Theobald, 1907 consisting of ten oriental and two Ethiopian species, and Mansonia Blanchard, 1901 consisting of fifteen Neotropical species [2,3,4,5].
At least twelve species of Mansonia have been recorded in Brazil, of which six are present in the Amazon region—Mansonia amazonensis (Theobald), Mansonia flaveola (Coquillett), Mansonia humeralis Dyar & Knab, Mansonia indubitans Dyar & Shannon, Mansonia pseudotitillans (Theobald), and Mansonia titillans (Walker) [3,4,5,6,7]—although molecular studies suggest that the diversity is greater [8,9]. Species of Mansonia are often found in forest environments, but they can also occur in urbanized areas [10]. Females are aggressive and voracious during hematophagy, with preferential nocturnal activity [10,11].
The epidemiological importance of Mansonia is related to the participation of some species in the transmission cycle of arboviruses and filariasis [12,13,14,15,16,17,18,19]. Furthermore, a new virus of the family Tymoviridae was recently isolated from Mansonia specimens collected in the city of Porto Velho; nonetheless, few studies have assessed the medical importance of these mosquitoes in Brazil [20]. Recently, De Sousa et al. (2023) [21] carried out an investigative study to evaluate the distribution of arboviruses in Mansonia humeralis and reported 34 positive pools for the Mayaro virus (MAYV), obtained from females captured in the district of Jaci Paraná, municipality of Porto Velho, in the region known as the Madeira River hydroelectric complex.
Uncontrolled reproduction of species of Mansonia is generally associated with the imbalance of aquatic flora in areas influenced by hydroelectric projects [5,20]. Macrophytes serve as reproduction sites in these areas, as they provide oxygen for the respiration of larvae and pupae [22,23,24]. This sequence of phenomena occurred in the area influenced by the Tucuruí hydroelectric plant in the state of Pará, with a marked population growth of species of Mansonia during the post-construction phase, when each collector captured 600 mosquitoes per hour [11].
In the Amazon, the presence of lakes with macrophytes in floodplain areas, associated with changes in land use and with the raising of domestic animals on farms, farmstead, and rural villages, contributes to the proliferation of Mansonia species. This is because there is an increase in the availability of blood sources for females, favoring the reproduction of these mosquitoes in the environment. Some species that are generalists in capturing blood for food, such as Ma. titillans and Ma. humeralis, benefit from these landscapes, as they consume the blood of domesticable animals such as cattle, horses, goats, birds, and also humans [25]. These interrelations are present in the areas covered by hydroelectric plants and are important for understanding the spatial distribution and the dispersion capacity of species in the environment [26].
The record of the relationship between Mansonia species and areas of influence of hydroelectric projects, first observed in Tucuruí, boosted several studies in the region of the so-called Madeira River hydroelectric complex, in order to understand different aspects related to taxonomy, vector competence of the species, seasonality, characteristic breeding grounds for immature forms, and dispersal patterns of Mansonia species, mostly carried out in the post-construction phase of the hydroelectric dams [8,9,20,21,22,26,27,28]. In this study, we approach pioneering data from the pre-construction phase of the Jirau and Santo Antonio hydroelectric plants, evaluating ecological aspects, space–time distribution, patterns of hematophagous activity, and the relationships of the species with the characteristics of the local landscape. This information can be used to design efficient population control measures and evaluate possible impacts generated from the construction of hydroelectric plants in the region. The present study, therefore, aimed to evaluate bioecological aspects of species of the subgenus Mansonia (Mansonia) in the region of the upper Madeira River, before the construction of the Jirau and Santo Antônio hydroelectric power plants.
2. Material and Methods
2.1. Study Area
The study took place in the state of Rondonia, which occupies 23.7 million hectares, representing 4.7% of the Legal Amazon. The climate is classified as Aw [29], tropical rainy, with an average annual temperature of 25 °C (20.7–31.5 °C), a relatively dry season and a rainy season, with marked rains between September and May, for a total annual rainfall of above 2000 mm [30]. The study was carried out in the districts of Porto Velho (capital), Jaci Paraná, Mutum Paraná, and Abunã, located in northern Rondônia. The population size of municipality in 2019 was estimated at 52,9544 inhabitants [31]. The districts of Porto Velho (capital) and Jaci Paraná are located in the area of influence of the Santo Antônio hydroelectric plant, while the districts of Mutum Paraná and Abunã are located in that of the Jirau hydroelectric plant (Figure 1). The district of Mutum Paraná was relocated after the filling of the reservoir, which took place in 2013, forming the village of Nova Mutum Paraná, located further downstream.
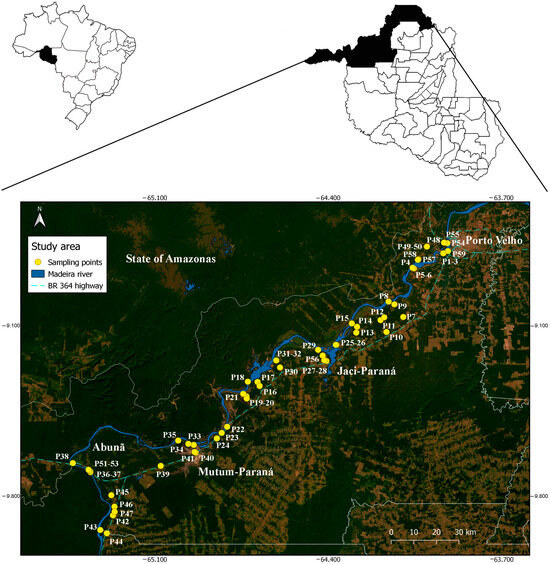
Figure 1.
Mosquito sampling points in the upper Madeira River region, Porto Velho, Rondonia, Brazil.
2.2. Mosquito Sampling
A total of 199 samplings were carried out at 59 points, according to land use, namely: Farm—places with cattle ranching (26 points); Periurban zone (5 points); Farmstead—places without cattle ranching (15 points); and Rural village (13 points). Sampling in peridomiciliary environments took place during monthly campaigns, carried out as follows: 1—November–December (2003); 2 to 8—February to August (2004)—totaling eight collection periods; most sampling points were located on the right bank of the Madeira River, where there is great anthropic pressure and several dwellings (Figure 2). For this, six field collectors were needed, performing six points visited per night. The collectors did not have a fixed point for sampling, that is, they were randomly distributed throughout the area; each collector remained at the sampling point for four consecutive hours (6:00 p.m. to 10:00 p.m.), and four 12-h collections were carried out, two in the rainy season and two in the dry season (6:00 p.m. to 6:00 a.m.). Differentiated times were established to evaluate the hematophagic activity patterns of Mansonia species.
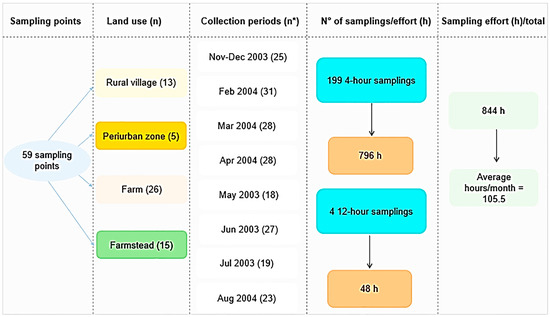
Figure 2.
Sampling design used to evaluate the bioecological aspects of Mansonia species, in the upper Madeira River region, Porto Velho, Rondonia, Brazil (* number of nights).
Adult specimens were acquired using the human landing catches (HLC) with protection and electronic entomological aspirators for capture (INPA—Ethics Committee—Opinion N° 3.474.088). Mosquitoes attracted by human odor (kairomone) were aspirated and transferred to paraffin cups with the help of a Castro capturer. The team involved in the sampling wore personal protective equipment that consisted of black socks, long denim pants, long-sleeved shirts, closed shoes, and a hat. Sampled mosquitoes were transported to the Laboratório de Malária e Dengue, of the Instituto Nacional de Pesquisas da Amazônia—INPA, and identified using the key of Forattini (2002) [32] and the descriptions of Barbosa (2007) [3]. Specimens were preserved following Belkin (1967) [33] and Forattini (1962) [34] and deposited in the INPA Invertebrate Collection.
2.3. Data Analysis
Monthly relative abundances of species of Mansonia were calculated to determine the species composition of the studied area. The temporal distribution of species was evaluated by calculating the bite rate per man/hour, obtained from the ratio between the number of mosquitoes collected (Nm), number of collectors (NC), and number of collection hours (NH) [35]. Sampling point data were used to evaluate the spatial distribution of the species according to district (Porto Velho, Jaci Paraná, Mutum Paraná and Abunã), in the area of the Madeira River. The pattern of hematophagous activity of species of Mansonia was evaluated considering collections of 12 (6:00 p.m. to 6:00 a.m.) and 4 (6:00 p.m. to 10:00 p.m.) consecutive hours. These hourly collections were essential to know the peaks of greater hematophagic activity, as the mosquitoes were quantified according to the time of capture. The information was recorded in field spreadsheets.
The sampling points were properly characterized according to the distance from the water (m) and the forest (m), number of residents, land use, and animal husbandry (goat, horse, cat, dog, chicken, and duck); this information was completed in field worksheets and subsequently inserted into the database. Subsequently, the data were subjected to negative binomial distribution analysis to assess the presence/absence of Mansonia mosquitoes based on the following predictors—distance from water (m), distance from forest (m), number of residents, land use, and livestock of animals. Initially, the data were analyzed using the Pascal distribution, through the negative binomial model, aiming to evaluate the influence of the predictors on the presence of Mansonia species. Afterwards, the data were submitted to analysis of Generalized Linear Models, with a Poisson distribution model, to evaluate the influence of the predictors on the abundance of Mansonia. The analyses were performed with the aid of the R software (version 4.0.2), “glm” function of the “gamlss” package.
3. Results
A total of 1479 specimens distributed in four species of Mansonia were collected: Ma. amazonensis, Ma. humeralis, Ma. indubitans and Ma. titillans. The most abundant species was Ma. titillans (86.6%), followed by Ma. humeralis (6.5%), Ma. amazonensis (6.2%), and Ma. indubitans (0.5%). There were specimens that could not be identified at a specific level, which were denominated as Mansonia spp. (0.2%). According to bite rate per man/hour, the incidence of Ma. indubitans ranged from 0 to 0.012, being highest in February, while that of Ma. amazonensis ranged from 0.006 to 0.1, being highest in June. The incidence of Ma. humeralis ranged from 0.025 to 0.055, while that of Ma. titillans ranged from 0.24 to 1, both being highest in July (Table 1). In the month of August, no specimens of Mansonia were captured.

Table 1.
Bite rate per man/hour and abundance (n) of species of Mansonia in the region of the upper Madeira River, prior to the construction of the Santo Antônio and Jirau hydroelectric plants, in Porto Velho, Rondônia, Brazil. RA = relative abundance.
The spatial distribution data show that Ma. titillans was widely dispersed among the four districts analyzed (Porto Velho 90%, Jaci Paraná 89%, Mutum Paraná 95%, and Abunã 78%), and present in the periurban area of the city of Porto Velho. On the other hand, Ma. humeralis occurred at low abundance in the periurban region (2%) but with increased abundance in the most distant points of the city (Abunã 15%). Mansonia amazonensis had higher abundance near the urban perimeter of Porto Velho, and lower in the other districts, while Ma. indubitans had low abundance in all the analyzed districts (Figure 3).
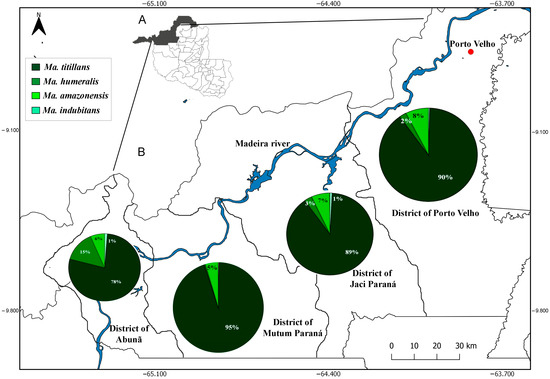
Figure 3.
Spatial distribution of species of Mansonia in the upper Madeira River region (A), according to the municipal districts of Porto Velho (B), Rondonia, Brazil.
The 12-h peridomicile sampling revealed that Ma. titillans had higher activity between 6:00 p.m. and 7:00 p.m., with a decrease in later hours. On the other hand, hematophagous activity of this species was observed in the interval between 3:00 and 4:00 a.m. The observed behavior for Ma. amazonensis was similar to that of Ma. titillans, with greater hematophagous activity between 6:00 and 7:00 p.m., but not extending beyond 8:00 to 9:00 p.m. Mansonia humeralis also showed hematophagous activity between 6:00 and 7:00 p.m., extending to the interval 2:00 to 3:00 a.m. Hematophagous activity of Ma. indubitans was only recorded between 6:00 and 7:00 p.m. (Figure 4a). The four-hour sampling revealed a peak of hematophagous activity for Ma. amazonensis between 6:00 and 7:00 p.m., with a decrease in later intervals. In contrast, peak hematophagous activity for Ma. titillans and Ma. humeralis was between 7:00 and 8:00 p.m., with a decrease in subsequent intervals. Mansonia indubitans had low hematophagous activity in all temporal intervals (Figure 4b–d).
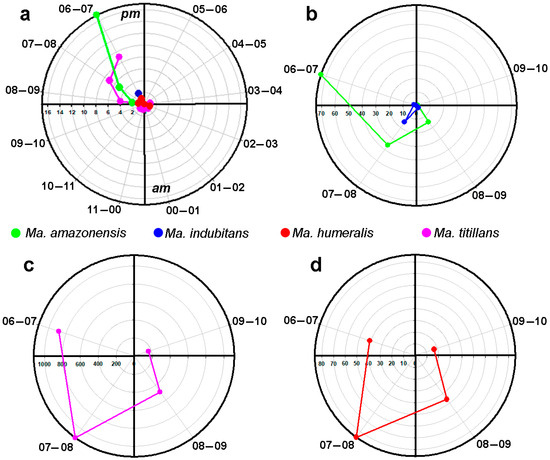
Figure 4.
Hematophagous activity of species of Mansonia collected in the upper Madeira River region, Porto Velho, Rondonia, Brazil, (a) 12 h of collection; (b–d) 4 h of collection.
Pascal’s distribution analysis indicated that among the analyzed predictors, the periurban area, the presence of horses and ducks were considered important for the presence of Mansonia mosquitoes (Figure 5A). On the other hand, Ma. titillans was present mainly in those places where horses and ducks were maintained or raised (Figure 5B).
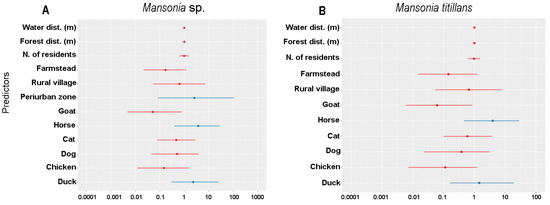
Figure 5.
Pascal distribution analysis, using a negative binomial model to evaluate the influence of predictors on the presence of Mansonia sp. (A) and Mansonia titillans (B), in the upper Madeira River region, Porto Velho, Rondonia, Brazil.
In the analysis of generalized linear models—GLM, two models were obtained that best explained the relationship between Mansonia and the analyzed predictors. The first was performed confronting predictors versus Mansonia spp. and the other with predictors versus Ma. titillans. We can observe that the first model demonstrates that there was a significant influence of the type of land use—farmstead (p = 0.027) and the presence of goat (p = 0.021) and chicken (p = 0.032)—on the abundance of Mansonia spp. in the studied area. These same predictors also influenced the abundance of Ma. titillans—land use—farmstead (p = 0.024) and the presence of goat (p = 0.023) and chicken (p = 0.026) (Table 2).

Table 2.
Influence of environmental predictors on the abundance of Mansonia spp. and Mansonia titillans in the Upper Rio Madeira region, Porto Velho, Rondonia, Brazil.
4. Discussion
Aspects of the bioecology, taxonomy, and behavior of Mansonia mosquitoes remain little studied, even after their proven relationship with hydroelectric projects in Brazil, as first observed in the Amazon region for the area of influence of the Tucuruí hydroelectric plant [12]. The present study found the abundance of species to be low, when compared to that found in the area of influence of the Tucuruí hydroelectric plant, where a total of 33,458 specimens of Mansonia were captured in 10 sampling points.
The dominance of Ma. titillans and Ma. humeralis (93.3%) recorded here corroborates the findings of Galardo et al. (2022) [27], for controlled studies in the same area, where the two species represented 90.1% of the specimens captured between 2015 and 2019, shortly after the construction of the Santo Antonio hydroelectric plant. Thus, the dominance of these species over other species of Mansonia did not change between pre- and post-construction on the Madeira River (Table 1). Similar results were also reported by Scarpassa et al. (2022) [9] after the construction of the Jirau hydroelectric plant.
The predominance of Ma. titillans reinforces the eclectic and opportunistic behavior of this species, which uses forested areas as shelter and open areas for foraging [36,37], feeding on several species of animals including birds, rodents, humans, horses, lizards, cattle, capybaras, and frogs [38], with high occurrence around rural villages, cities, and on farms [39] (Table 1; Figure 3). Conversely, Ma. titillans was abundant at points in periurban areas, corroborating Navarro-Silva et al. (2004) [10], who found this species inhabiting a forest fragment in the urban area of Curitiba, state of Paraná, southern Brazil. Data obtained by Scarpassa et al. (2022) [8] studying the DNA barcode of species of Mansonia collected in the same area (Jirau hydroelectric plant) suggest that Ma. titillans represents a species complex. Therefore, the occurrence of this species in different types of environmental conditions may be due to the existence of cryptic species in the area, which is reflected in greater adaptive capacity, factors that must be considered when control measures are implemented.
Prior to the construction of the hydroelectric plants, the right bank of the Madeira River suffered intense anthropic pressure from deforestation and mining, which likely influenced the diversity of species obtained in the present study. Hutchings et al. (2008) [40] reported a total of 1802 specimens of Mansonia in 367 samplings carried out in 50 locations along the Solimões–Amazon River channel, representing six species—Ma. amazonensis, Ma. flaveola, Ma. humeralis, Ma. indubitans, Ma. pseudotitillans, and Ma. titillans. Of these, Ma. amazonensis was the most abundant, differing from the present study, which found four species, and Ma. titillans accounting for almost 90% of the mosquitoes collected (Table 1; Figure 3). These differences between may be explained because the authors sampled in a preserved environment (six species), whereas the present study sampled an anthropized environment, resulting in lower species diversity (four species) and greater dominance (e.g., Ma. titillans). Based on the results of Tadei et al. (1991) [11] and of the present study, Ma. titillans seems to benefit from anthropized environments, whereas Ma. amazonensis may benefit from more preserved environments.
Species of Mansonia showed higher densities in February, March, and April, corresponding to the rainy season, except for Ma. indubitans, which had low incidence throughout the studied period (Table 1). According to Fearnside (2014) [41], there is higher incidence of macrophytes in the upper Madeira River during the high-water period, when these plant groups are present on both banks of the river, opposite to what was observed in the low-water period. The macrophyte species in the region of the upper Madeira River with the highest abundance of Mansonia immatures were Eichhornia crassipes (Mart.) Solms (n = 14,438), followed by Salvinia sp. (n = 2292) and Pistia stratiotes (Jalkumbhi) (n = 1877), with a peak population of immatures during the rainy season (Gil et al. 2021) [22]. In the month of August, no specimens of Mansonia were collected, due to the dry period that reduces the number of available breeding sites, and several fires with large amounts of smoke, these factors may have negatively influenced the density of Mansonia.
The 4-h and 12-h samplings revealed higher hematophagous activity of Mansonia females between 6:00 and 8:00 p.m. (Figure 4a–d). These findings differ from those of Cruz et al. (2009) [42], who observed greater activity of mosquitoes of the subfamily Culicinae between 8:00 p.m. and 9:00 p.m., in peridomicile areas located in the region of the Madeira River hydroelectric complex. This may have occurred because the authors included Culex, Coquillettidia, and Psorophora specimens in the analyses, resulting in variations in patterns of hematophagic activity, since biting activity varies within the Culicinae subfamily. On the other hand, the biting activity data recorded in the present study corroborate Galardo et al. (2022) [27], who also observed peaks of hematophagous activity between 7:00 and 8:00 p.m. for Ma. titillans and Ma. humeralis in the same region.
The biting activity patterns of culicids show great interspecific and even intraspecific variation, as observed by Zimmerman et al. (2013) [43], studying the nocturnal cycles of malaria vector bites in Brazil. Still in this sense, Charlwood (1996) [44] suggests that the age of the population, the phase of the moon and the distance from the oviposition or mating site can influence the patterns of hematophagic activity.
The studied predictors responded differently for the variables of presence/absence and abundance of Mansonia species (Table 2; Figure 5). The presence of Mansonia spp. was associated with duck and horse rearing in the vicinity of the peri-urban area and the presence of Ma. titillans was also associated with the rearing of these animals. According to the data obtained by Alonso et al. (2023) [25], carried out in the same region, the hematophagous behavior of Mansonia species was heterogeneous, where Ma. amazonensis showed high affinity for Bos taurus Linnaeus, Ma. humeralis for Cannis familiaris Linnaeus and B. taurus, in addition to Homo sapiens and Equus caballus Linnaeus, while Ma. titillans was highly opportunistic, feeding on the blood of Gallus gallus Linnaeus and Homo sapiens Linnaeus.
The analysis of generalized linear models showed that the abundance of Mansonia spp. and Ma. titillans were influenced by the site environment, with goat and chicken rearing. These data indicate the presence of Mansonia in anthropized environments, adapting to feed on different blood sources and favoring the dispersal process of the species, especially if these locations are close to lakes with macrophytes, oviposition sites for this group of mosquitoes. According to De Mello and Alencar (2021) [26], they tend to remain close to their breeding sites, but distances of up to 2000 m from the release point were recorded for Ma. amazonensis and Ma. humeralis.
The results observed in this study, carried out before the construction of the hydroelectric plants, indicated that the density of Mansonia species increased after construction. The studies conducted by Galardo et al. (2022) [27] in the area of influence of the Santo Antônio hydroelectric plant, also located in the same region, recorded 96,766 specimens captured over a period of five years, between 2015 and 2019, carrying out three annual collections, with a total of 15 collection periods, while the results presented here between 2003 and 2004 recorded 1479 specimens, at the end of eight collection periods. The collection effort of the authors mentioned above was 192 h/month while in this study it was 105.5 h/month (Figure 2). The transformations that occurred in the region with the expansion of floodable areas made the environment favorable for the reproduction of macrophytes, positively reflecting on the increase in the population density of Mansonia species.
It should be noted that there is currently no official population control program for Mansonia species in Brazil. Currently, some initiatives have been adopted by the private sector to expand studies on these mosquitoes, but there is still no consensus on the best strategies that can be implemented to reduce the population. Challenges include the difficulty in identifying the species, especially immature forms, and the extensive breeding sites, making it difficult to mechanically remove macrophytes or even apply larvicides. Therefore, we recommend the installation of mosquito nets on doors and windows and indoor spraying in homes close to breeding sites, constituted by farms, especially during the rainy season to reduce the successive bites from female Mansonia.
In addition to mosquito population control techniques, it is important to consider measures to reduce the biomass of macrophytes in the area of influence of the hydroelectric plant. There is no consensus on the best technique to be used; however, it is known that the use of herbicides poses a risk to the aquatic environment, as does the introduction of species that act in biological control. Therefore, it is necessary to strengthen ecological knowledge of macrophyte species, as each aquatic ecosystem has unique characteristics with distinct limnological properties. This will make it possible to carry out modeling studies to predict the proliferation potential of macrophyte species with a view to controlling and consequently reducing the development habitats of immature of Mansonia.
5. Conclusions
The findings of this study indicated that Mansonia species showed a high incidence in the rainy season, with peaks of twilight hematophagic activity. In addition, Ma. titillans was observed to be predominant species in the region, demonstrating highly adaptive plasticity in these environments, occupying niches near the urban perimeter as well as more distant places. The presence and abundance of Mansonia mosquitoes was strongly influenced by human activities and transformation of the landscape on the banks of the Madeira River, involving land use and animal husbandry, consequently increasing the sources of blood meal for these species in this environment. In this study, the density of Mansonia species was influenced by the flood and ebb pulses of the Madeira River and the Ma. titillans population responds positively to environmental changes related to land use. Furthermore, the low density found in the pre-construction period contrasts with studies carried out in the same region after the construction of the hydroelectric plants, indicating that the environmental transformations that occurred benefited the Mansonia mosquito population, despite not altering the composition of the species.
Author Contributions
F.A.d.S.F.—Substantial contribution in the concept and design of the study. F.M.d.C.—Contribution to critical revision, adding intellectual content. A.S.G.—Contribution to data analysis and interpretation. R.A.R.—Contribution to manuscript preparation. V.M.S.—Contribution to data analysis, interpretation, and critical revision. W.P.T.—Management of the research project that sponsored the activities of this study. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação de Amparo à Pesquisa do Estado do Amazonas—FAPEAM, Programa de Apoio a Pesquisa UNIVERSAL AMAZONAS - Edital n.002/2018, The Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Code 001) And Furnas Centrais Elétricas S/A.
Institutional Review Board Statement
The study was conducted in accordance with INPA—Ethics Committee—Opinion N° 3.474.088.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data analyzed in this study can be accessed in more detail at https://repositorio.inpa.gov.br/handle/1/38744 (accessed on 15 August 2023).
Acknowledgments
We are grateful to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Code 001) for the scholarship granted to the first author and also to Furnas Centrais Elétricas S/A for making the fieldwork possible. We also thank the technicians involved in the field collections and identification of the material collected—Elias Pacheco, Raimundo Nonato, Acilino Carvalho, and Carlos Praia. We also thank Wanderli Pedro Tadei (in memoriam), who greatly contributed to the bioecological knowledge of Culicidae in the Brazilian Amazon.
Conflicts of Interest
The authors declare no conflicts of interest related to the publication of this manuscript.
References
- Harbach, R.E. Mosquito Taxonomic Inventory. 2022. Available online: http://mosquito-taxonomic-inventory.info/ (accessed on 17 June 2022).
- Harbach, R.E.; Kitching, I.J. Phylogeny and classification of the Culicidae (Diptera). Syst. Entomol. 1998, 23, 327–370. [Google Scholar] [CrossRef]
- Barbosa, A.A. Revisão do Subgênero Mansonia Blanchard, 1901 (Diptera, Culicidae) e Estudo Filogenético de Mansoniini. Ph.D. Thesis, Universidade Federal do Paraná, Curitiba, Paraná, 2007; 158p. [Google Scholar]
- Ferreira, F.A.S.; Simões, R.C.; Ferreira-Keppler, R.L.; Alencar, J.; Scarpassa, V.M.; Tadei, W.P. Scanning Electron Microscopy and Geometric Contour Morphometry for identifying eggs of three Amazonian Species of Mansonia (Diptera: Culicidae). J. Med. Entomol. 2019, 57, 745–754. [Google Scholar] [CrossRef]
- WRBU. Systematic Catalog of Culicidae; Walter Reed Biosystematics Unit: Washington, DC, USA, 2022; Available online: https://wrbu.si.edu/ (accessed on 12 June 2022).
- Tadei, W.P. O gênero Mansonia (Diptera: Culicidae) e a proliferação de mosquitos na usina hidrelétrica de Tucuruí. In Energia na Amazônia; Magalhães, S.B., Brito, R.C., Castro, E.R., Eds.; MPEG/FPA/UNAMAZ: Belém, Brazil, 1996; Volume 1, pp. 311–318. [Google Scholar]
- Ferreira, R.L.M.; Nunes de Mello, J.A.S. Aspectos biológicos de três espécies de Mansonia (Mansonia) Blanchard, 1901 (Diptera, Culicidae) em laboratório. Rev. Bras. Entomol. 1999, 43, 29–34. [Google Scholar]
- Scarpassa, V.M.; Batista, E.T.; Ferreira, V.C.; Santos-Neto, V.A.; Roque, R.A.; Tadei, W.P.; Ferreira, F.A.S.; Costa, F.M. DNA barcoding suggests new species for the Mansonia subgenus (Mansonia, Mansoniini, Culicidae, Diptera) in the area surrounding the Jirau hydroelectric dam, Porto Velho, Rondônia state, Brazil. Acta Trop. 2022, 233, 106574. [Google Scholar] [CrossRef]
- Amorim, J.Á.; Oliveira, T.M.P.; Sá, I.L.R.; Silva, T.P.; Sallum, M.A.M. DNA Barcodes of Mansonia (Mansonia) Blanchard, 1901 (Diptera, Culicidae). Genes 2023, 14, 1127. [Google Scholar] [CrossRef] [PubMed]
- Tadei, W.P.; Scarpassa, V.M.; Rodrigues, I.B. Evolução das populações de Anopheles e Mansonia, na área de influência da Usina Hidrelétrica de Tucuruí (Pará). Ciência Cult. 1991, 43, 639–640. [Google Scholar]
- Navarro-Silva, M.A.; Barbosa, A.A.; Calado, D. Atividade de Mansonia spp. (Mansoniini, Culicidae) em fragmento florestal na área urbana de Curitiba, Paraná, Brasil. Rev. Bras. Zool. 2004, 21, 243–247. [Google Scholar] [CrossRef]
- Converse, J.D.; Ratna, I.T.; Iman, T.R.; Vernon, H.L.; Robert, E.S. Ingwawma virus (Simbu group) from Culex and Mansonia mosquitoes (Diptera: Culicidae) in Indonesia. J. Med. Entomol. 1985, 22, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Karabastos, N.E. International Catalogue of Arbovírus including Certain Other Viruses of Vertebrates, 4th ed; American Society of Tropical Medicine and Hygiene: San Antonio, TX, USA, 1985. [Google Scholar]
- Mitchell, C.J.; Monath, T.P.; Sabattini, M.S.; Cropp, C.B.; Daffner, J.I.; Calisher, C.H.; Jakob, W.L.C. Arbovirus investigation in Argentine, 1977–1980. II Arthropods Collections and arbovírus isolations from Argentine mosquitoes. Am. J. Trop. Med. Hyg. 1985, 34, 945–955. [Google Scholar] [CrossRef]
- Cupp, E.W.; Scherer, W.F.; Lok, J.B.; Brenner, R.J.; Dziem, G.M.; Ordonez, J.V. Entomological studies at on enzootic Venezuelan an equine encephalitis virus focus in Guatemala. Am. J. Trop. Med. Hyg. 1986, 35, 851–859. [Google Scholar] [CrossRef]
- Mitchell, C.J.; Monath, T.P.; Sabattini, M.S.; Christensen, H.A.; Darsi, R.F.; Jakob, W.L.; Daffner, J.F. Host-feeding pattern of Argentine mosquitoes (Diptera: Culicidae) collected during and after an epizootic of western equine encephalitis. J. Med. Entomol. 1987, 24, 260–267. [Google Scholar]
- Vasconcelos, P.F.D.C.; Travassos da Rosa, J.F.S.; Travassos da Rosa, A.P.D.A.; Dégallier, N.; Pinheiro, F.D.P.; Sá-Filho, G.C. Epidemiologia das encefalites por arbovírus na Amazônia brasileira. Rev. Inst. Med. Trop. São Paulo 1991, 33, 465–476. [Google Scholar] [CrossRef]
- Althouse, B.M.; Hanley, K.A.; Diallo, M.; Sall, A.A.; Ba, Y.; Faye, O.; Diallo, D.; Watts, D.M.; Weaver, S.C.; Cummings, D.A.; et al. Impact of climate and mosquito vector abundance on sylvatic arbovirus circulation dynamics in Senegal. Am. J. Trop. Med. Hyg. 2015, 92, 88–97. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, F.B.; de Curcio, J.S.; Silva, L.D.C.; da Silva, D.M.F.; Salem-Izacc, S.M.; Anunciação, C.E.; Ribeiro, B.M.; Garcia-Zapata, M.T.A.; Silveira-Lacerda, E.d.P. Zika virus emergence in mosquitoes in southeastern Senegal, 2011. PLoS ONE 2014, 9, e109442. [Google Scholar]
- Miranda, K.K.P.; Galvão, G.J.P.; Araújo, P.A.d.S.; Ribeiro, A.C.d.S.; da Silva, S.P.; Lemos, P.d.S.; Martins, L.C.; Nunes, M.R.T.; Vasconcelos, P.F.d.C.; Ferreira, V.d.C.; et al. Discovery and genome sequencing of a new virus related to members of the family Tymoviridae, isolated from mosquitoes of the genus Mansonia in Brazil. Arch. Virol. 2022, 167, 1889–1892. [Google Scholar] [CrossRef]
- De Sousa, F.B.; Curcio, J.S.; Silva, L.C.; Silva, D.M.F.; Salem-Izacc, S.M.; Anunciação, C.E.; Ribeiro, B.M.; Garcia-Zapata, M.T.A.; Silveira-Lacerda, E.P. Report of natural Mayaro vírus infection in Mansonia humeralis (Dyar & Knab, Diptera: Culicidae). Parasit. Vetores 2023, 16, 140. [Google Scholar] [CrossRef]
- Gil, L.H.S.; Mello, C.F.; Silva, J.S.; Oliveira, J.S.; Silva, S.O.F.; Rodríguez-Planes, L.; Costa, F.M.; Alencar, J. Evaluation of Mansonia spp. infestation on aquatic plants in lentic and lotic environments of the Madeira River basin in Porto Velho, Rondônia, Brazil. J. Am. Mosq. Control Assoc. 2021, 37, 143–151. [Google Scholar]
- Ferreira, R.L.M.; Pereira, E.S.; Har, N.T.F.; Hamada, N. Mansonia spp. (Diptera: Culicidae) associated with two species of macrophytes in a Varzea lake, Amazonas, Brazil. Entomotropica 2007, 18, 21–25. [Google Scholar]
- D’avila, F.A.; Gomes, A.C. Seasonality of Mansonia titillans during dam construction, Biritiba-Mirim, São Paulo State, Brazil. Biota Neotrop. 2013, 13, 70–73. [Google Scholar] [CrossRef]
- Alonso, D.P.; Amorim, J.A.; de Oliveira, T.M.P.; de Sá, I.L.R.; Possebon, F.S.; de Carvalho, D.P.; Ribeiro, K.A.N.; Ribolla, P.E.M.; Sallum, M.A.M. Host Feeding Patterns of Mansonia (Diptera, Culicidae) in Rural Settlements near Porto Velho, State of Rondonia, Brazil. Biomolecules 2023, 13, 553. [Google Scholar] [CrossRef]
- De Mello, C.F.; Alencar, J. Dispersion pattern of Mansonia in the surroundings of the Amazon Jirau Hydroelectric Power Plant. Sci. Rep. 2021, 11, 24273. [Google Scholar] [CrossRef] [PubMed]
- Galardo, A.K.R.; Hijjar, A.V.; Falcão, L.L.O.; Carvalho, D.P.; Ribeiro, K.A.N.; Silveira, G.A.; Santos-Neto, N.F.; Saraiva, J.F. Seasonality and biting behavior of Mansonia (Diptera, Culicidae) in rural settlements near Porto Velho, State od Rondônia, Brazil. J. Med. Entomol. 2022, 59, 883–890. [Google Scholar] [CrossRef]
- Alonso, D.P.; Alvarez, M.V.N.; Amorim, J.A.; de Sá, I.L.R.; Carvalho, D.P.; Ribeiro, K.A.N.; Ribolla, P.E.M.; Sallum, M.A.M. Mansonia spp. population genetics based on mitochondrion whole-genome sequencing alongside the Madeira River near Porto Velho, Rondonia, Brazil. Infect. Genet. Evol. 2022, 103, 105341. [Google Scholar] [CrossRef] [PubMed]
- Koppen, N.W. Climatologia, Com um Estudio de los Climas de la Tierra; Fondo Cultural Econômico: Parroquia, México, 1948; 478p. [Google Scholar]
- Ab’Sáber, A.N. Os Domínios de Natureza No Brasil: Potencialidades Paisagísticas; Ateliê Editorial: Cotia, Brazil, 2003; 144p. [Google Scholar]
- IBGE—Instituto Brasileiro de Geografia e Estatística. Diretoria de Pesquisas, Coordenação de População e Indicadores Sociais, Estimativas da População Residente com. 2022. Available online: https://cidades.ibge.gov.br/brasil/ro/porto-velho/panorama (accessed on 25 August 2023).
- Forattini, O.P. Culicidologia Médica; EDUSP: São Paulo, Brazil, 2002; Volume 2, 860p. [Google Scholar]
- Belkin, J.N. Estudios sobre Mosquitos (Diptera, Culicidae) Ia. un Proyecto para un Estudio Sistemático de los Mosquitos de Meso-America. IIa. Métodos para Coleccionar, Criar y Preservar Mosquitos; Contributions of the American Entomological Institute: Ann Arbor, MI, USA, 1967; Volume 1, pp. 1–89. [Google Scholar]
- Forattini, O.P. Entomologia Médica Vol. 1 Parte Geral, Diptera, Anophelini; Faculdade de Higiene, Saúde Pública, Depto. de Parasitologia: São Paulo, Brazil, 1962; 662p. [Google Scholar]
- Service, M.W. Medical Entomology for Students; Chapman & Hall: London, UK, 1996; 278p. [Google Scholar]
- Méndez, W.; Liria, J.; Navarro, J.C.; Garciá, C.Z.; Freier, J.E.; Salas, R.; Weaver, S.C.; Barrera, R. Spatial Dispersion of Adult Mosquitoes (Diptera: Culicidae) in a Sylvatic Focus of Venezuelan Equine Encephalitis Virus. J. Med. Entomol. 2001, 38, 813–819. [Google Scholar] [CrossRef][Green Version]
- Alencar, J.; Lorosa, E.S.; Silva, J.S.; Lopes, C.M.; Guimarães, A.E. Observações sobre padrões alimentares de mosquitos (Diptera: Culicidae) no Pantanal Mato-Grossense. Neotrop. Entomol. 2005, 34, 681–687. [Google Scholar] [CrossRef]
- Lorosa, E.S.; Faria, M.S.; Oliveira, L.C.; Alencar, J.; Marcondes, C.B. Blood meal identification of selected mosquitoes in Rio de Janeiro, Brazil. J. Am. Mosq. Control Assoc. 2010, 26, 18–23. [Google Scholar] [CrossRef]
- Klein, T.A.; Lima, J.B.P.; Tang, A.T. Seasonal distribution and diel biting patterns of culicine mosquitoes in Costa Marques, Rondônia, Brasil. Memórias Inst. Oswaldo Cruz 1992, 87, 141–148. [Google Scholar] [CrossRef]
- Hutchings, R.W.; Hutchings, R.S.G.; Sallum, M.A.M. Distribuição de Culicidae na várzea, ao longo da calha dos rios Solimões-Amazonas. In Conservação da Várzea–Identificação e Caracterização de Regiões Biogeográficas; Ministério do Meio Ambiente: PróVarzea, Brazil, 2008; 45p, ISBN 978-85-7300-267-6. [Google Scholar]
- Fearnside, P.M. Análisis de los Principales Proyectos Hidro-Energéticos en la Región Amazónica; Derecho, Ambiente y Recursos Naturales (DAR), Centro Latinoamericano de Ecología Social (CLAES), & Panel Internacional de Ambiente y Energia en la Amazonia: Lima, Peru, 2014; 55p. [Google Scholar]
- Cruz, R.M.B.; Gil, L.H.S.; Silva, A.A.; Araújo, M.S.; Katsuragawa, T.H. Mosquito abundance and behavior in the influence area of the hydroelectric complex on the Madeira River, Western Amazon, Brazil. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 1174–1176. [Google Scholar] [CrossRef]
- Zimmerman, R.H.; Lounibos, L.P.; Nishimura, N.; Galardo, A.K.; Galardo, C.D.; Arruda, M.E. Nightly biting cycles of malaria vectors in a heterogeneous transmission area of eastern Amazonian Brazil. Malar. J. 2013, 12, 262. [Google Scholar] [CrossRef]
- Charlwood, J.D. Biological variation in Anopheles darlingi Root. Memórias Do Inst. Oswaldo Cruz 1996, 91, 391–398. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).