Prevalence and Molecular Identification of Schistosoma haematobium among Children in Lusaka and Siavonga Districts, Zambia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Design
2.2. Sample Collection
2.3. Sample Preparation and Processing
2.3.1. Microscopic Examination
2.3.2. DNA Extraction and Amplification
2.3.3. Purification of the PCR Products and Cycle Sequencing
2.4. Data Analysis
2.4.1. Epidemiological Data Analysis
2.4.2. Sequence Analysis
3. Results
3.1. Demographic Characteristics of the Study Participants
3.2. Prevalence of Schistosomiasis Based on Microscopic Examination
3.3. Distribution of Potential Risk Factors
3.4. Analysis of the Association between Potential Risk Factors and Schistosoma Positivity
3.5. Maximum Likelihood Estimates of Risk Factors for Schistosomiasis
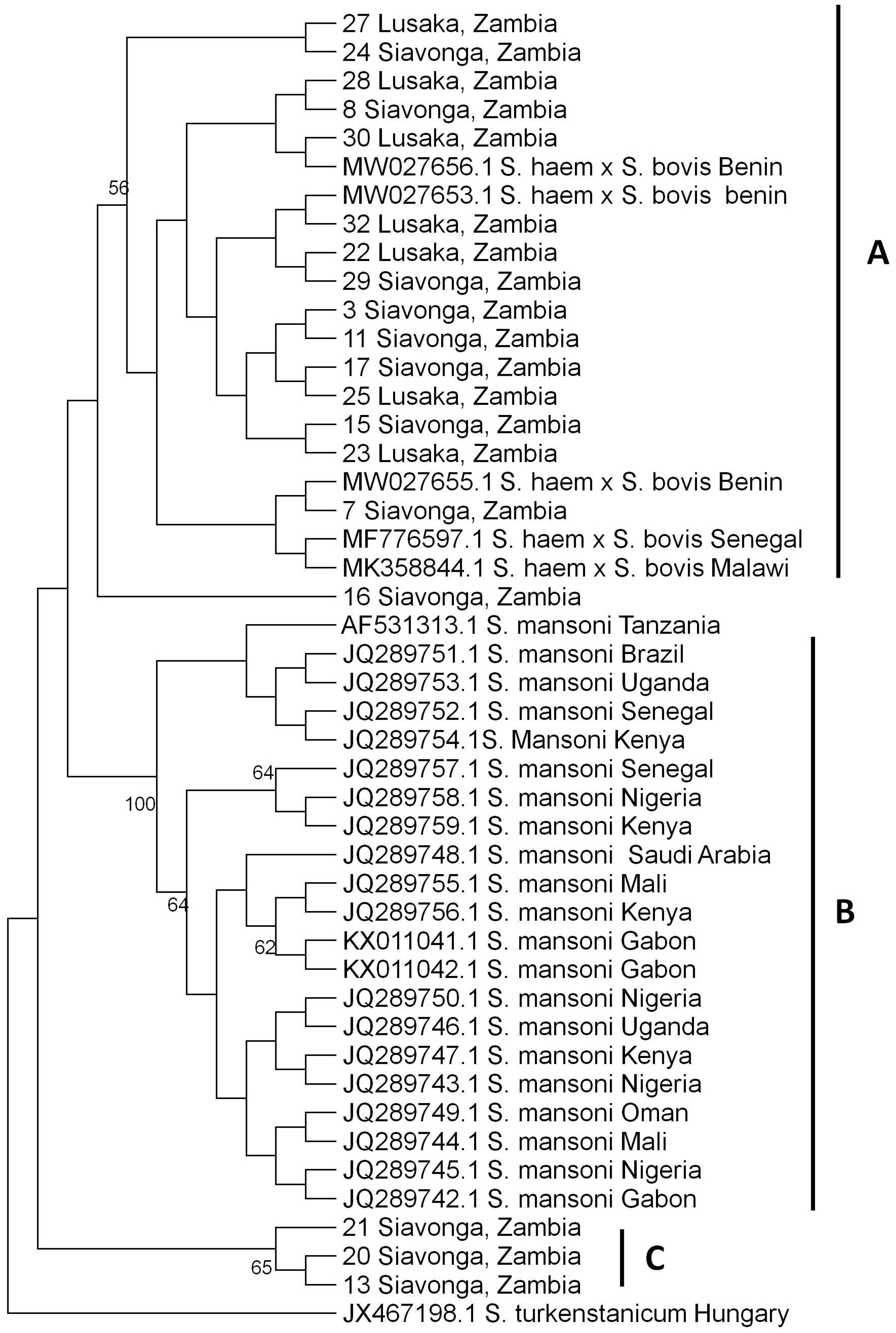
3.6. Phylogenetic Tree Analysis of S. haematobium nuclear ITS gene
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Golan, R.; Gower, C.M.; Emery, A.M.; Rollinson, D.; Webster, J.P. Europe PMC Funders Group Isolation and characterization of the first polymorphic microsatellite markers for Schistosoma haematobium and their application in multiplex reactions of larval stages. Mol. Ecol. Resour. 2014, 8, 647–649. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aula, O.P.; McManus, D.P.; Jones, M.K.; Gordon, C.A. Schistosomiasis with a focus on Africa. Trop. Med. Infect. Dis. 2021, 6, 109. [Google Scholar] [CrossRef]
- Weerakoon, K.G. DNA Diagnostics for Schistosomiasis Control. Trop. Med. Infect. Dis. 2018, 3, 81. [Google Scholar] [CrossRef]
- Mutengo, M.M.; Mwansa, J.C.L.; Mduluza, T.; Sianongo, S.; Chipeta, J. High Schistosoma mansoni disease burden in a rural district of Western Zambia. Am. J. Trop. Med. Hyg. 2014, 91, 965–972. [Google Scholar] [CrossRef]
- Lawiye, J.; Vandi, P.V.; Godly, C.; Midala, A.; Watirahel, P.; Enamola, W. Prevalence and risk factors of Schistosoma haematobium infections among primary school children in Igbokuta Village, Ikorodu North Local Government, Lagos State. IOSR J. Nurs. Health Sci. 2020, 2, 62–68. [Google Scholar]
- Kalinda, C.; Chimbari, M.J.; Mukaratirwa, S. Schistosomiasis in Zambia: A systematic review of past and present experiences. Infect. Dis. Poverty 2018, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Monde, C.; Syampungani, S.; van den Brink, P.J. Natural and human induced factors influencing the abundance of Schistosoma host snails in Zambia. Environ. Monit. Assess. 2016, 188, 370. [Google Scholar] [CrossRef]
- Kalungwana, N.; Mwakazanga, D.; Mwansa, J.; Mutengo, M.M.; Siziya, S. Prevalence and factors associated with Schistosomiasis in Ng’ombe Township of Lusaka urban district. JABS 2012, 1, 7–11. [Google Scholar]
- Agnew-Blais, J.; Carnevale, J.; Gropper, A.; Shilika, E.; Bail, R.; Ngoma, M. Schistosomiasis haematobium prevalence and risk factors in a school-age population of peri-urban Lusaka, Zambia. J. Trop. Pediatr. 2009, 56, 247–253. [Google Scholar] [CrossRef]
- Saathoff, E.; Olsen, A.; Magnussen, P.; Becker, W.; Appleton, C.C. Patterns of Schistosoma haematobium infection, impact of praziquantel treatment and re-infection after treatment in a cohort of schoolchildren from rural KwaZulu-Natal/South Africa. BMC Infect. Dis. 2004, 4, 40. [Google Scholar] [CrossRef]
- Monde, C.; Syampungani, S.; Van den Brink, P.J. Exploring the potential of host-environment relationships in the control of schistosomiasis in Africa. Afr. J. Aquat. Sci. 2015, 40, 47–55. [Google Scholar] [CrossRef]
- Aryeetey, Y.A.; Essien-Baidoo, S.; Larbi, I.A.; Ahmed, K.; Amoah, A.S.; Obeng, B.B.; Van Lieshout, L.; Yazdanbakhsh, M.; Boakye, D.A.; Verweij, J.J. Molecular diagnosis of Schistosoma infections in urine samples of school children in Ghana. Am. J. Trop. Med. Hyg. 2013, 88, 1028–1031. [Google Scholar] [CrossRef]
- Kosinski, K.C.; Bosompem, K.M.; Stadecker, M.J.; Wagner, A.D.; Plummer, J.; Durant, J.L.; Gute, D.M. Diagnostic accuracy of urine filtration and dipstick tests for Schistosoma haematobium infection in a lightly infected population of Ghanaian schoolchildren. Acta Trop. 2011, 118, 123–127. [Google Scholar] [CrossRef]
- Mendy, A.; Kargbo, A.; Ibrahim, Y.K.E.; Entonu, M.E.; Gbem, T.T. Molecular epidemiology of schistosomiasis in Central River Region of The Gambia. Afr. J. Biotechnol. 2020, 19, 508–519. [Google Scholar]
- Leger, E.; Webster, J.P. Hybridizations within the Genus Schistosoma: Implications for evolution, epidemiology and control. Parasitology 2017, 144, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Hessler, M.J.; Cyrs, A.; Krenzke, S.C.; Mahmoud, E.S.; Sikasunge, C.; Mwansa, J.; Lodh, N. Detection of duo-schistosome infection from filtered urine samples from school children in Zambia after MDA. PLoS ONE 2017, 12, e0189400. [Google Scholar] [CrossRef]
- Halwindi, H.; Magnussen, P.; Olsen, A.; Lisulo, M. Potential contribution of adult populations to the maintenance of schistosomiasis and soil-transmitted helminth infections in the Siavonga and Mazabuka Districts of Zambia. J. Biosoc. Sci. 2017, 49, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Webster, B.L.; Emery, A.M.; Webster, J.P.; Gouvras, A.; Garba, A.; Diaw, O.; Seye, M.M.; Tchuente, L.A.; Simoonga, C.; Mwanga, J.; et al. Genetic Diversity within Schistosoma haematobium: DNA Barcoding Reveals Two Distinct Groups. PLoS Negl. Trop. Dis. 2012, 6, e1882. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Shawa, S.T.; Mwase, E.T.; Simonsen, P.E. Surveys for Schistosomiasis and Soil Transmitted Helminths in Luangwa, Kalabo and Serenje Districts of Zambia. Med. J. Zambia 2014, 41, 174–180. [Google Scholar]
- Kabuyaya, M.; Chimbari, M.J.; Manyangadze, T.; Mukaratirwa, S. Efficacy of praziquantel on Schistosoma haematobium and re-infection rates among school-going children in the Ndumo area of uMkhanyakude district, KwaZulu-Natal, South Africa. Infect. Dis. Poverty 2017, 6, 83. [Google Scholar] [CrossRef]
- N’goran, E.K.; Utzinger, J.; N’guessan, A.N.; Müller, I.; Zamblé, K.; Lohourignon, K.L.; Traoré, M.; Sosthène, B.A.; Lengeler, C.; Tanner, M. Reinfection with Schistosoma haematobium following school-based chemotherapy with praziquantel in four highly endemic villages in Côte d’Ivoire. Trop. Med. Int. Health 2001, 6, 817–825. [Google Scholar] [CrossRef]
- Hajissa, K.; Muhajir, A.E.; Eshag, H.A.; Alfadel, A.; Nahied, E.; Dahab, R.; Ali, S.M.; Mohammed, M.; Gaafar, M.; Mohamed, Z. Prevalence of schistosomiasis and associated risk factors among school children in Um-Asher Area, Khartoum, Sudan. BMC Res. Notes 2018, 11, 779. [Google Scholar] [CrossRef] [PubMed]
- Angora, E.K.; Boissier, J.; Menan, H.; Rey, O.; Tuo, K.; Touré, A.O.; Coulibaly, J.T.; Méité, A.; Raso, G.; N’goran, E.K.; et al. Prevalence and risk factors for schistosomiasis among schoolchildren in two settings of Côte d’Ivoire. Trop. Med. Infect. Dis. 2019, 4, 110. [Google Scholar] [CrossRef] [PubMed]
- Joof, E.; Sanyang, A.M.; Camara, Y.; Sey, A.P.; Baldeh, I.; Jah, S.L.; Ceesay, S.J.; Sambou, S.M.; Sanyang, S.; Wade, C.M.; et al. Prevalence and risk factors of schistosomiasis among primary school children in four selected regions of the gambia. PLoS Negl. Trop. Dis. 2021, 15, e0009380. [Google Scholar] [CrossRef]
- Senghor, B.; Diallo, A.; Sylla, S.N.; Doucouré, S.; Ndiath, M.O.; Gaayeb, L.; Djuikwo-Teukeng, F.F.; Bâ, C.T.; Sokhna, C. Prevalence and intensity of urinary schistosomiasis among school children in the district of Niakhar, region of Fatick, Senegal. Parasites Vectors 2014, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Sam-Wobo, S.O.; Idowu, J.M.; Adeleke, M.A. Urinary schistosomiasis among children and teenagers near Oyan dam, Abeokuta, Nigeria. J. Rural Trop. Public Health 2011, 10, 57–60. Available online: http://www.jcu.edu.au/jrtph/vol/JRTPHVol10p57-60Adeleke.pdf (accessed on 25 June 2022).
- Noriode, R.M.; Idowu, E.T.; Otubanjo, O.A.; Mafe, M.A. Urinary schistosomiasis in school aged children of two rural endemic communities in Edo State, Nigeria. J. Infect. Public Health 2018, 11, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Amuta, E.U.; Houmsou, R.S. Prevalence, intensity of infection and risk factors of urinary schistosomiasis in pre-school and school aged children in Guma Local Government Area, Nigeria. Asian Pac. J. Trop. Med. 2014, 7, 34–39. [Google Scholar] [CrossRef]
- Poole, H.; Terlouw, D.J.; Naunje, A.; Mzembe, K.; Stanton, M.; Betson, M.; Lalloo, D.G.; Stothard, J.R. Schistosomiasis in pre-school-age children and their mothers in Chikhwawa district, Malawi with notes on characterization of schistosomes and snails. Parasites Vectors 2014, 7, 153. [Google Scholar] [CrossRef]
- Betson, M.; Sousa-figueiredo, J.C.; Kabatereine, N.B.; Stothard, J.R. New Insights into the Molecular Epidemiology and Population Genetics of Schistosoma mansoni in Ugandan Pre-school Children and Mothers. PLoS Negl. Trop. Dis. 2013, 7, e2561. [Google Scholar] [CrossRef]
- Simoonga, C.; Kazembe, L.N.; Kristensen, T.K.; Olsen, A.; Appleton, C.C.; Mubita, P.; Mubila, L. The epidemiology and small-scale spatial heterogeneity of urinary schistosomiasis in Lusaka province, Zambia. Geospatial Health 2008, 3, 57–67. [Google Scholar] [CrossRef]
- Zida, A.; Briegel, J.; Kabré, I.; Sawadogo, M.P.; Sangaré, I.; Bamba, S.; Yacouba, A.; Ouédraogo, A.; Yonli, D.; Drabo, F.; et al. Epidemiological and clinical aspects of urogenital schistosomiasis in women, in Burkina Faso, West Africa. Infect. Dis. Poverty 2016, 5, 81. [Google Scholar] [CrossRef] [PubMed]
- Ndassi, V.D.; Anchang-Kimbi, J.K.; Sumbele, I.U.N.; Wepnje, G.B.; Kimbi, H.K. Prevalence and risk factors associated with S. haematobium Egg Excretion during the Dry Season, Six Months following Mass Distribution of Praziquantel (PZQ) in 2017 in the Bafia Health Area, South West Region Cameroon: A Cross-Sectional Study. J. Parasitol. Res. 2019, 2019, 4397263. [Google Scholar] [CrossRef] [PubMed]
- Ogbonna, C.C.; Dori, G.U.; Nweze, E.I.; Muoneke, G.; Nwankwo, I.E.; Akputa, N. Comparative analysis of urinary schistosomiasis among primary school children and rural farmers in Obollo-Eke, Enugu State, Nigeria: Implications for control. Asian Pac. J. Trop. Med. 2012, 5, 796–802. [Google Scholar] [CrossRef]
- Atalabi, T.E.; Adoh, S.D.; Eze, K.M. The current epidemiological status of urogenital schistosomiasis among primary school pupils in Katsina State, Nigeria: An imperative for a scale up of water and sanitation initiative and mass administration of medicines with Praziquantel. PLoS Negl. Trop. Dis. 2018, 12, e0006636. [Google Scholar] [CrossRef]
- Umar, S.; Shinfafi, S.H.; Hudu, S.A.; Neela, V.; Suresh, K.; Nordin, S.A.; Malina, O. Prevalence and molecular characterisation of Schistosoma haematobium among primary school children in Kebbi State, Nigeria. Ann. Parasitol. 2017, 63, 133–139. Available online: http://www.ncbi.nlm.nih.gov/pubmed/28822206 (accessed on 16 July 2022). [PubMed]
- Cisse, M.; Sangare, I.; Djibougou, A.D.; Tahita, M.C.; Gnissi, S.; Bassinga, J.K.; Konda, S.; Diallo, A.H. Prevalence and risk factors of Schistosoma mansoni infection among preschool-aged children from Panamasso village, Burkina Faso. Parasites Vectors 2021, 14, 185. [Google Scholar] [CrossRef] [PubMed]
- Kabuyaya, M.; Chimbari, M.J.; Manyangadze, T.; Mukaratirwa, S. Schistosomiasis risk factors based on the infection status among school-going children in the Ndumo area, uMkhanyakude district, South Africa. S. Afr. J. Infect. Dis. 2017, 32, 67–72. [Google Scholar]
- Kapito-Tembo, A.P.; Mwapasa, V.; Meshnick, S.R.; Samanyika, Y.; Banda, D.; Bowie, C.; Radke, S. Prevalence distribution and risk factors for Schistosoma hematobium infection among school children in Blantyre, Malawi. PLoS Negl. Trop. Dis. 2009, 3, e361. [Google Scholar]
- Ismail, H.A.; Hong, S.T.; Babiker, A.T.; Hassan, R.M.; Sulaiman, M.A.; Jeong, H.G.; Kong, W.H.; Lee, S.H.; Cho, H.I.; Nam, H.S.; et al. Prevalence, risk factors, and clinical manifestations of schistosomiasis among school children in the White Nile River basin, Sudan. Parasites Vectors 2014, 7, 478. [Google Scholar] [CrossRef]
- Angelo, T.; Buza, J.; Kinung’hi, S.M.; Kariuki, H.C.; Mwanga, J.R.; Munisi, D.Z.; Wilson, S. Geographical and behavioral risks associated with Schistosoma haematobium infection in an area of complex transmission. Parasites Vectors 2018, 11, 481. [Google Scholar] [CrossRef]
- Angora, E.K.; Allienne, J.F.; Rey, O.; Menan, H.; Touré, A.O.; Coulibaly, J.T.; Raso, G.; Yavo, W.; N’Goran, E.K.; Utzinger, J.; et al. High prevalence of Schistosoma haematobium × Schistosoma bovis hybrids in schoolchildren in Côte d’Ivoire. Parasitology 2020, 147, 287–294. [Google Scholar] [CrossRef]
- Tian-Bi, Y.N.; Webster, B.; Konan, C.K.; Allan, F.; Diakité, N.R.; Ouattara, M.; Salia, D.; Koné, A.; Kakou, A.K.; Rabone, M.; et al. Molecular characterization and distribution of Schistosoma cercariae collected from naturally infected bulinid snails in northern and central Côte d’Ivoire. Parasites Vectors 2019, 12, 117. [Google Scholar] [CrossRef]
- Kincaid-Smith, J.; Tracey, A.; de Carvalho Augusto, R.; Bulla, I.; Holroyd, N.; Rognon, A.; Rey, O.; Chaparro, C.; Oleaga, A.; Mas-Coma, S.; et al. Morphological and genomic characterisation of the Schistosoma hybrid infecting humans in Europe reveals admixture between Schistosoma haematobium and Schistosoma bovis. PLoS Negl. Trop. Dis. 2021, 15, e0010062. [Google Scholar] [CrossRef]
- Bonnie LWebster, M.H.A.; Sekeleghe Kayuni, P.M.; Fenella Halstead, R.C.; Lazarus Juziwelo, M.C.S.; EJames LaCourse, D.R.; Khumbo Kalua, J.R.S. No TitleSchistosome Interactions within the Schistosoma haematobium Group, Malawi. Emerg. Infect. Dis. 2019, 25, 1245–1247. [Google Scholar]
- Onyekwere, A.M.; Rey, O.; Allienne, J.F.; Nwanchor, M.C.; Alo, M.; Uwa, C.; Boissier, J. Population Genetic Structure and Hybridization of Schistosoma haematobium in Nigeria. Pathogens 2022, 11, 425. [Google Scholar] [CrossRef] [PubMed]
- Barber, K.E.; Mkoji, G.M.; Loker, E.S. PCR-RFLP analysis of the ITS2 region to identify Schistosoma haematobium and S. Bovis from Kenya. Am. J. Trop. Med. Hyg. 2000, 62, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Huyse, T.; Webster, B.L.; Geldof, S.; Stothard, J.R.; Diaw, O.T.; Polman, K.; Rollinson, D. Bidirectional introgressive hybridization between a cattle and human schistosome species. PLoS Pathog. 2009, 5, e1000571. [Google Scholar] [CrossRef] [PubMed]

| Demographic Characteristics | Category | Frequency (n = 421) | Proportion (%) | 95% CI |
|---|---|---|---|---|
| Gender | Male | 232 | 55.1 | 50.21–59.91 |
| Female | 189 | 44.9 | 40.09–49.79 | |
| Age (years) | ≤10 | 32 | 7.6 | 5.34–10.67 |
| 11–15 | 359 | 85.3 | 81.44–88.45 | |
| ≥16 | 30 | 7.1 | 4.94–10.13 | |
| School Grade | 1–4 | 42 | 10.0 | 7.36–13.34 |
| 5–7 | 379 | 90.0 | 86.66–92.64 | |
| School | Nabutezi | 47 | 11.2 | 8.93–14.66 |
| Chininde | 46 | 10.9 | 8.18–14.40 | |
| Mpango | 44 | 10.5 | 7.77–13.87 | |
| Siamatika | 42 | 10.0 | 7.36–13.34 | |
| Butete | 41 | 9.7 | 7.16–13.08 | |
| Ng’ombe | 201 | 47.7 | 42.90–52.63 |
| Character | Categories | Frequency (n = 421) | Prevalence | 95% CI |
|---|---|---|---|---|
| District | Lusaka | 24 | 5.7 | 3.76–8.48 |
| Siavonga | 17 | 4.0 | 2.44–6.51 | |
| Overall | 41 | 9.7 | 7.16–13.08 | |
| Sex | Male | 26 | 6.2. | 4.15–9.03 |
| Female | 15 | 3.5 | 2.01–5.94 | |
| Age | ≤10 | 4 | 0.9 | 0.30–2.58 |
| 11–15 | 35 | 8.3 | 5.94–11.48 | |
| ≥16 | 2 | 0.5 | 0.08–1.89 | |
| School | Nabutezi | 2 | 0.5 | 0.08–1.89 |
| Chininde | 4 | 0.9 | 0.30–2.58 | |
| Mpango | 3 | 0.7 | 0.18–2.25 | |
| Siamatika | 5 | 1.2 | 0.44–2.91 | |
| Butete | 3 | 0.7 | 0.18–2.25 | |
| Ng’ombe | 24 | 5.7 | 3.76–8.48 | |
| Grade | 1–4 | 3 | 0.7 | 0.18–2.25 |
| 5–7 | 38 | 9.0 | 6.54–12.28 |
| Character | Categories | Frequency | n | Prevalence | 95% CI |
|---|---|---|---|---|---|
| District | Lusaka | 24 | 201 | 11.9 | 7.95–17.43 |
| Siavonga | 17 | 220 | 7.7 | 4.70–12.29 | |
| Sex | Male | 26 | 232 | 11.2 | 7.58–16.16 |
| Female | 15 | 189 | 7.9 | 4.67–12.99 | |
| Age | ≤10 | 4 | 32 | 12.5 | 4.08–29.93 |
| 11–15 | 35 | 359 | 9.7 | 6.97–13.41 | |
| ≥16 | 2 | 30 | 6.7 | 1.16–25.51 | |
| School | Nabutezi | 2 | 47 | 4.3 | 0.74–15.73 |
| Chininde | 4 | 46 | 8.7 | 2.82–21.69 | |
| Mpango | 3 | 44 | 6.8 | 1.78–19.71 | |
| Siamatika | 5 | 42 | 11.9 | 4.47–26.43 | |
| Butete | 3 | 41 | 7.3 | 1.91–21.01 | |
| Ng’ombe | 24 | 201 | 11.9 | 7.95–17.43 | |
| Grade | 1–4 | 3 | 42 | 7.1 | 1.86–20.55 |
| 5–7 | 38 | 379 | 10.0 | 7.28–13.61 |
| Potential Risk Factors | Category | Frequency (n = 421) | Proportion (%) | 95% CI |
|---|---|---|---|---|
| Swimming | Yes | 250 | 59.4 | 54.51–64.08 |
| No | 171 | 40.6 | 35.92–45.49 | |
| Bathing | Yes | 217 | 51.5 | 46.66–56.40 |
| No | 204 | 48.5 | 43.60–53.34 | |
| Fishing | Yes | 261 | 62.0 | 57.15–66.62 |
| No | 160 | 38.0 | 33.38–42.85 | |
| Washing/water fetching | Yes | 279 | 66.3 | 61.50–70.74 |
| No | 142 | 33.7 | 29.26–38.50 | |
| Macroscopic results | Clear/Amber | 327 | 77.7 | 73.33–81.50 |
| Cloudy/Bloody | 94 | 23.3 | 18.50–26.67 | |
| Prior treatment | No | 155 | 36.8 | 32.23–41.64 |
| Yes | 226 | 53.7 | 48.79–58.51 | |
| Distance to water | Far (>5 km) | 365 | 86.7 | 82.99–89.72 |
| Near (<5 km) | 56 | 13.3 | 10.28–17.01 | |
| Knowledge about disease | Yes | 375 | 89.1 | 85.60–91.81 |
| No | 46 | 10.9 | 8.19–14.40 | |
| Herding livestock | Yes | 230 | 54.6 | 49.74–59.44 |
| No | 191 | 45.4 | 40.56–50.26 | |
| Sanitation | Good | 360 | 85.5 | 81.70–88.66 |
| Poor | 61 | 14.5 | 11.34–18.30 | |
| Prior infection | Yes | 173 | 41.1 | 36.38–45.97 |
| No | 248 | 58.9 | 54.03–53.62 |
| Variable | Level | aOR | 95% CI | p-Value |
|---|---|---|---|---|
| Fishing (n = 421) | Yes | Ref | - | - |
| No | 0.008 | 0.001–0.071 | 0.001 ** | |
| Macroscopic results (n = 421) | Clear | Ref | - | - |
| Not clear | 9.98 | 3.222–30.937 | 0.001 ** | |
| Distance to water bodies (n = 421) | Far | Ref | - | - |
| Near | 11.66 | 3.290–41.310 | 0.001 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tembo, R.; Muleya, W.; Yabe, J.; Kainga, H.; Nalubamba, K.S.; Zulu, M.; Mwaba, F.; Saad, S.A.; Kamwela, M.; Mukubesa, A.N.; et al. Prevalence and Molecular Identification of Schistosoma haematobium among Children in Lusaka and Siavonga Districts, Zambia. Trop. Med. Infect. Dis. 2022, 7, 239. https://doi.org/10.3390/tropicalmed7090239
Tembo R, Muleya W, Yabe J, Kainga H, Nalubamba KS, Zulu M, Mwaba F, Saad SA, Kamwela M, Mukubesa AN, et al. Prevalence and Molecular Identification of Schistosoma haematobium among Children in Lusaka and Siavonga Districts, Zambia. Tropical Medicine and Infectious Disease. 2022; 7(9):239. https://doi.org/10.3390/tropicalmed7090239
Chicago/Turabian StyleTembo, Rabecca, Walter Muleya, John Yabe, Henson Kainga, King S. Nalubamba, Mildred Zulu, Florence Mwaba, Shereen Ahmed Saad, Moses Kamwela, Andrew N. Mukubesa, and et al. 2022. "Prevalence and Molecular Identification of Schistosoma haematobium among Children in Lusaka and Siavonga Districts, Zambia" Tropical Medicine and Infectious Disease 7, no. 9: 239. https://doi.org/10.3390/tropicalmed7090239
APA StyleTembo, R., Muleya, W., Yabe, J., Kainga, H., Nalubamba, K. S., Zulu, M., Mwaba, F., Saad, S. A., Kamwela, M., Mukubesa, A. N., Monde, N., Kallu, S. A., Mbewe, N., & Phiri, A. M. (2022). Prevalence and Molecular Identification of Schistosoma haematobium among Children in Lusaka and Siavonga Districts, Zambia. Tropical Medicine and Infectious Disease, 7(9), 239. https://doi.org/10.3390/tropicalmed7090239










