Entomological Surveillance Activities in Regions in Greece: Data on Mosquito Species Abundance and West Nile Virus Detection in Culex pipiens Pools (2019–2020)
Abstract
:1. Introduction
2. Materials and Methods
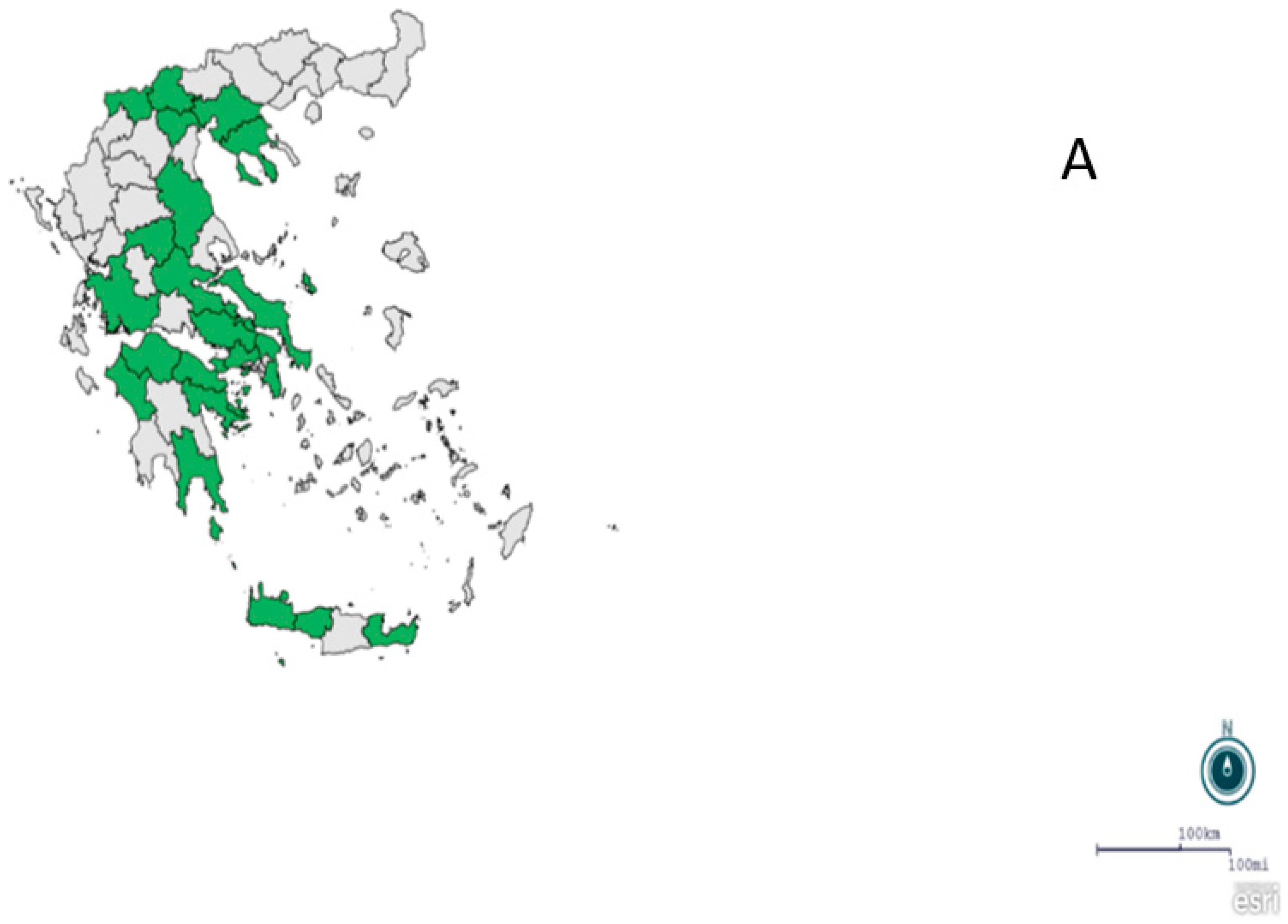
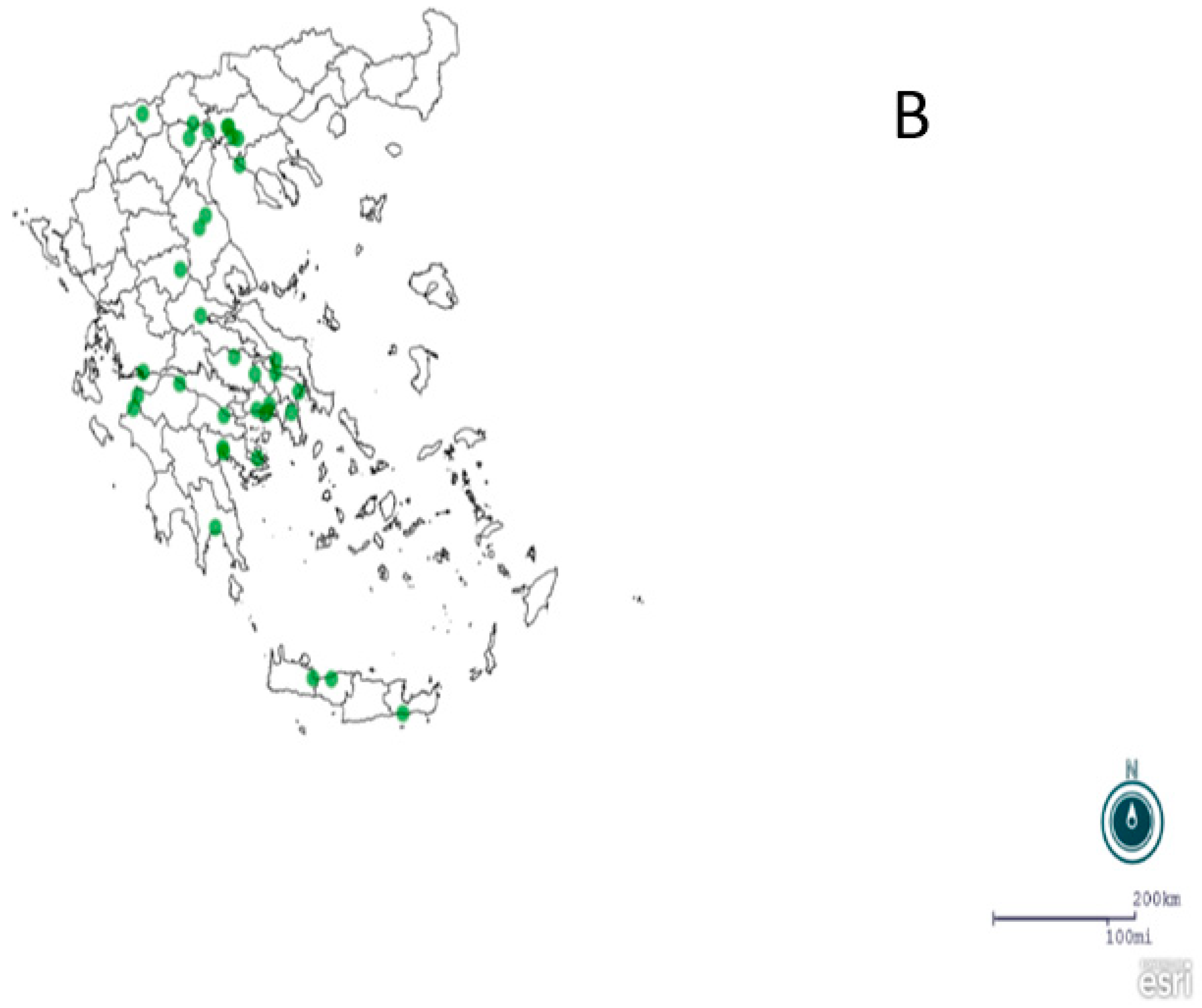
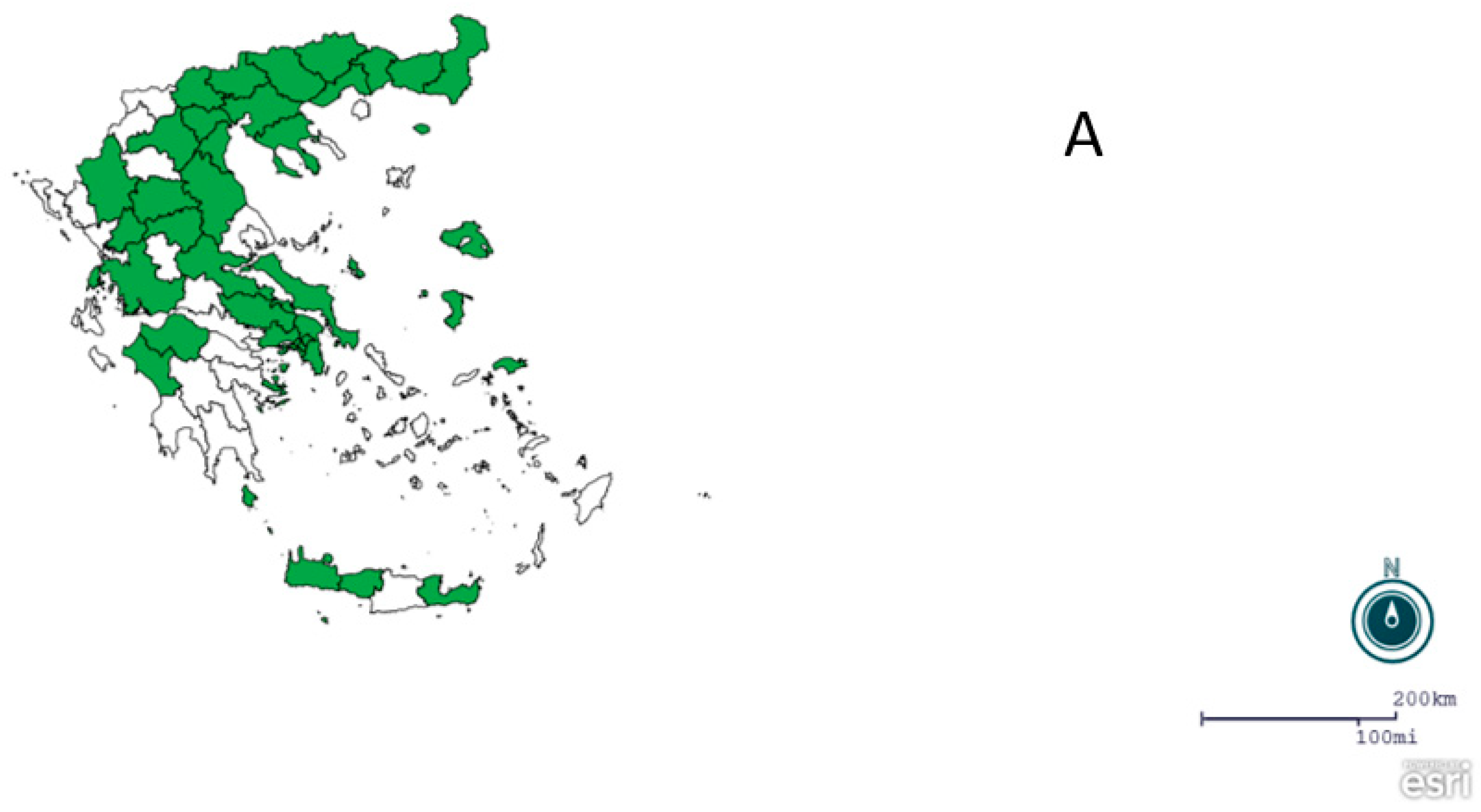
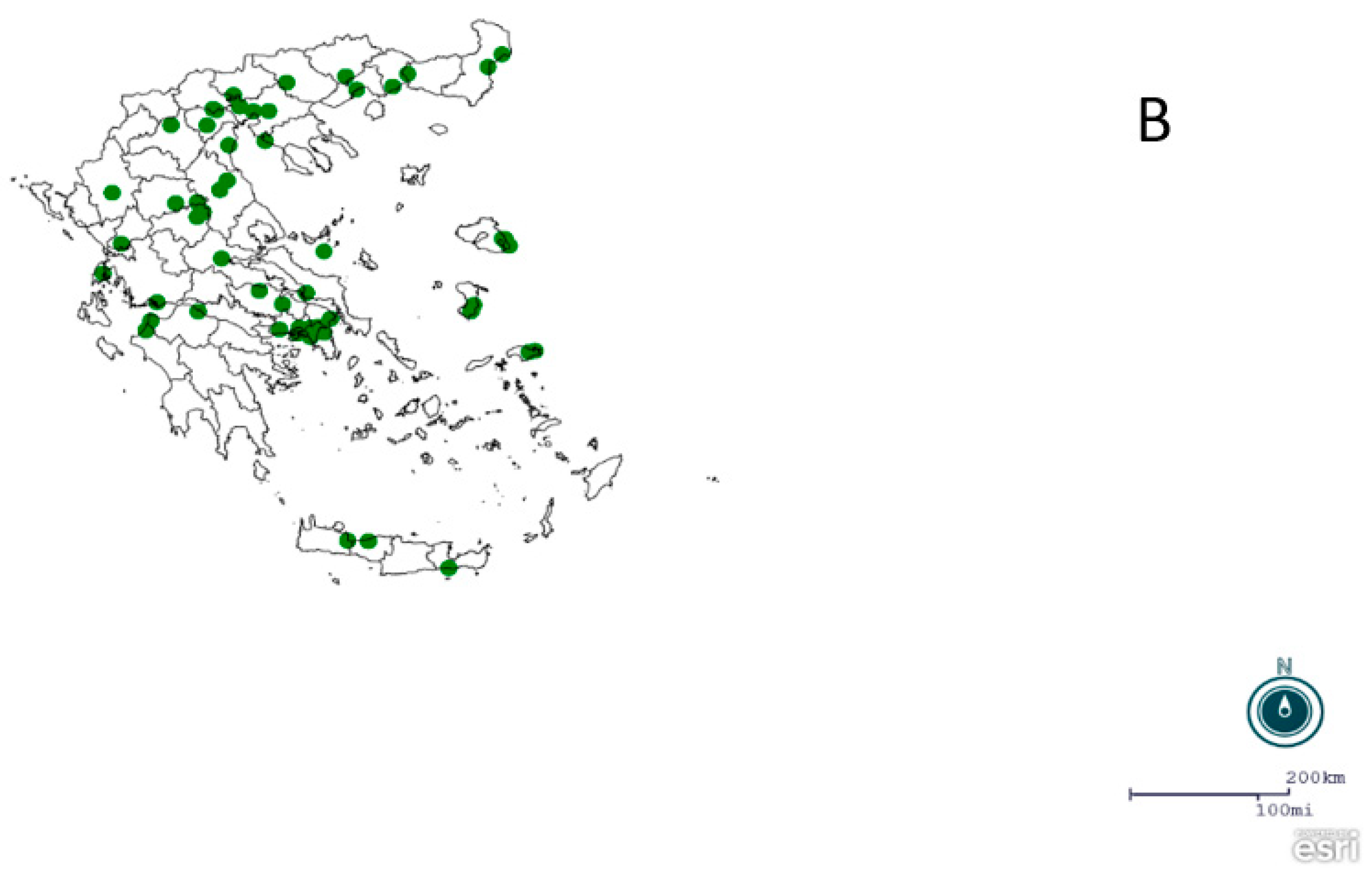
2.1. Regions, Mosquito Collection and Identification
2.2. WNV in Mosquito Pools
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petersen, L.R.; Brault, A.C.; Nasci, R.S. West Nile virus: Review of the literature. JAMA 2013, 310, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Fall, G.; Di Paola, N.; Faye, M.; Dia, M.; Freire, C.C.D.M.; Loucoubar, C.; Zanotto, P.M.D.A.; Faye, O.; Sall, A.A. Biological and phylogenetic characteristics of West African lineages of West Nile virus. PLoS Negl. Trop. Dis. 2017, 11, e0006078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, N.; Petric, D.; Zgomba, M.; Boase, C.; Madon, M.; Dahl, C.; Kaiser, A. Mosquitoes and Their Control, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- World Health Organization (WHO). A Global Brief on Vector-Borne Diseases; World Health Organization: Geneva, Switzerland, 2014.
- World Health Organization (WHO). Vector-Borne Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 20 June 2022).
- May, F.J.; Davis, C.T.; Tesh, R.B.; Barrett, A.D. Phylogeography of West Nile virus. J. Virol. 2011, 85, 2964–2974. [Google Scholar] [CrossRef] [Green Version]
- Sambri, V.; Capobianchi, M.; Charrel, R.; Fyodorova, M.; Gaibani, P.; Gould, E.; Niedrig, M.; Papa, A.; Pierro, A.; Rossini, G.; et al. West Nile virus in Europe: Emergence, epidemiology, diagnosis, treatment, and prevention. Clin. Microbiol. Infect. 2013, 19, 699–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Centre for Disease Prevention and Control (ECDC). West Nile Virus Infection. Stockholm: ECDC. Available online: https://www.ecdc.europa.eu/en/news-events/epidemiological-update-west-nile-virus-transmission-season-europe-2020 (accessed on 25 June 2022).
- Pervanidou, D.; Vakali, A.; Georgakopoulou, T.; Panagiotopoulos, T.; Patsoula, E.; Koliopoulos, G.; Politis, C.; Stamoulis, K.; Gavana, E.; Pappa, S.; et al. West Nile virus in humans, Greece, 2018: The largest seasonal number of cases, 9 years after its emergence in the country. Euro Surveill. 2020, 25, 1900543–1900555. [Google Scholar] [CrossRef] [PubMed]
- National Public Health Organization (NPHO). West Nile Virus. Available online: https://eody.gov.gr/en/disease/west-nile-virus/ (accessed on 10 July 2022).
- Young, J.J.; Haussig, J.M.; Aberle, S.W.; Pervanidou, D.; Riccardo, F.; Sekulić, N.; Bakonyi, T.; Gossner, C.M. Epidemiology of human West Nile virus infections in the European Union and European Union enlargement countries, 2010 to 2018. Euro Surveill. 2021, 26, 2001095. [Google Scholar] [CrossRef] [PubMed]
- Higgs, S.; Snow, K.; Gould, E.A. The potential for West Nile virus to establish outside of its natural range: A consideration of potential mosquito vectors in the United Kingdom. Trans. R. Soc.Trop. Med. Hyg. 2004, 98, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Martinet, J.P.; Ferté, H.; Failloux, A.B.; Schaffner, F.; Depaquit, J. Mosquitoes of North-Western Europe as Potential Vectors of Arboviruses: A Review. Viruses 2019, 11, 1059. [Google Scholar] [CrossRef] [Green Version]
- Farajollahi, A.; Fonseca, D.M.; Kramer, L.D.; Kilpatrick, M.A. “Bird biting” mosquitoes and human disease: A review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect. Genet. Evol. 2011, 11, 1577–1585. [Google Scholar] [CrossRef] [Green Version]
- Vogels, C.B.F.; Hartemink, N.; Koenraadt, C.J.M. Modelling West Nile Virus transmission risk in Europe: Effect of temperature and mosquito biotypes on the basic reproduction number. Sci. Rep. 2017, 7, 5022–5032. [Google Scholar] [CrossRef]
- Brustolin, M.; Talavera, S.; Santamaría, C.; Rivas, R.; Pujol, N.; Aranda, C.; Marquès, E.; Valle, M.; Verdún, M.; Pagès, N.; et al. Culex pipiens and Stegomyia albopicta (Aedes albopictus) populations as vectors for lineage 1 and 2 West Nile virus in Europe. Med. Vet. Entomol. 2016, 30, 166–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balenghien, T.; Vazeille, M.; Reiter, P.; Schaffner, F.; Zeller, H.; Bicout, D.J. Evidence of laboratory vector competence of Culex modestus for West Nile virus. J. Am. Mosq. Control Assoc. 2007, 23, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Vogels, C.B.F.; Göertz, G.P.; Pijlman, G.P.; Koenraadt, C.J.M. Vector competence of European mosquitoes for West Nile virus. Emerg. Microbes Infect. 2017, 6, e96–e108. [Google Scholar] [CrossRef] [Green Version]
- Papa, A.; Xanthopoulou, K.; Gewehr, S.; Mourelatos, S. Detection of West Nile virus lineage 2 in mosquitoes during a human out-break in Greece. Clin. Microbiol. Infect. 2011, 17, 1176–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patsoula, E.; Vakali, A.; Balatsos, G.; Pervanidou, D.; Beleri, S.; Tegos, N.; Baka, A.; Spanakos, G.; Georgakopoulou, T.; Tserkezou, P.; et al. West Nile Virus Circulation in Mosquitoes in Greece (2010–2013). Biomed. Res. Int. 2016, 2016, 2450682. [Google Scholar] [CrossRef] [Green Version]
- Chaskopoulou, A.; L’Ambert, G.; Petric, D.; Bellini, R.; Zgomba, M.; Groen, T.A. Ecology of West Nile fever across four European countries: History of WNV transmission, vector population dynamics & vector control response. Parasites Vectors 2016, 9, 482–490. [Google Scholar]
- Samanidou-Voyadjoglou, A.; Harbach, R.E. Keys to the adult female mosquitoes (Culicidae) of Greece. Eur. Mosq. Bull. 2001, 10, 13–20. [Google Scholar]
- Darsie, R.F.J.; Samanidou-Voyadjoglou, A. Keys for the identification of the mosquitoes of Greece. J. Am. Mosq. Control Assoc. 1997, 13, 247–254. [Google Scholar]
- Beleri, S.; Chatzinikolaou, S.; Nearchou, A.; Patsoula, E. Entomological Study of the Mosquito Fauna in the Regional Unit of Drama, Region of East Macedonia-Thrace, Greece (2015 to 2016). Vector Borne Zoonotic Dis. 2017, 17, 665–671. [Google Scholar] [CrossRef]
- Patsoula, E.; Samanidou-Voyadjoglou, A.; Spanakos, G.; Kremastinou, J.; Nasioulas, G.; Vakalis, N.C. Molecular characterization of the Anopheles maculipennis complex during surveillance for the 2004 Olympic Games in Athens. Med. Vet. Entomol. 2007, 21, 36–43. [Google Scholar] [CrossRef]
- Tang, Y.; Hapip, A.C.; Liu, B.; Fang, C.T. Highly sensitive TaqMan RT-PCR assay for detection and quantification of both lineages of West Nile virus RNA. J. Clin. Virol. 2006, 36, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Eiden, M.; Vina-Rodriguez, A.; Hoffmann, B.; Ziegler, U.; Groschup, M.H. Two new real-time quantitative reverse transcription polymerase chain reaction assays with unique target sites for the specific and sensitive detection of lineages 1 and 2 West Nile Virus Strains. J. Vet. Diagn. Investig. 2010, 22, 748–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biggerstaff, B.J. PooledInfRate, Version 4.0: A Microsoft® Office Excel© Add-In to Compute Prevalence Estimates from Pooled Samples; Centers for Disease Control and Prevention: Fort Collins, CO, USA, 2009.
- Bertola, M.; Mazzucato, M.; Pombi, M.; Montarsi, F. Updated occurrence and bionomics of potential malaria vectors in Europe: A systematic review (2000–2021). Parasites Vectors 2022, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Schaffner, F.; Angel, G.; Geoffroy, B.; Hervy, J.P.; Rhaeim, A. The Mosquitoes of Europe/Les Moustiques d’Europe [Computer Program]; IRD Editions: Montpellier, France, 2001. [Google Scholar]
- Patsoula, E.; Beleri, S.; Tegos, N.; Mkrtsian, R.; Vakali, A.; Pervanidou, D. Entomological Data and Detection of West Nile Virus in Mosquitoes in Greece (2014–2016), before Disease Re-Emergence in 2017. Vector Borne Zoonotic Dis. 2020, 20, 60–70. [Google Scholar] [CrossRef]
- Patsoula, E.; Samanidou-Voyadjoglou, A.; Spanakos, G.; Kremastinou, J.; Nasioulas, G.; Vakalis, N.C. Molecular and morphological characterization of Aedes albopictus in northwestern Greece and differentiation from Aedes cretinus and Aedes aegypti. J. Med. Entomol. 2006, 43, 40–54. [Google Scholar] [CrossRef]
- Badieritakis, Ε.; Papachristos, D.; Latinopoulos, D.; Stefopoulou, A.; Kolimenakis, A.; Bithas, K.; Patsoula, Ε.; Beleri, S.; Maselou, D.; Balatsos, G.; et al. Aedes albopictus (Skuse, 1895) (Diptera: Culicidae) in Greece: 13 years of living with the Asian tiger mosquito. Parasitol. Res. 2018, 117, 453–460. [Google Scholar] [CrossRef]
- Beleri, S.; Balatsos, G.; Karras, V.; Tegos, N.; Sereti, F.; Rachiotis, G.; Hadjichristodoulou, C.; Papadopoulos, N.; Papachristos, D.; Michaelakis, A.; et al. Seasonal Phenological Patterns and Flavivirus Vectorial Capacity of Medically Important Mosquito Species in a Wetland and an Urban Area of Attica, Greece. Trop. Med. Infect. Dis. 2021, 6, 176. [Google Scholar] [CrossRef]
- Stefopoulou, A.; Balatsos, G.; Papadopoulos, N.T.; Daskalakis, D.; Chatzidaki, A.; Milonas, P.; Papachristos, D.; Michaelakis, A. Spatial and temporal dynamics of Aedes albopictus populations in rural and agricultural areas in Chania, Greece after its invasion. Front. Trop. Dis. 2022, 3, 811945. [Google Scholar] [CrossRef]
- Rueda, L.M.; Kim, H.C.; Klein, T.A.; Pecor, J.E.; Li, C.; Sithiprasasna, R.; Debboun, M.; Wilkerson, R. Distribution and larval habitat characteristics of members of the Anopheles hyrcanus group and related mosquito species (Diptera: Culicidae) in South Korea. J. Vector Ecol. 2006, 31, 198–205. [Google Scholar] [CrossRef]
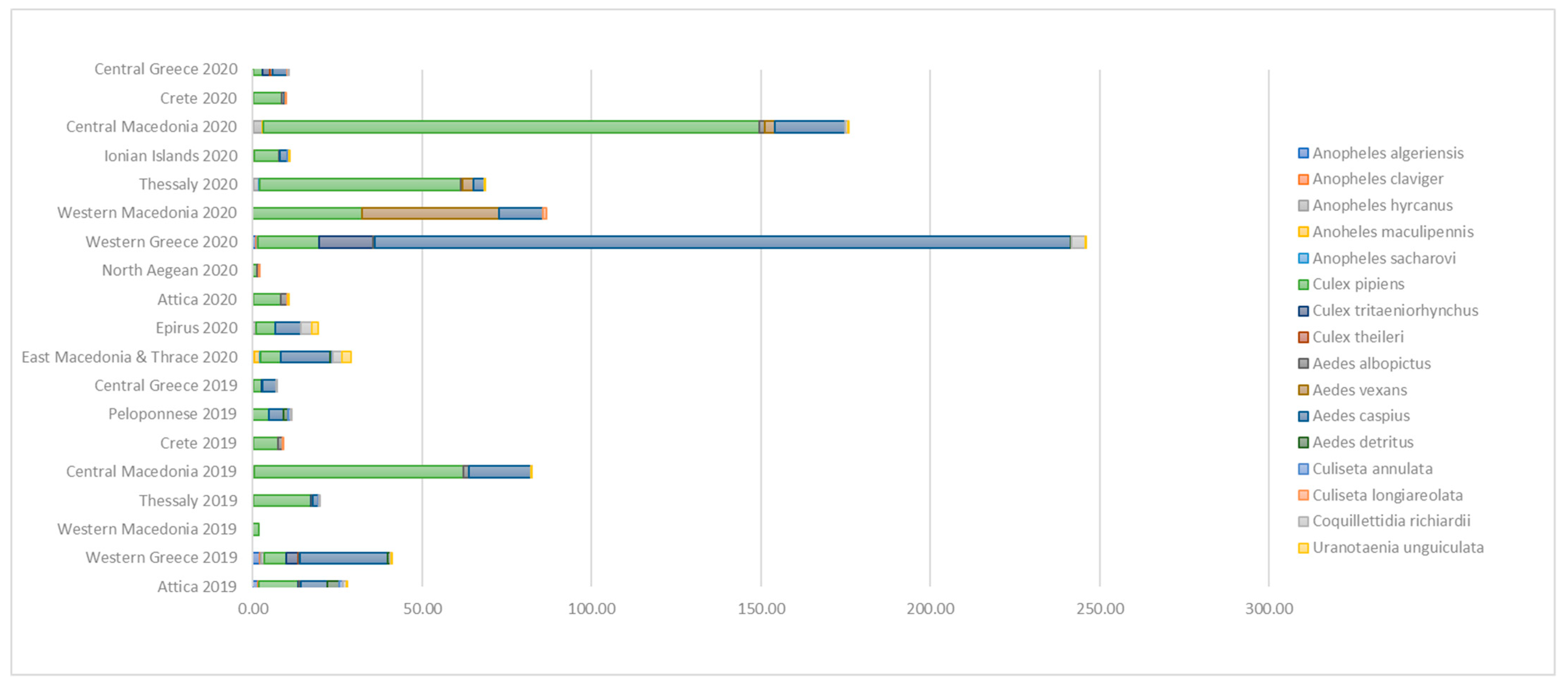
| Region | 2019 | 2020 | p-Value for the 2020 vs. 2019 Comparison |
|---|---|---|---|
| Central Greece | 2.65 (6.56; 0–37) § | 2.61 (6.56; 0–40) § | 0.551 |
| Peloponnese | 4.42 (3.35; 0–13) † | - | Not estimable |
| Crete | 5.33 (7.58; 0–29) ** | 7.42 (9.78; 0–39) † | 0.265 |
| Western Greece | 4.75 (6.69; 0–33) ** | 16.52 (19.71; 0–82) † | 0.0001 |
| Thessaly | 15.33 (32.14; 0–160) * | 58.17 (200.23; 0–1441) † | 0.002 |
| Central Macedonia | 60.19 (153.30; 0–948) † | 143.79 (269.19; 0–1283) † | 0.027 |
| Western Macedonia | 0.43 (0.51; 0–1) | 30 (26.46; 0–95) † | 0.0002 |
| Attica | 9.67 (22.04; 0–158) † | 4.64 (6.20; 0–35) † | 0.0005 |
| Epirus | - | 1.75 (3.44; 0–11) § | Not estimable |
| East Macedonia and Thrace | - | 3.31 (4.31; 0–17) *** | Not estimable |
| Ionian Islands | - | 3.6 (3.78; 0–8) * | Not estimable |
| North Aegean | - | 0.77 (1.59; 0–7) | Not estimable |
| Regions | West Nile Virus | ||||||
|---|---|---|---|---|---|---|---|
| 2019 | 2020 | p-Value for the 2020 vs. 2019 Comparison in Np/Nt Rates † | |||||
| Np/Nt | MIR (95% CI) | MLE (95% CI) | Np/Nt * | MIR (95% CI) | MLE (95% CI) | ||
| Central Greece | 1/12 | 8.47 (0.00–25.01) | 9.17 (0.52–49.16) | 0/19 | 0.00 (NE) | 0.00 (0.00–25.98) | 0.387 |
| Peloponnese | 1/16 | 9.71 (0.00–28.64) | 9.89 (0.57–48.49) | − | - | - | Not estimable |
| Crete | 2/14 | 14.18 (0.00–33.70) | 13.89 (2.72–44.46) | 0/30 | 0.00 (NE) | 0.00 (0.00–12.60) | 0.096 |
| Western Greece | 0/11 | 0.00 (NE) | 0.00 (0.00–24.84) | 0/40 | 0.00 (NE) | 0.00 (0.00–4.95) | Not estimable due to zero events |
| Thessaly | 1/12 | 2.81 (0.00–8.31) | 2.61 (0.17–12.68) | 4/75 | 1.04 (0.02–2.06) | 1.07 (0.35–2.55) | 0.533 |
| Central Macedonia | 11/59 | 2.31 (0.95–3.68) | 2.58 (1.41–4.43) | 8/171 | 0.46 (0.14–0.78) | 0.47 (0.22–0.90) | 0.002 |
| Western Macedonia | 1/4 | 250.0 (0.00–674.34) | 250.0 (15.22–737.44) | 0/10 | 0.00 (NE) | 0.00 (0.00–9.14) | 0.286 |
| Attica | 1/19 | 2.64 (0.00–7.80) | 2.51 (0.16–12.11) | 0/110 | 0.00 (NE) | 0.00 (0.00–5.18) | 0.147 |
| Epirus | − | - | - | 0/5 | 0.00 (NE) | 0.00 (0.00–88.75) | Not estimable |
| East Macedonia and Thrace | − | - | - | 0/26 | 0.00 (NE) | 0.00 (0.00–21.68) | Not estimable |
| Ionian Islands | − | - | - | 0/3 | 0.00 (NE) | 0.00 (0.00–124.18) | Not estimable |
| North Aegean | − | - | - | 0/23 | 0.00 (NE) | 0.00 (0.00–56.28) | Not estimable |
| No. of WNV positive/tested mosquito pools | 18/147 | 12/512 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vakali, A.; Beleri, S.; Tegos, N.; Fytrou, A.; Mpimpa, A.; Sergentanis, T.N.; Pervanidou, D.; Patsoula, E. Entomological Surveillance Activities in Regions in Greece: Data on Mosquito Species Abundance and West Nile Virus Detection in Culex pipiens Pools (2019–2020). Trop. Med. Infect. Dis. 2023, 8, 1. https://doi.org/10.3390/tropicalmed8010001
Vakali A, Beleri S, Tegos N, Fytrou A, Mpimpa A, Sergentanis TN, Pervanidou D, Patsoula E. Entomological Surveillance Activities in Regions in Greece: Data on Mosquito Species Abundance and West Nile Virus Detection in Culex pipiens Pools (2019–2020). Tropical Medicine and Infectious Disease. 2023; 8(1):1. https://doi.org/10.3390/tropicalmed8010001
Chicago/Turabian StyleVakali, Annita, Stavroula Beleri, Nikolaos Tegos, Anastasia Fytrou, Anastasia Mpimpa, Theodoros N. Sergentanis, Danai Pervanidou, and Eleni Patsoula. 2023. "Entomological Surveillance Activities in Regions in Greece: Data on Mosquito Species Abundance and West Nile Virus Detection in Culex pipiens Pools (2019–2020)" Tropical Medicine and Infectious Disease 8, no. 1: 1. https://doi.org/10.3390/tropicalmed8010001
APA StyleVakali, A., Beleri, S., Tegos, N., Fytrou, A., Mpimpa, A., Sergentanis, T. N., Pervanidou, D., & Patsoula, E. (2023). Entomological Surveillance Activities in Regions in Greece: Data on Mosquito Species Abundance and West Nile Virus Detection in Culex pipiens Pools (2019–2020). Tropical Medicine and Infectious Disease, 8(1), 1. https://doi.org/10.3390/tropicalmed8010001










