Spatial and Temporal Distribution of Aedes aegypti and Aedes albopictus Oviposition on the Coast of Paraná, Brazil, a Recent Area of Dengue Virus Transmission
Abstract
1. Introduction
2. Materials and Methods
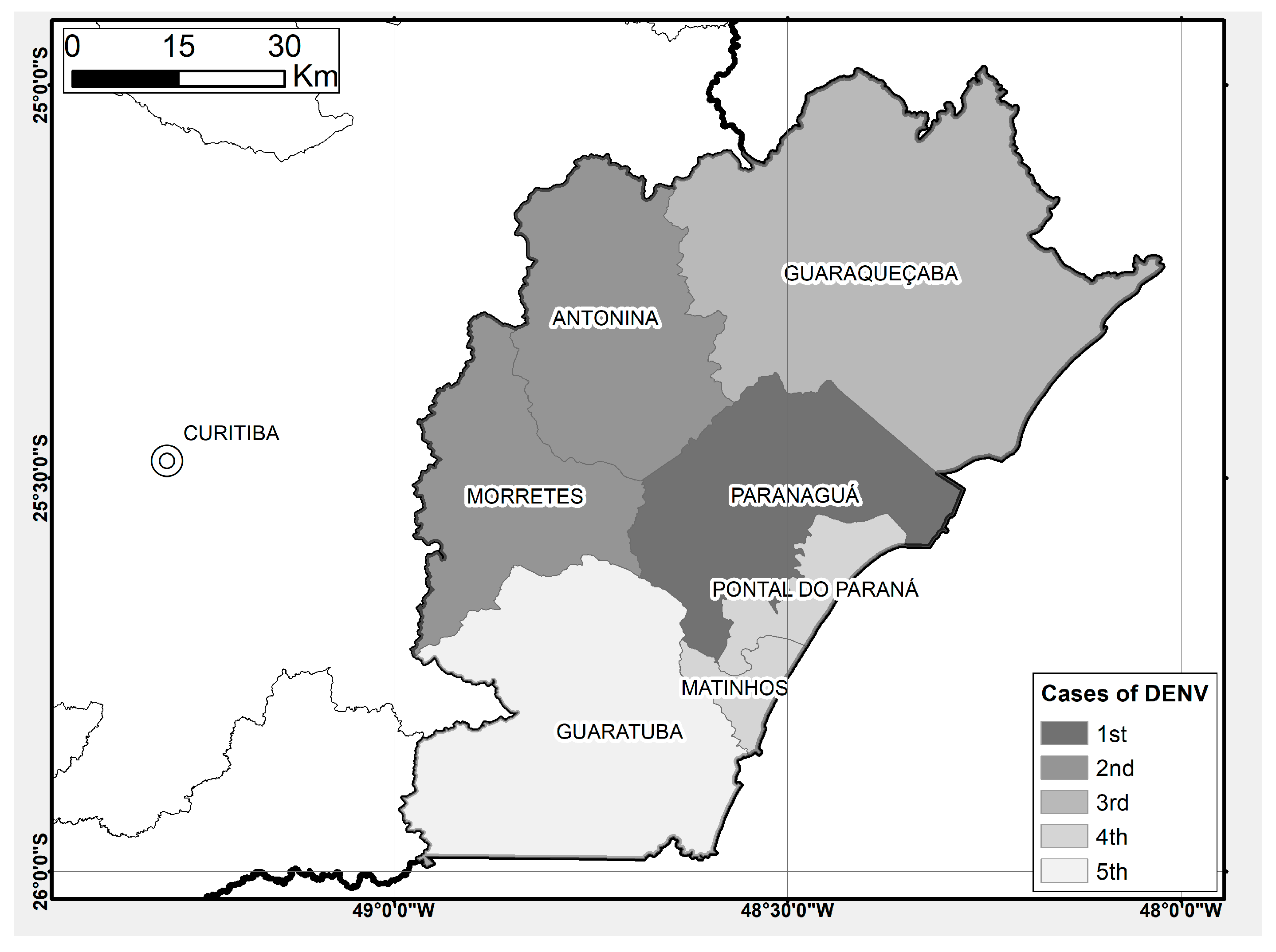
2.1. Study Area
2.2. Entomological Survey and DENV Dengue Fever Cases
2.3. Climate Variables
2.4. Statistical Analysis
3. Results
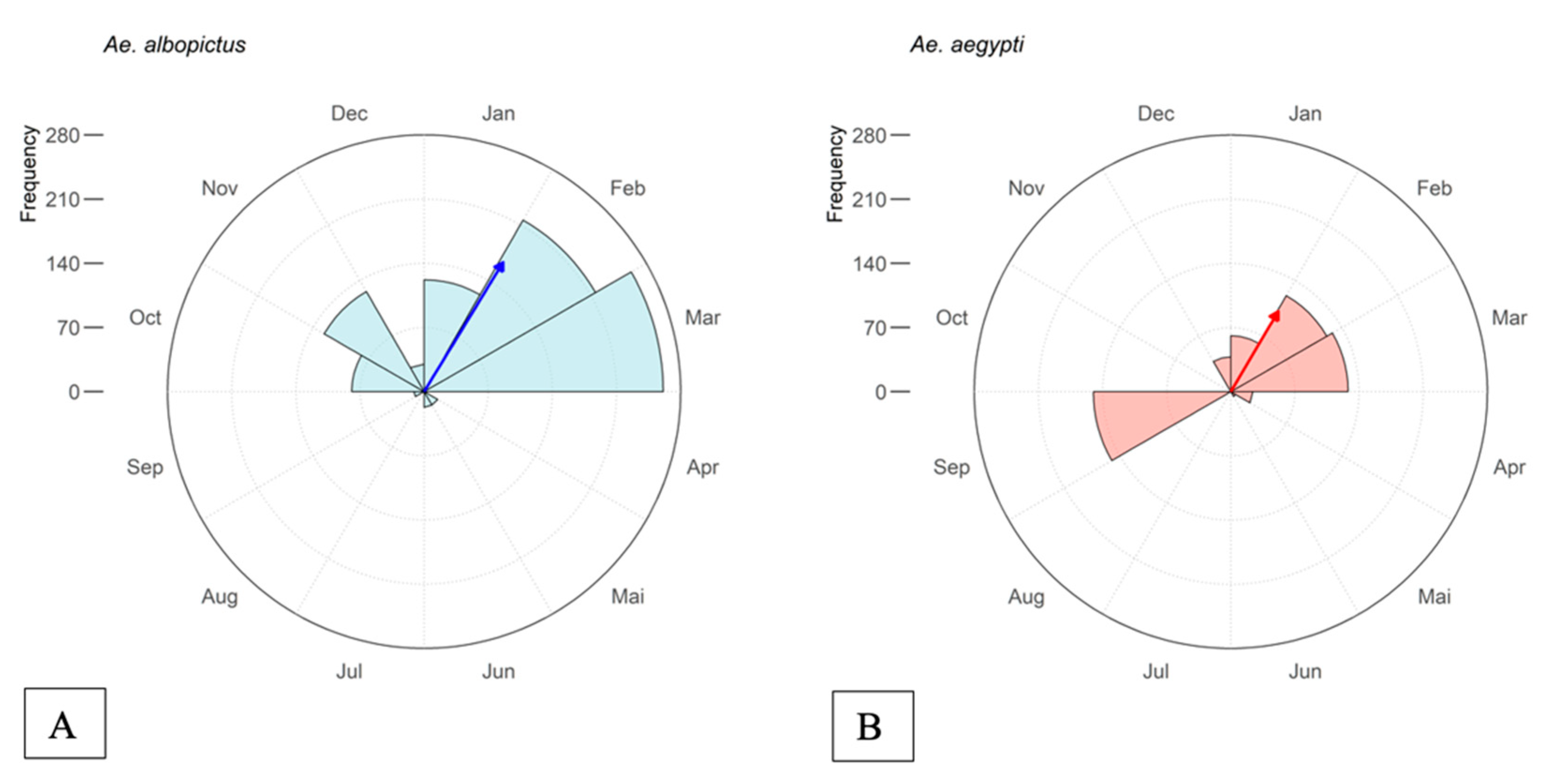
3.1. Spatial and Temporal Distribution of Mosquitoes and DENV Cases
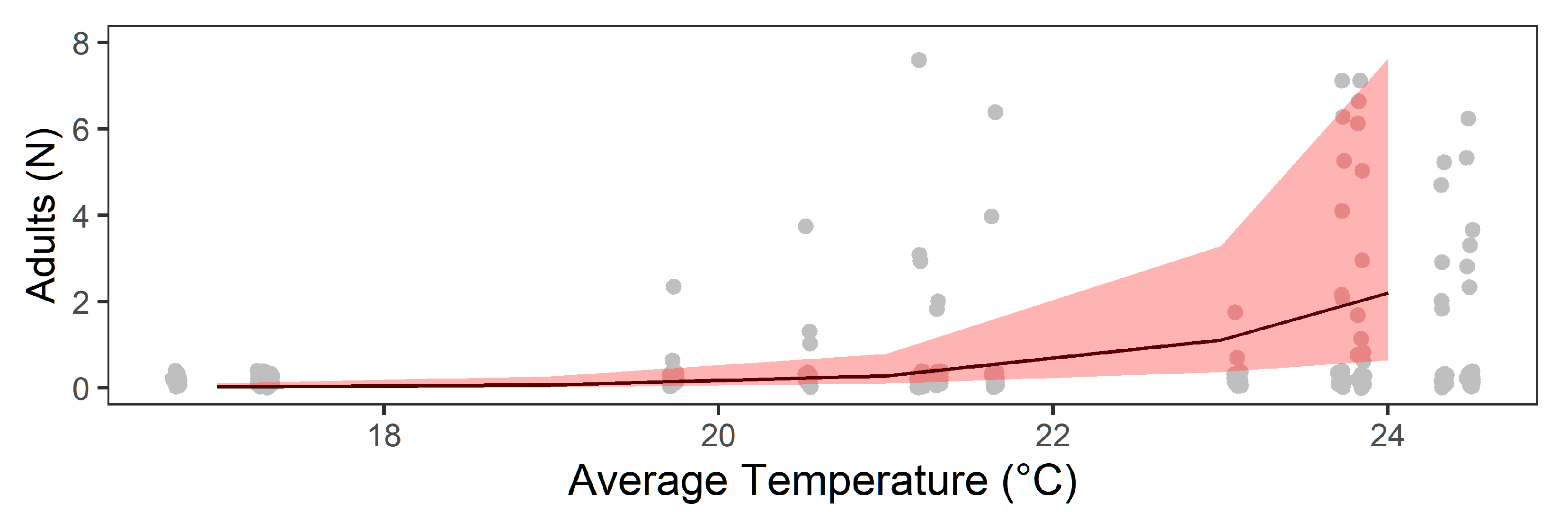
3.2. Abiotic Factors
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Honório, N.A.; Câmara, D.C.P.; Calvet, G.A.; Brasil, P. Chikungunya: An Arbovirus Infection in the Process of Establishment and Expansion in Brazil. Cad. de Saude Publica 2015, 31, 906–908. [Google Scholar] [CrossRef] [PubMed]
- Heinisch, M.R.S.; Diaz-Quijano, F.A.; Chiaravalloti-Neto, F.; Menezes Pancetti, F.G.; Rocha Coelho, R.; dos Santos Andrade, P.; Urbinatti, P.R.; de Almeida, R.M.M.S.; Lima-Camara, T.N. Seasonal and Spatial Distribution of Aedes Aegypti and Aedes Albopictus in a Municipal Urban Park in São Paulo, SP, Brazil. Acta Trop. 2019, 189, 104–113. [Google Scholar] [CrossRef]
- Consoli, R.A.G.B.; Lourenço de Oliveira, R. Principais Mosquitos de Importância Sanitária No Brasil; Editora Fiocruz: Rio de Janeiro, Brasil, 1994. [Google Scholar]
- Rey, J.R.; Lounibos, P. Ecología de Aedes Aegypti y Aedes Albopictus En América y Transmisión Enfermedades. Biomedica 2015, 35, 177–185. [Google Scholar] [CrossRef][Green Version]
- Camara, D.C.P.; Codeço, C.T.; Juliano, S.A.; Lounibos, L.P.; Riback, T.I.S.; Pereira, G.R.; Honorio, N.A. Seasonal Differences in Density but Similar Competitive Impact of Aedes Albopictus (Skuse) on Aedes Aegypti (L.) in Rio de Janeiro, Brazil. PLoS ONE 2016, 11, e0157120. [Google Scholar] [CrossRef] [PubMed]
- Lounibos, L.P.; Juliano, S.A. Where Vectors Collide: The Importance of Mechanisms Shaping the Realized Niche for Modeling Ranges of Invasive Aedes Mosquitoes. Biol. Invasions 2018, 20, 1913–1929. [Google Scholar] [CrossRef] [PubMed]
- Shragai, T.; Harrington, L.; Alfonso-Parra, C.; Avila, F. Oviposition Site Attraction of Aedes Albopictus to Sites with Conspecific and Heterospecific Larvae during an Ongoing Invasion in Medellín, Colombia. Parasites Vectors 2019, 12, 455. [Google Scholar] [CrossRef] [PubMed]
- Leta, S.; Beyene, T.J.; de Clercq, E.M.; Amenu, K.; Kraemer, M.U.G.; Revie, C.W. Global Risk Mapping for Major Diseases Transmitted by Aedes Aegypti and Aedes Albopictus. Int. J. Infect. Dis. 2018, 67, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.G.; Reiner, R.C.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and Future Spread of the Arbovirus Vectors Aedes Aegypti and Aedes Albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Nsoesie, E.O.; Kraemer, M.U.; Golding, N.; Pigott, D.M.; Brady, O.J.; Moyes, C.L.; Johansson, M.A.; Gething, P.W.; Velayudhan, R.; Khan, K.; et al. Global Distribution and Environmental Suitability for Chikungunya Virus, 1952 to 2015. Eurosurveillance 2016, 21, 30234. [Google Scholar] [CrossRef]
- Cavalcante, K.R.L.J.; Tauil, P.L. Características Epidemiológicas Da Febre Amarela No Brasil, 2000–2012. Epidemiol. E Serv. De Saúde Rev. Do Sist. Único De Saúde Do Bras. 2016, 25, 11–20. [Google Scholar] [CrossRef]
- Zara, A.; dos Santos, S.; Fernandes-Oliveira, E.; Carvalho, R.; Coelho, G. Estratégias de Controle Do Aedes Aegypti: Uma Revisão. Epidemiol. Serviços Saúde 2016, 25, 391–404. [Google Scholar] [CrossRef]
- Lopes, J.; Silva, M.N.; Borsato, Â.M.; Oliveira, V.D.R.B.D.; Oliveira, F.J. Aedes (Stegomyia) Aegypti L. e a Culicideofauna Associada Em Área Urbana Da Região Sul, Brasil. Rev. Saúde Pública 1993, 27, 326–333. [Google Scholar] [CrossRef]
- Paraná, Secretaria de Estado da Saúde do Paraná (SESA/PR). Boletim Epidemiológico Dengue_Combate À Dengue. Available online: https://www.dengue.pr.gov.br/Pagina/Boletins-da-Dengue (accessed on 11 June 2022).
- Forattini, O.P. Identificação de Aedes (Stegomyia) Albopictus (Skuse) No Brasil. Rev. Saúde Pública 1986, 20, 244–245. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Nunes, V.; Lopes, J. Culicídeos Associados a Entrenós de Bambu e Bromélias, Com Ênfase Em Aedes (Stegomyia) Albopictus (Diptera, Culicidae) Na Mata Atlântica, Paraná, Brasil. Iheringia. Série Zoologia 2004, 94, 63–66. [Google Scholar] [CrossRef]
- Costa-Ribeiro, M.C.V.; Santos-Neto, L.G. dos Primeiro Registro de Aedes (Stegomyia) Albopictus (Skuse) (Diptera, Culicidae) Em Morretes, Planície Litorânea Do Estado Do Paraná, Brasil. Rev. Bras. de Zool. 2001, 18, 347–348. [Google Scholar] [CrossRef]
- Wrege, M.S.; Steinmetz, S.; Júnior, C.R.; de Almeida, I.R. Atlas Climático Da Região Sul Do Brasil: Estados Do Paraná, Santa Catarina e Rio Grande Do Sul; Embrapa: Brasília, Spain, 2012; ISBN 9788570350138. [Google Scholar]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and Future Köppen-Geiger Climate Classification Maps at 1-Km Resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; de Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s Climate Classification Map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Köppen, W.G.; Geiger, R.M. Das Geographische System Der Klimate. Handb. der Klimatol. 1936, 1, 1–44. [Google Scholar]
- Vanhoni, F.; Mendonça, F. O Clima Do Litoral Do Estado Do Paraná. Rev. Bras. de Climatol. 2008, 3, 1–15. [Google Scholar] [CrossRef]
- Fritzsons, E.; Mantovani, L.E.; Wrege, M.S.; Neto, A.C. Análise Da Pluviometria Para Definição de Zonas Homogêneas No Estado Do Paraná Rainfall Analysis to Define Homogeneous Pluvometric Areas in the State of Paraná; 2011; pp. 555–572. Available online: https://revistas.ufpr.br/raega/article/view/24921 (accessed on 13 August 2022).
- Nitsche, P.R.; Caramori, P.H.; da Ricce, W.S.; Pinto, L.F.D. Atlas Climático Do Estado Do Paraná; Instituto Agronômico do Paraná: Londrina, Spain, 2019; ISBN 978-85-88184-58-3.
- de Cavalcanti, I.F.A.; Ferreira, N.J.; da Silva, M.G.A.J.; da Dias, M.A.F.S. Tempo e Clima No Brasil, 1st ed.; Oficina de Textos: São Paulo, Spain, 2009; ISBN 978-85-86238-92-5. [Google Scholar]
- SIMEPAR. Sistema de Tecnologia e Monitoramento Ambiental do Paraná Dados Das Estações Meteorológicas Do Simepar No Paraná. Available online: http://www.simepar.br (accessed on 31 December 2021).
- IBGE—Instituto Brasileiro de Geografia e Estatística Estimativas de População. 2021. Available online: http://www.ibge.gov.br:estatisticas:sociais:populacao:9103-estimativas-de-populacao.html?=&t=resultados (accessed on 31 December 2021).
- IPARDES—Instituto Paranaense de Desenvolvimento Econômico e Social. Caderno Estatístico Município de Morretes; 2022. Available online: http://www.ipardes.gov.br/cadernos/MontaCadPdf1.php?Municipio=83350 (accessed on 13 August 2022).
- Braga, I.A.; Valle, D. Aedes Aegypti—Histórico Do Controle No Brasil. Epidemiol. Serviços Saúde 2007, 16, 113–118. [Google Scholar] [CrossRef]
- Valle, D.; Aguiar, R.; Pimenta, D.N.; Ferreira, V. Aedes de A a Z.; Editora Fiocruz: Rio de Janeiro, Brazil, 2021; ISBN 9786557080986. [Google Scholar]
- Valle, D.; Codeço, C.; da Lima Silva, A.W.; Araújo, S.C.; Lima, J.B.P.; Honório, N.; de Freitas, R. Allan Kardec Ribeiro Galardo NOTA TÉCNICA N.o 3:2014:IOC—Avaliação de Armadilhas Para a Vigilância Entomológica de Aedes Aegypti Com Vistas À Elaboração de Novos Índices de Infestação. Fiocruz Diretoria 2014, 1, 1–7. [Google Scholar]
- Braks, M.A.H.; Honório, N.A.; Lourenço-De-Oliveira, R.; Juliano, S.A.; Lounibos, L.P. Convergent Habitat Segregation of Aedes Aegypti and Aedes Albopictus (Diptera: Culicidae) in Southeastern Brazil and Florida. J. Med. Entomol. 2003, 40, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Honório, N.A.; Castro, M.G.; de Barros, F.S.M.; de Magalhães, M.A.F.M.; Sabroza, P.C. Padrões Da Distribuição Espacial Do Aedes Aegypti e Aedes Albopictus Em Uma Zona de Transição No Rio de Janeiro, Brasil. Cad. de Saude Publica 2009, 25, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Fay, R.; Eliason, D. A Preferred Oviposition Site as a Surveillance Method for Aedes Aegypti. Mosq. News 1966, 26, 531–535. [Google Scholar]
- Agostinelli, C.; Lund, U. R Package Circular: Circular Statistics (Version 0.4-7); CA: Department of Environmental Sciences, Informatics and Statistics, Ca’ Foscari University, Venice, Italy; UL: Department of Statistics, California Polytechnic State University, San Luis Obispo, California, USA, 2013; Available online: https://CRAN.R-project.org/package=circular (accessed on 5 September 2019).
- Core Team. R: A Language and Environment for Statistical Computing (Version 3.4.2); R Foundation for Statistical Computing: Vienna, Austria, 2018. Available online: https://www.R-project.org (accessed on 5 September 2019).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Lme4: Linear Mixed-Effects Models Using S4 Classes; 2014; Available online: http://lme4.r-forge.r-project.org/ (accessed on 5 September 2019).
- Russell, A.; Lenth, V.; Buerkner, P.; Herve, M.; Love, J.; Singmann, H.; Lenth, M.R. V Package ‘Emmeans’ R Topics Documented. 2021, 34, 216–221. Available online: https://github.com/rvlenth/emmeans (accessed on 19 April 2022).
- Wilke, C. Cowplot: Streamlined Plot Theme and Plot Annotations for “Ggplot2”; 2017. Available online: https://github.com/wilkelab/cowplot (accessed on 5 September 2019).
- Wickham, H. Ggplot2; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-24275-0. [Google Scholar]
- Signorelli, M.; Spitali, P.; Tsonaka, R. Poisson–Tweedie Mixed-Effects Model: A Flexible Approach for the Analysis of Longitudinal RNA-Seq Data. Stat. Model. 2021, 21, 520–545. [Google Scholar] [CrossRef]
- Environmental Systems Research Institute (ESRI). Spatial Analysis and Data Science—ArcGis 10.5; 2017; Available online: http://www.esri.com/software/arcgis/index.html (accessed on 1 December 2019).
- Secretaria de Municipal da Saúde. Comunicando a Identificação de Larvas Do Mosquito Aedes aegypti No Município de Morretes. Nota No18/2014. Secretaria de Municipal da Saúde: Morretes, Parana, Brazil, 2014; not published. [Google Scholar]
- Paranaguá, Secretaria de Municipal da Saúde. Plano Municipal de Contingência Da Dengue; Secretaria de Municipal da Saúde; Paranaguá, Secretaria de Municipal da Saúde: Paranaguá, Brasil, 2014. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/plano_contingencia_nacional_epidemias_dengue.pdf (accessed on 30 May 2018).
- Wilson-Bahun, T.A.; Kamgang, B.; Lenga, A.; Wondji, C.S. Larval Ecology and Infestation Indices of Two Major Arbovirus Vectors, Aedes Aegypti and Aedes Albopictus (Diptera: Culicidae), in Brazzaville, the Capital City of the Republic of the Congo. Parasites Vectors 2020, 13, 492. [Google Scholar] [CrossRef]
- Alfsnes, K.; Eldholm, V.; Gaunt, M.W.; de Lamballerie, X.; Gould, E.A.; Pettersson, J.H.O. Tracing and Tracking the Emergence, Epidemiology and Dispersal of Dengue Virus to Africa during the 20th Century. One Health 2021, 13, 100337. [Google Scholar] [CrossRef]
- Dalpadado, R.; Amarasinghe, D.; Gunathilaka, N.; Ariyarathna, N. Bionomic Aspects of Dengue Vectors Aedes Aegypti and Aedes Albopictus at Domestic Settings in Urban, Suburban and Rural Areas in Gampaha District, Western Province of Sri Lanka. Parasites Vectors 2022, 15, 148. [Google Scholar] [CrossRef]
- Pereira dos Santos, T.; Roiz, D.; Santos de Abreu, F.V.; Luz, S.L.B.; Santalucia, M.; Jiolle, D.; Santos Neves, M.S.A.; Simard, F.; Lourenço-de-Oliveira, R.; Paupy, C. Potential of Aedes Albopictus as a Bridge Vector for Enzootic Pathogens at the Urban-Forest Interface in Brazil. Emerg. Microbes Infect. 2018, 7, 1–8. [Google Scholar] [CrossRef]
- Lima-Camara, T.N.; Urbinatti, P.R.; Chiaravalloti-Neto, F. Finding Aedes Aegypti in a Natural Breeding Site in an Urban Zone, Sao Paulo, Southeastern Brazil. Rev. de Saúde Pública 2016, 50. [Google Scholar] [CrossRef] [PubMed]
- Ayllón, T.; Câmara, D.C.P.; Morone, F.C.; da Silva Gonçalves, L.; de Barros, F.S.M.; Brasil, P.; Carvalho, M.S.; Honório, N.A. Dispersion and Oviposition of Aedes Albopictus in a Brazilian Slum: Initial Evidence of Asian Tiger Mosquito Domiciliation in Urban Environments. PLoS ONE 2018, 13, e0195014. [Google Scholar] [CrossRef] [PubMed]
- Ferraguti, M.; Martínez-De La Puente, J.; Roiz, D.; Ruiz, S.; Soriguer, R.; Figuerola, J. Effects of landscape anthropization on mosquito community composition and abundance. Sci. Rep. 2016, 6, 29002. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca Júnior, D.P.; Serpa, L.L.N.; Barbosa, G.L.; Pereira, M.; Holcmam, M.M.; Voltolini, J.C.; Marques, G.R.A.M. Vectors of Arboviruses in the State of São Paulo: 30 Years of Aedes Aegypti and Aedes Albopictus. Rev. de Saúde Pública 2019, 53, 84. [Google Scholar] [CrossRef]
- Barbosa, R.M.R.; de Melo-Santos, M.A.V.; Silveira, J.C., Jr.; Silva-Filha, M.H.N.L.; Souza, W.V.; de Oliveira, C.M.F.; Ayres, C.F.J.; do Xavier, M.N.; Rodrigues, M.P.; dos Santos, S.A.; et al. Infestation of an Endemic Arbovirus Area by Sympatric Populations of Aedes Aegypti and Aedes Albopictus in Brazil. Memórias do Inst. Oswaldo Cruz 2020, 115, e190437. [Google Scholar] [CrossRef]
- Juliano, S.A.; Philip Lounibos, L. Ecology of Invasive Mosquitoes: Effects on Resident Species and on Human Health. Ecol. Lett. 2005, 8, 558–574. [Google Scholar] [CrossRef]
- Bezerra, J.; Araújo, R.; Melo, F.; Gonçalves, C.; Chaves, B.; Silva, B.; Silva, L.; Brandão, S.; Secundino, N.; Norris, D.; et al. Aedes (Stegomyia) Albopictus Dynamics Influenced by Spatiotemporal Characteristics in a Brazilian Dengue-Endemic Risk City. Acta Trop. 2016, 164, 431–437. [Google Scholar] [CrossRef]
- Ferreira-de-Lima, V.H.; Câmara, D.C.P.; Honório, N.A.; Lima-Camara, T.N. The Asian Tiger Mosquito in Brazil: Observations on Biology and Ecological Interactions since Its First Detection in 1986. Acta Trop. 2020, 205, 105386. [Google Scholar] [CrossRef]
- de Moura, M.C.B.M.; de Oliveira, J.V.; Pedreira, R.M.; de Tavares, A.M.; de Souza, T.A.; de Lima, K.C.; Barbosa, I.R. Spatio-Temporal Dynamics of Aedes Aegypti and Aedes Albopictus Oviposition in an Urban Area of Northeastern Brazil. Trop. Med. Int. Health 2020, 25, 1510–1521. [Google Scholar] [CrossRef]
- Lourenço de Oliveira, R.; Vazeille, M.; de Filippis, A.M.B.; Failloux, A.-B. Large Genetic Differentiation and Low Variation in Vector Competence for Dengue and Yellow Fever Viruses of Aedes Albopictus from Brazil, the United States, and the Cayman Islands. Am. J. Trop. Med. Hyg. 2003, 69, 105–114. [Google Scholar] [CrossRef]
- Amraoui, F.; Pain, A.; Piorkowski, G.; Vazeille, M.; Couto-Lima, D.; de Lamballerie, X.; Lourenço-de-Oliveira, R.; Failloux, A.B. Experimental Adaptation of the Yellow Fever Virus to the Mosquito Aedes Albopictus and Potential Risk of Urban Epidemics in Brazil, South America. Sci. Rep. 2018, 8, 14337. [Google Scholar] [CrossRef]
- Couto-Lima, D.; Madec, Y.; Bersot, M.I.; Campos, S.S.; de Motta, M.A.; dos Santos, F.B.; Vazeille, M.; da Vasconcelos, P.F.C.; Lourenço-de-Oliveira, R.; Failloux, A.-B. Potential Risk of Re-Emergence of Urban Transmission of Yellow Fever Virus in Brazil Facilitated by Competent Aedes Populations. Sci. Rep. 2017, 7, 4848. [Google Scholar] [CrossRef] [PubMed]
- Gabiane, G.; Yen, P.S.; Failloux, A.B. Aedes Mosquitoes in the Emerging Threat of Urban Yellow Fever Transmission. Rev. Med. Virol. 2022, 32, e2333. [Google Scholar] [CrossRef]
- Gu, H.; Leung, R.; Jing, Q.; Zhang, W.; Yang, Z.; Lu, J.; Hao, Y.; Zhang, D. Meteorological Factors for Dengue Fever Control and Prevention in South China. Int. J. Environ. Res. Public Health 2016, 13, 867. [Google Scholar] [CrossRef] [PubMed]
- de Custódio, J.M.O.; Nogueira, L.M.S.; Souza, D.A.; Fernandes, M.F.; Oshiro, E.T.; de Oliveira, E.F.; Piranda, E.M.; de Oliveira, A.G. Abiotic Factors and Population Dynamic of Aedes Aegypti and Aedes Albopictus in an Endemic Area of Dengue in Brazil. Rev. do Inst. de Med. Trop. de Sao Paulo 2019, 61, e18. [Google Scholar] [CrossRef]
- Da Cruz Ferreira, D.A.; Degener, C.M.; de Almeida Marques-Toledo, C.; Bendati, M.M.; Fetzer, L.O.; Teixeira, C.P.; Eiras, Á.E. Meteorological Variables and Mosquito Monitoring Are Good Predictors for Infestation Trends of Aedes Aegypti, the Vector of Dengue, Chikungunya and Zika. Parasites Vectors 2017, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Kramer, I.M.; Pfeiffer, M.; Steffens, O.; Schneider, F.; Gerger, V.; Phuyal, P.; Braun, M.; Magdeburg, A.; Ahrens, B.; Groneberg, D.A.; et al. The Ecophysiological Plasticity of Aedes Aegypti and Aedes Albopictus Concerning Overwintering in Cooler Ecoregions is Driven by Local Climate and Acclimation Capacity. Sci. Total Environ. 2021, 778, 146128. [Google Scholar] [CrossRef] [PubMed]
- Chouin-Carneiro, T.; Vega-Rua, A.; Vazeille, M.; Yebakima, A.; Girod, R.; Goindin, D.; Dupont-Rouzeyrol, M.; Lourenço-de-Oliveira, R.; Failloux, A.-B. Differential Susceptibilities of Aedes Aegypti and Aedes Albopictus from the Americas to Zika Virus. PLoS Negl. Trop. Dis. 2016, 10, e0004543. [Google Scholar] [CrossRef]
- Rezende, H.R.; Romano, C.M.; Claro, I.M.; Caleiro, G.S.; Sabino, E.C.; Felix, A.C.; Bissoli, J.; Hill, S.; Faria, N.R.; Cardoso da Silva, T.C.; et al. First Report of Aedes Albopictus Infected by Dengue and Zika Virus in a Rural Outbreak in Brazil. PLoS ONE 2020, 15, e0229847. [Google Scholar] [CrossRef] [PubMed]
- Unlu, I.; Faraji, A.; Indelicato, N.; McNelly, J.R. Do Tigers Hunt during the Day? Diel Activity of the Asian Tiger Mosquito, Aedes Albopictus (Diptera: Culicidae), in Urban and Suburban Habitats of North America. PLoS Negl. Trop. Dis. 2021, 15, e0009438. [Google Scholar] [CrossRef] [PubMed]
- Gratz, N.G. Critical Review of the Vector Status of Aedes Albopictus. Med. Vet. Entomol. 2004, 18, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Hawley, W.A. The Biology of Aedes Albopictus. J. Am. Mosq. Control Assoc. Suppl. 1988, 1, 1–39. [Google Scholar]
- Monge, S.; García-Ortúzar, V.; López Hernández, B.; Lopaz Pérez, M.Á.; Delacour-Estrella, S.; Sánchez-Seco, M.P.P.; Fernández Martinez, B.; García San Miguel, L.; García-Fulgueiras, A.; Sierra Moros, M.J.J. Characterization of the First Autochthonous Dengue Outbreak in Spain (August–September 2018). Acta Trop. 2020, 205, 105402. [Google Scholar] [CrossRef] [PubMed]
- Laycock, T.; Ureña Paniego, C.; Javier, J. The Threat of Mosquito-Borne Arboviral Disease in Spain: A Bibliographic Review. Med. Clínica 2022, 158, 378–386. [Google Scholar] [CrossRef]
- Mariconti, M.; Obadia, T.; Mousson, L.; Malacrida, A.; Gasperi, G.; Failloux, A.-B.; Yen, P.-S. Estimating the Risk of Arbovirus Transmission in Southern Europe Using Vector Competence Data. Sci. Rep. 2019, 9, 17852. [Google Scholar] [CrossRef]
- Bellini, R.; Michaelakis, A.; Petrić, D.; Schaffner, F.; Alten, B.; Angelini, P.; Aranda, C.; Becker, N.; Carrieri, M.; di Luca, M.; et al. Practical Management Plan for Invasive Mosquito Species in Europe: I. Asian Tiger Mosquito (Aedes Albopictus). Travel Med. Infect. Dis. 2020, 35, 101691. [Google Scholar] [CrossRef]
- Barzon, L.; Gobbi, F.; Capelli, G.; Montarsi, F.; Martini, S.; Riccetti, S.; Sinigaglia, A.; Pacenti, M.; Pavan, G.; Rassu, M.; et al. Autochthonous Dengue Outbreak in Italy 2020: Clinical, Virological and Entomological Findings. J. Travel Med. 2021, 28, taab130. [Google Scholar] [CrossRef]
- Grandadam, M.; Caro, V.; Plumet, S.; Thiberge, J.M.; Souarès, Y.; Failloux, A.B.; Tolou, H.J.; Budelot, M.; Cosserat, D.; Leparc-Goffart, I.; et al. Chikungunya Virus, Southeastern France. Emerg. Infect. Dis. 2011, 17, 910–913. [Google Scholar] [CrossRef] [PubMed]




| Collection Points Positive | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Months | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | Total |
| Sep/17 | + | + | + | 3 | ||||||||||||||||||||
| Oct/17 | + | + | + | + | + | + | 6 | |||||||||||||||||
| Nov/17 | + | + | + | + | + | + | + | + | 8 | |||||||||||||||
| Dec/17 | + | + | + | + | + | + | + | + | + | + | 10 | |||||||||||||
| Jan/18 | + | + | + | + | + | + | + | + | + | + | + | 11 | ||||||||||||
| Feb/18 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 18 | |||||
| Mar/18 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 19 | ||||
| Apr/18 | + | + | + | + | + | + | 6 | |||||||||||||||||
| May/18 | + | + | + | + | + | 5 | ||||||||||||||||||
| Jun/18 | + | 1 | ||||||||||||||||||||||
| Jul/18 | + | + | 2 | |||||||||||||||||||||
| Aug/18 | 0 | |||||||||||||||||||||||
| Sep/18 | + | + | + | + | 4 | |||||||||||||||||||
| Total | 3 | 4 | 4 | 5 | 3 | 3 | 2 | 3 | 7 | 4 | 5 | 0 | 4 | 0 | 8 | 5 | 6 | 4 | 4 | 2 | 3 | 6 | 8 | Positives sites |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, S.J.P.; de Camargo Guaraldo, A.; Honório, N.A.; Câmara, D.C.P.; Sukow, N.M.; Machado, S.T.; Duarte dos Santos, C.N.; da Costa-Ribeiro, M.C.V. Spatial and Temporal Distribution of Aedes aegypti and Aedes albopictus Oviposition on the Coast of Paraná, Brazil, a Recent Area of Dengue Virus Transmission. Trop. Med. Infect. Dis. 2022, 7, 246. https://doi.org/10.3390/tropicalmed7090246
de Souza SJP, de Camargo Guaraldo A, Honório NA, Câmara DCP, Sukow NM, Machado ST, Duarte dos Santos CN, da Costa-Ribeiro MCV. Spatial and Temporal Distribution of Aedes aegypti and Aedes albopictus Oviposition on the Coast of Paraná, Brazil, a Recent Area of Dengue Virus Transmission. Tropical Medicine and Infectious Disease. 2022; 7(9):246. https://doi.org/10.3390/tropicalmed7090246
Chicago/Turabian Stylede Souza, Silvia Jaqueline Pereira, André de Camargo Guaraldo, Nildimar Alves Honório, Daniel Cardoso Portela Câmara, Natali Mary Sukow, Sarita Terezinha Machado, Claudia Nunes Duarte dos Santos, and Magda Clara Vieira da Costa-Ribeiro. 2022. "Spatial and Temporal Distribution of Aedes aegypti and Aedes albopictus Oviposition on the Coast of Paraná, Brazil, a Recent Area of Dengue Virus Transmission" Tropical Medicine and Infectious Disease 7, no. 9: 246. https://doi.org/10.3390/tropicalmed7090246
APA Stylede Souza, S. J. P., de Camargo Guaraldo, A., Honório, N. A., Câmara, D. C. P., Sukow, N. M., Machado, S. T., Duarte dos Santos, C. N., & da Costa-Ribeiro, M. C. V. (2022). Spatial and Temporal Distribution of Aedes aegypti and Aedes albopictus Oviposition on the Coast of Paraná, Brazil, a Recent Area of Dengue Virus Transmission. Tropical Medicine and Infectious Disease, 7(9), 246. https://doi.org/10.3390/tropicalmed7090246










