Coagulopathy of Dengue and COVID-19: Clinical Considerations
Abstract
1. Introduction
2. Virology of DENV
3. Virology of COVID-19
4. Epidemiology of Dengue
5. Epidemiology of COVID-19
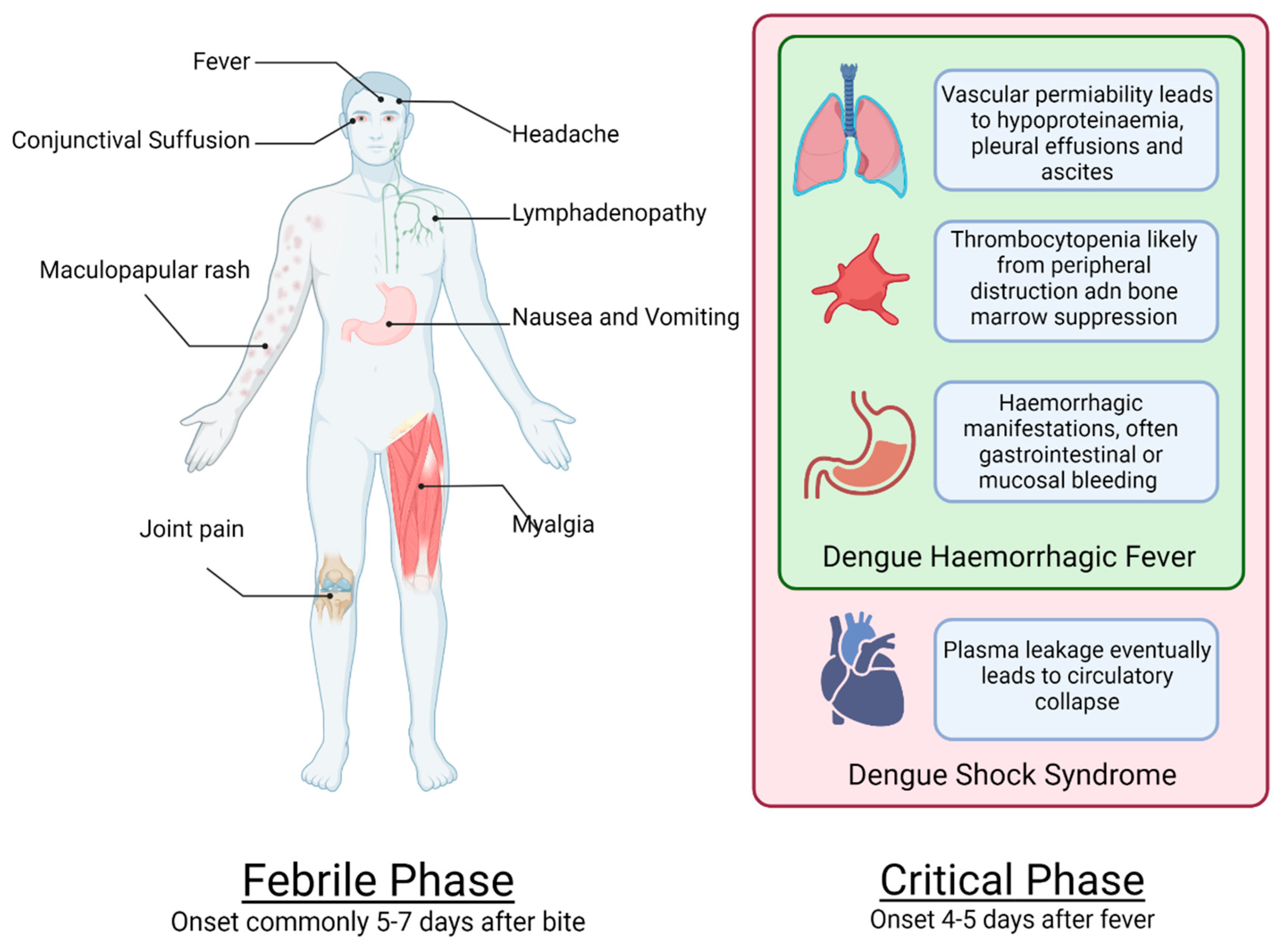
6. Severe Dengue in Adults and Infants
6.1. Antibody-Dependent Enhancement and Severe Dengue
6.2. Severe Dengue among Infants
7. General Aspects of Platelets and Haemostasis
8. Coagulopathy in Dengue
9. Thrombocytopenia Associated with COVID Vaccines
10. Coagulopathy of Dengue versus COVID-19
11. Prior Exposure to DENV and COVID-19 Severity
12. Clinical Manifestations
12.1. Haemorrhagic Manifestations of Dengue
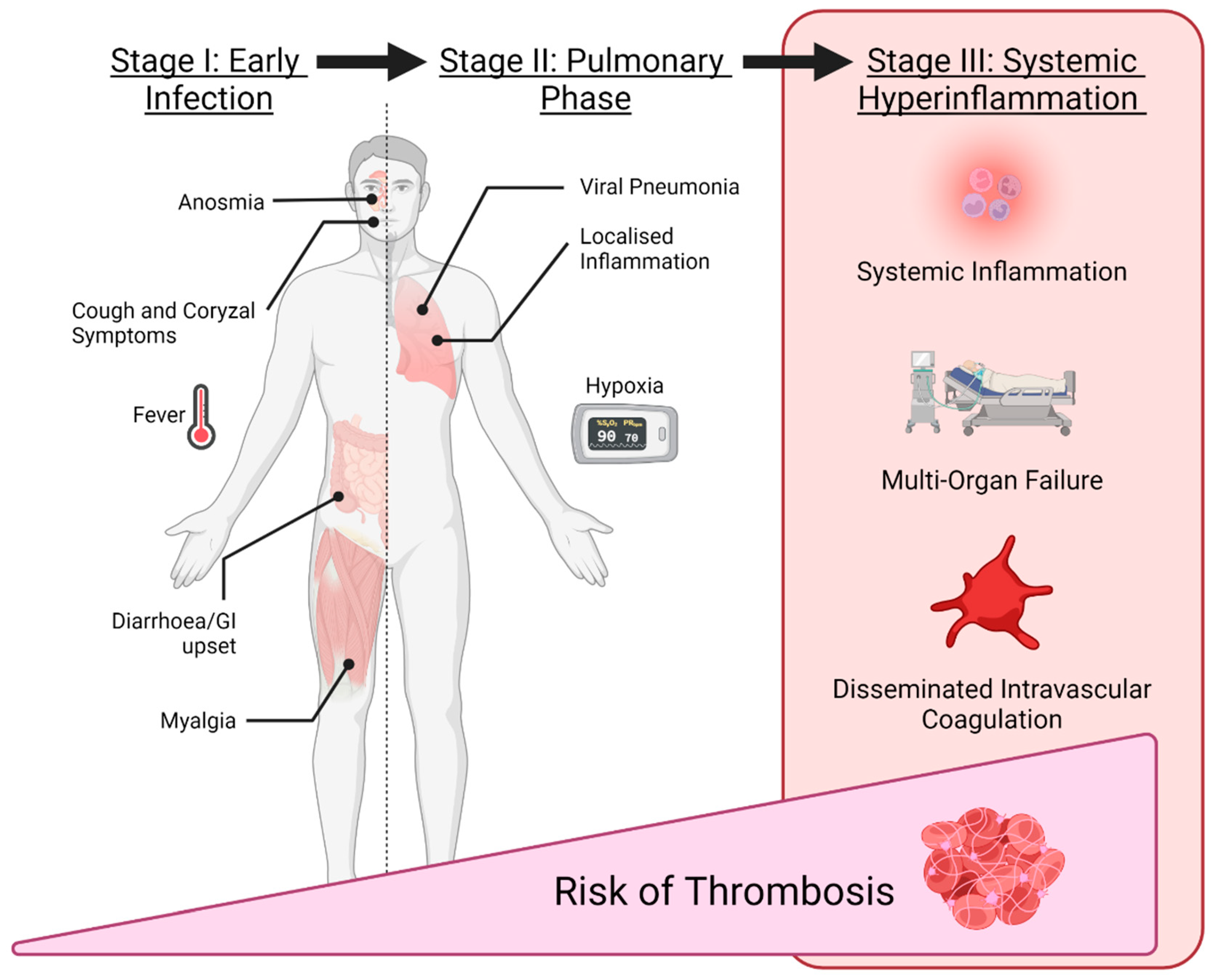
12.2. Thrombosis in COVID-19
13. Serological Cross-Reactivity between Dengue and COVID-19
14. Management Recommendations for COVID-19 Coagulopathy
14.1. Mild COVID-19 Infection
14.2. Moderate to Severe COVID-19 Infection
15. Management Recommendations in Dengue
General Aspects of Management of Dengue
16. Management of Haemorrhage in Dengue
- Prophylactic platelet transfusion should not be routinely prescribed based on low platelet counts in patients with dengue and no bleeding.
- Therapeutic platelet transfusion should not be routinely prescribed in patients with dengue with thrombocytopaenia and mild bleeding.
- There is insufficient evidence to support or refute the use of platelet transfusion in patients with severe bleeding in dengue.
- There is a need for further, well-designed RCTs to evaluate the role of platelets and plasma transfusion in patients in both the prevention of bleeding and in the setting of clinically significant bleeding in dengue infection.
- There is currently insufficient evidence regarding the role of rFVIIa, anti-D globulin, Ig or tranexamic acid in the prevention or treatment of bleeding in dengue infection and there is a need for further research on these therapeutic agents.
17. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harapan, H.; Ryan, M.; Yohan, B.; Abidin, R.S.; Nainu, F.; Rakib, A.; Jahan, I.; Emran, T.B.; Ullah, I.; Panta, K.; et al. COVID-19 and Dengue: Double Punches for Dengue-Endemic Countries in Asia. Rev. Med. Virol. 2021, 31, e2161. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, T.; Han, M.; Li, X.; Wu, D.; Xu, Y.; Zhu, Y.; Liu, Y.; Wang, X.; Wang, L. Diagnostic Utility of Clinical Laboratory Data Determinations for Patients with the Severe COVID-19. J. Med. Virol. 2020, 92, 791–796. [Google Scholar] [CrossRef]
- Kellstein, D.; Fernandes, L. Symptomatic treatment of dengue: Should the NSAID contraindication be reconsidered? Postgrad. Med. 2019, 131, 109–116. [Google Scholar] [CrossRef]
- Simmons, C.P.; Farrar, J.J.; van Vinh Chau, N.; Wills, B. Dengue. N. Engl. J. Med. 2012, 366, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Kembuan, G.J. Dengue Serology in Indonesian COVID-19 Patients: Coinfection or Serological Overlap? IDCases 2020, 22, e00927. [Google Scholar] [CrossRef]
- Wilder-Smith, A.; Tissera, H.; Ooi, E.E.; Coloma, J.; Scott, T.W.; Gubler, D.J. Preventing Dengue Epidemics during theCOVID-19 Pandemic. Am. J. Trop. Med. Hyg. 2020, 103, 570–571. [Google Scholar] [CrossRef]
- Henrina, J.; Putra, I.C.S.; Lawrensia, S.; Handoyono, Q.F.; Cahyadi, A. Coronavirus Disease of 2019: A Mimicker of Dengue Infection? SN Compr. Clin. Med. 2020, 2, 1109–1119. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Is It Dengue or Is It COVID-19? Available online: https://www.cdc.gov/dengue/healthcare-providers/dengue-or-covid.html (accessed on 18 August 2022).
- Vaughn, D.W.; Green, S.; Kalayanarooj, S.; Innis, B.L.; Nimmannitya, S.; Suntayakorn, S.; Endy, T.P.; Raengsakulrach, B.; Rothman, A.L.; Ennis, F.A.; et al. Dengue Viremia Titer, Antibody Response Pattern, and Virus Serotype Correlate with Disease Severity. J. Infect. Dis. 2000, 181, 2–9. [Google Scholar] [CrossRef]
- Peeling, R.W.; Artsob, H.; Pelegrino, J.L.; Buchy, P.; Cardosa, M.J.; Devi, S.; Enria, D.A.; Farrar, J.; Gubler, D.J.; Guz-man, M.G.; et al. Evaluation of Diagnostic Tests: Dengue. Nat. Rev. Microbiol. 2010, 8, S30–S37. [Google Scholar] [CrossRef]
- Mohamadian, M.; Chiti, H.; Shoghli, A.; Biglari, S.; Parsamanesh, N.; Esmaeilzadeh, A. COVID-19: Virology, biology and novel laboratory diagnosis. J. Gene. Med. 2021, 23, e3303. [Google Scholar] [CrossRef]
- Rowe, B.; Canosa, A.; Meslem, A.; Rowe, F. Increased airborne transmission of COVID-19 with new variants, implications for health policies. Build. Environ. 2022, 219, 109132. [Google Scholar] [CrossRef]
- Waggoner, J.J.; Gresh, L.; Vargas, M.J.; Ballesteros, G.; Tellez, Y.; Soda, K.J.; Sahoo, M.K.; Nuñez, A.; Balmaseda, A.; Harris, E.; et al. Viremia and Clinical Presentation in Nicaraguan Patients Infected with Zika Virus, Chikungunya Virus, and Dengue Virus. Clin. Infect. Dis. 2016, 63, 1584–1590. [Google Scholar] [CrossRef]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The Global Distribution and Burden of Dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Brady, O.J.; Gething, P.W.; Bhatt, S.; Messina, J.P.; Brownstein, J.S.; Hoen, A.G.; Moyes, C.L.; Farlow, A.W.; Scott, T.W.; Hay, S.I. Refining the Global Spatial Limits of Dengue Virus Transmission by Evidence-Based Consensus. PLoS Negl. Trop. Dis. 2012, 6, e1760. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Dengue and Severe Dengue. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 18 August 2022).
- Sharma, H.; Ilyas, A.; Chowdhury, A.; Poddar, N.K.; Chaudhary, A.A.; Shilbayeh, S.A.R.; Ibrahim, A.A.; Khan, S. Does COVID-19 lockdowns have impacted on global dengue burden? A special focus to India. BMC Public Health 2022, 22, 1402. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Aditya, G.; Saha, G.K. Household Wastes as Larval Habitats of Dengue Vectors: Comparison between Urban and Rural Areas of Kolkata, India. PLoS ONE 2015, 10, e0138082. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Weekly epidemiological update on COVID-19-29 June 2022. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---29-june-2022 (accessed on 18 August 2022).
- Rehman, S.; Shafique, L.; Ihsan, A.; Liu, Q. Evolutionary Trajectory for the Emergence of Novel Coronavirus SARS-CoV-2. Pathogens 2020, 9, 240. [Google Scholar] [CrossRef]
- Centres of Disease Control and Prevention (CDC). COVID-19 Overview and Infection Prevention and Control Priorities in Non-U.S. Healthcare Settings. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/non-us-settings/overview (accessed on 18 August 2022).
- Bulut, C.; Kato, Y. Epidemiology of COVID-19. Turk. J. Med. Sci. 2020, 50, 563–570. [Google Scholar] [CrossRef]
- Tsai, P.; Lai, W.; Lin, Y.; Luo, Y.; Lin, Y.; Chen, H.; Chen, Y.M.; Lai, Y.C.; Kuo, L.C.; Chen, S.D.; et al. Clinical manifestation and disease progression in COVID-19 infection. J. Chin. Med. Assoc. 2021, 84, 3–8. [Google Scholar] [CrossRef]
- Siordia, J. Epidemiology and clinical features of COVID-19: A review of current literature. J. Clin. Virol. 2020, 127, 104357. [Google Scholar] [CrossRef]
- Hu, Z.; Song, C.; Xu, C.; Jin, G.; Chen, Y.; Xu, X.; Ma, H.; Chen, W.; Lin, Y.; Zheng, Y.; et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci. China Life Sci. 2020, 63, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Robertson, C.; Stowe, J.; Tessier, E.; Simmons, R.; Cottrell, S.; Roberts, R.; O’Doherty, M.; et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in England: Test negative case-control study. BMJ 2021, 373, n1088. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Dengue Clinical Case Management Course. Available online: https://www.cdc.gov/dengue/training/cme.html (accessed on 18 August 2022).
- Centers for Disease Control and Prevention (CDC). COVID-19 Information for Specific Groups of People. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/index.html (accessed on 18 August 2022).
- Centers for Disease Control and Prevention (CDC). Serology Testing for COVID-19 at CDC. Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/serology-testing.html (accessed on 18 August 2022).
- Coller, B.S.; Shattil, S.J. The GPIIb/IIIa (Integrin AlphaIIbbeta3) Odyssey: A Technology-Driven Saga of a Receptor with Twists, Turns, and Even a Bend. Blood 2008, 112, 3011–3025. [Google Scholar] [CrossRef] [PubMed]
- Gawaz, M.; Langer, H.; May, A.E. Platelets in Inflammation and Atherogenesis. J. Clin. Investig. 2005, 115, 3378–3384. [Google Scholar] [CrossRef]
- Kaushansky, K. Lineage-Specific Hematopoietic Growth Factors. N. Engl. J. Med. 2006, 354, 2034–2045. [Google Scholar] [CrossRef]
- Semple, J.W.; Italiano, J.E.; Freedman, J. Platelets and the Immune Continuum. Nat. Rev. Immunol. 2011, 11, 264–274. [Google Scholar] [CrossRef]
- Trzeciak-Ryczek, A.; Tokarz-Deptuła, B.; Deptuła, W. Platelets-an Important Element of the Immune System. Pol. J. Vet. Sci. 2013, 16, 407–413. [Google Scholar] [CrossRef]
- Kasthuri, R.S.; Glover, S.L.; Boles, J.; Mackman, N. Tissue Factor and Tissue Factor Pathway Inhibitor as Key Regulators of Global Hemostasis: Measurement of Their Levels in Coagulation Assays. Semin. Thromb. Hemost. 2010, 36, 764–771. [Google Scholar] [CrossRef]
- Daubie, V.; Pochet, R.; Houard, S.; Philippart, P. Tissue Factor: A Mini-Review. J. Tissue Eng. Regen. Med. 2007, 1, 161–169. [Google Scholar] [CrossRef]
- Danielli, J.F. Capillary Permeability and Oedema in the Perfused Frog. J. Physiol. 1940, 98, 109–129. [Google Scholar] [CrossRef]
- Andia, I.; Abate, M. Platelet-Rich Plasma: Underlying Biology and Clinical Correlates. Regen. Med. 2013, 8, 645–658. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Dengue Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition. Available online: https://apps.who.int/iris/handle/10665/44188 (accessed on 18 August 2022).
- Rothman, A.L.; Ennis, F.A. Immunopathogenesis of Dengue Hemorrhagic Fever. Virology 1999, 257, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mitrakul, C. Bleeding Problem in Dengue Haemorrhagic Fever: Platelets and Coagulation Changes. Southeast Asian J. Trop. Med. Public Health 1987, 18, 407–412. [Google Scholar]
- Malavige, G.N.; Ranatunga, P.K.; Velathanthiri, V.G.N.S.; Fernando, S.; Karunatilaka, D.H.; Aaskov, J.; Seneviratne, S.L. Patterns of Disease in Sri Lankan Dengue Patients. Arch. Dis. Child. 2006, 91, 396–400. [Google Scholar] [CrossRef][Green Version]
- Chaudhary, R.; Khetan, D.; Sinha, S.; Sinha, P.; Sonkar, A.; Pandey, P.; Das, S.; Agarwal, P.; Ray, V. Transfusion Support to Dengue Patients in a Hospital Based Blood Transfusion Service in North India. Transf. Apheresis Sci. 2007, 35, 239–244. [Google Scholar] [CrossRef]
- Makroo, R.N.; Raina, V.; Kumar, P.; Kanth, R.K. Role of Platelet Transfusion in the Management of Dengue Patients in a Tertiary Care Hospital. Asian J. Transf. Sci. 2007, 1, 4. [Google Scholar] [CrossRef]
- Lum, L.C.S.; Abdel-Latif, M.E.-A.; Goh, A.Y.T.; Chan, P.W.K.; Lam, S.K. Preventive Transfusion in Dengue Shock Syndrome-Is It Necessary? J. Pediatr. 2003, 143, 682–684. [Google Scholar] [CrossRef]
- Murgue, B.; Cassar, O.; Guigon, M.; Chungue, E. Dengue Virus Inhibits Human Hematopoietic Progenitor Growth in Vitro. J. Infect. Dis. 1997, 175, 1497–1501. [Google Scholar] [CrossRef]
- Nakao, S.; Lai, C.J.; Young, N.S. Dengue Virus, a Flavivirus, Propagates in Human Bone Marrow Progenitors and Hematopoietic Cell Lines. Blood 1989, 74, 1235–1240. [Google Scholar] [CrossRef]
- Edelman, R.; Nimmannitya, S.; Colman, R.W.; Talamo, R.C.; Top, F.H. Evaluation of the Plasma Kinin System in Dengue Hemorrhagic Fever. J. Lab. Clin. Med. 1975, 86, 410–421. [Google Scholar]
- Hottz, E.D.; Oliveira, M.F.; Nunes, P.C.G.; Nogueira, R.M.R.; Valls-de-Souza, R.; Da Poian, A.T.; Weyrich, A.S.; Zimmerman, G.A.; Bozza, P.T.; Bozza, F.A. Dengue Induces Platelet Activation, Mitochondrial Dysfunction and Cell Death through Mechanisms That Involve DC-SIGN and Caspases. J. Thromb. Haemost. 2013, 11, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Laur, F.; Murgue, B.; Deparis, X.; Roche, C.; Cassar, O.; Chungue, E. Plasma Levels of Tumour Necrosis Factor Alpha and Transforming Growth Factor Beta-1 in Children with Dengue 2 Virus Infection in French Polynesia. Trans. R. Soc. Trop. Med. Hyg. 1998, 92, 654–656. [Google Scholar] [CrossRef]
- Pang, X.; Zhang, R.; Cheng, G. Progress towards understanding the pathogenesis of dengue hemorrhagic fever. Virol. Sin. 2017, 32, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Jumnainsong, A.; Onsirisakul, N.; Fitton, P.; Vasanawathana, S.; Limpitikul, W.; Puttikhunt, C.; Edwards, C.; Duangchinda, T.; Supasa, S. Cross-Reacting Antibodies Enhance Dengue Virus Infection in Hu-mans. Science 2010, 328, 745–748. [Google Scholar] [CrossRef] [PubMed]
- De Azeredo, E.L.; Monteiro, R.Q.; de-Oliveira Pinto, L.M. Thrombocytopenia in Dengue: Interrelationship between Virus and the Imbalance between Coagulation and Fibrinolysis and Inflammatory Mediators. Mediat. Inflamm. 2015, 2015, 313842. [Google Scholar] [CrossRef]
- Islam, A.; Bashir, M.S.; Joyce, K.; Rashid, H.; Laher, I.; Elshazly, S. An Update on COVID-19 Vaccine Induced Thrombotic Thrombocytopenia Syndrome and Some Management Recommendations. Molecules 2021, 26, 5004. [Google Scholar] [CrossRef]
- Geisbert, T.W.; Jahrling, P.B. Exotic Emerging Viral Diseases: Progress and Challenges. Nat. Med. 2004, 10, 110–121. [Google Scholar] [CrossRef]
- Hidalgo, J.; Richards, G.A.; Jiménez, J.I.S.; Baker, T.; Amin, P. Viral Hemorrhagic Fever in the Tropics: Report from the Task Force on Tropical Diseases by the World Federation of Societies of Intensive and Critical Care Medicine. J. Crit. Care. 2017, 42, 366–372. [Google Scholar] [CrossRef]
- Buonsenso, D.; Riitano, F.; Valentini, P. Pediatric Inflammatory Multisystem Syndrome Temporally Related with SARS-CoV-2: Immunological Similarities with Acute Rheumatic Fever and Toxic Shock Syndrome. Front. Pediatr. 2020, 8, 574. [Google Scholar] [CrossRef]
- Schnittler, H.-J.; Feldmann, H. Viral Hemorrhagic Fever–a Vascular Disease? Thromb. Haemost. 2003, 89, 967–972. [Google Scholar] [CrossRef]
- Zapata, J.C.; Cox, D.; Salvato, M.S. The Role of Platelets in the Pathogenesis of Viral Hemorrhagic Fevers. PLoS Negl. Trop. Dis. 2014, 8, e2858. [Google Scholar] [CrossRef] [PubMed]
- Kularatne, S.A.M. Dengue Fever. BMJ 2015, 351, h4661. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention, and Control, 2nd ed.; World Health Organization: Geneva, Switzerland, 1997; ISBN 978-92-4-154500-6. Available online: https://apps.who.int/iris/handle/10665/41988 (accessed on 18 August 2022).
- Nopp, S.; Moik, F.; Jilma, B.; Pabinger, I.; Ay, C. Risk of Venous Thromboembolism in Patients with COVID-19: A Systematic Review and Meta-Analysis. Res. Pract. Thromb. Haemost. 2020, 4, 1178–1191. [Google Scholar] [CrossRef] [PubMed]
- Smilowitz, N.R.; Subashchandran, V.; Yuriditsky, E.; Horowitz, J.M.; Reynolds, H.R.; Hochman, J.S.; Berger, J.S. Thrombosis in Hospitalized Patients with Viral Respiratory Infections versus COVID-19. Am. Heart J. 2021, 231, 93–95. [Google Scholar] [CrossRef]
- Katz, D.; Maher, P.; Getrajdman, C.; Hamburger, J.; Zhao, S.; Madek, J.; Bhatt, H.; Levin, M.; Görlinger, K. Monitoring of COVID-19-Associated Coagulopathy and Anticoagulation with Thromboelastometry. Transfus. Med. Hemother. 2021, 48, 168–172. [Google Scholar] [CrossRef]
- Wibowo, A.; Pranata, R.; Lim, M.A.; Akbar, M.R.; Martha, J.W. Endotheliopathy Marked by High von Willebrand Factor (VWF) Antigen in COVID-19 Is Associated with Poor Outcome: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2021, 117, 267–273. [Google Scholar] [CrossRef]
- Al-Samkari, H.; Karp Leaf, R.S.; Dzik, W.H.; Carlson, J.C.T.; Fogerty, A.E.; Waheed, A.; Goodarzi, K.; Bendapudi, P.K.; Bornikova, L.; Gupta, S.; et al. COVID-19 and Coagulation: Bleeding and Thrombotic Manifestations of SARS-CoV-2 Infection. Blood 2020, 136, 489–500. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Levi, M. Viral-Induced Inflammatory Coagulation Disorders: Preparing for Another Epidemic. Thromb. Haemost. 2021, 122, 8–19. [Google Scholar] [CrossRef]
- Nath, H.; Mallick, A.; Roy, S.; Sukla, S.; Basu, K.; De, A.; Biswas, S. 2021 Archived Dengue Serum Samples Produced False-Positive Results in SARS-CoV-2 Lateral Flow-Based Rapid Antibody Tests. J. Med. Microbiol. 2021, 70, 001369. [Google Scholar] [CrossRef]
- Nath, H.; Mallick, A.; Roy, S.; Sukla, S.; Biswas, S. Computational Modelling Supports That Dengue Virus Envelope Antibodies Can Bind to SARS-CoV-2 Receptor Binding Sites: Is Pre-Exposure to Dengue Virus Protective against COVID-19 Severity? Comput. Struct. Biotechnol. J. 2021, 19, 459–466. [Google Scholar] [CrossRef]
- Carlos, C.C.; Oishi, K.; Cinco, M.T.D.D.; Mapua, C.A.; Inoue, S.; Cruz, D.J.M.; Pancho, M.A.M.; Tanig, C.Z.; Matias, R.R.; Morita, K.; et al. Comparison of Clinical Features and Hematologic Abnormalities between Dengue Fever and Dengue Hemorrhagic Fever among Children in the Philippines. Am. J. Trop. Med. Hyg. 2005, 73, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.M.; Mejally, M.A.A.; Hussain, W.M.; Maimani, W.A.; Hanif, S.; Khoujah, A.M.; Siddiqi, A.; Akhtar, M.; Bafaraj, M.G.; Fareed, K. Skin Rash, Headache and Abnormal Behaviour: Unusual Presentation of Intracranial Haemorrhage in Dengue Fever. BMJ Case Rep. 2010, 2010, bcr0620091949. [Google Scholar] [CrossRef] [PubMed]
- Hanff, T.; Mohareb, A.; Giri, J.; Cohen, J.; Chirinos, J. Thrombosis in COVID-19. Am. J. Hematol. 2020, 95, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- England, J.T.; Abdulla, A.; Biggs, C.M.; Lee, A.Y.Y.; Hay, K.A.; Hoiland, R.L.; Wellington, C.L.; Sekhon, M.; James, S.; Shojania, K.; et al. Weathering the COVID-19 storm: Lessons from hematologic cytokine syndromes. Blood Rev. 2021, 45, 100707. [Google Scholar] [CrossRef]
- Gomez-Mesa, J.; Galindo-Coral, S.; Montes, M.; Martin, M. Thrombosis and Coagulopathy in COVID-19. Curr. Prob. Cardiol. 2021, 46, 100742. [Google Scholar] [CrossRef]
- Karim, S.; Islam, A.; Rafiq, S.; Laher, I. The COVID-19 Pandemic: Disproportionate Thrombotic Tendency and Management Recommendations. Trop. Med. Infect. Dis. 2021, 6, 26. [Google Scholar] [CrossRef]
- Tsantes, A.E.; Frantzeskaki, F.; Tsantes, A.G.; Rapti, E.; Rizos, M.; Kokoris, S.I.; Paramythiotou, E.; Katsadiotis, G.; Karali, V.; Flevari, A.; et al. The haemostatic profile in critically ill COVID-19 patients receiving therapeutic anticoagulant therapy: An observational study. Medicine 2020, 99, e23365. [Google Scholar] [CrossRef]
- Meizoso, J.; Moore, H.; Moore, E. Fibrinolysis Shutdown in COVID-19: Clinical Manifestations, Molecular Mechanisms, and Therapeutic Implications. J. Am. Coll. Surg. 2021, 232, 995–1003. [Google Scholar] [CrossRef]
- De Brune, S.; Bos, L.; van Roon, M.; Tuip-de Boer, A.; Schuurman, A.; Koel-Simmelink, M.; Bogaard, H.J.; Tuinman, P.R.; van Agtmael, M.A.; Hamann, J.; et al. Clinical features and prognostic factors in COVID-19: A prospective cohort study. EBioMedicine 2021, 67, 103378. [Google Scholar] [CrossRef]
- Lustig, Y.; Keler, S.; Kolodny, R.; Ben-Tal, N.; Atias-Varon, D.; Shlush, E.; Gerlic, M.; Munitz, A.; Doolman, R.; Asraf, K.; et al. Potential Antigenic Cross-Reactivity Between Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Dengue Viruses. Clin. Infect. Dis. 2021, 73, 2444–2449. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Clinical Excellence (NICE). COVID-19 Rapid Guidelines: Managing COVID-19; Section 8.3: Venous Thromboembolism (VTE) Prophylaxis. Available online: https://app.magicapp.org/#/guideline/L4Qb5n/rec/LwomXL (accessed on 18 August 2022).
- Halstead, S.B.; Lum, L.C. Assessing the Prognosis of Dengue-Infected Patients. F1000 Med. Rep. 2009, 1, 73. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, D.W.; Green, S.; Kalayanarooj, S.; Innis, B.L.; Nimmannitya, S.; Suntayakorn, S.; Rothman, A.L.; Ennis, F.A.; Nisalak, A. Dengue in the Early Febrile Phase: Viremia and Antibody Responses. J. Infect. Dis. 1997, 176, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J. Dengue and Dengue Hemorrhagic Fever. Clin. Microbiol. Rev. 1998, 11, 480–496. [Google Scholar] [CrossRef]
- Kalayanarooj, S. Dengue Classification: Current WHO vs. the Newly Suggested Classification for Better Clinical Application? J. Med. Assoc. Thai 2011, 94, 74–84. [Google Scholar]
- Rajapakse, S.; de Silva, N.L.; Weeratunga, P.; Rodrigo, C.; Fernando, S.D. Prophylactic and Therapeutic Interventions for Bleeding in Dengue: A Systematic Review. Trans. R. Soc. Trop. Med. Hyg. 2017, 111, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Lye, D.C.; Lee, V.J.; Sun, Y.; Leo, Y.S. Lack of Efficacy of Prophylactic Platelet Transfusion for Severe Thrombocytopenia in Adults with Acute Uncomplicated Dengue Infection. Clin. Infect. Dis. 2009, 48, 1262–1265. [Google Scholar] [CrossRef]
- Lye, D.C.; Archuleta, S.; Syed-Omar, S.F.; Low, J.G.; Oh, H.M.; Wei, Y.; Fisher, D.; Ponnampalavanar, S.S.L.; Wijaya, L.; Lee, L.K.; et al. Prophylactic Platelet Transfusion plus Supportive Care versus Supportive Care Alone in Adults with Dengue and Thrombocytopenia: A Multicentre, Open-Label, Randomised, Superiority Trial. Lancet 2017, 389, 1611–1618. [Google Scholar] [CrossRef]
| General Features: | Dengue | COVID-19 |
|---|---|---|
| Virology | ||
| Family | Flaviviridae | Coronaviridae |
| Diameter | 50 nm | 65–125 nm |
| Genetic Material | ssRNA | ssRNA |
| Presentation | ||
| Incubation | 3–10 days | 2 to 14 (median 4–5) days |
| Fever | Saddleback fever (with 2 peaks) | No specific fever patterns. Defervescence after 6 days of illness |
| Headache | 45–95% | 6.5–13.6% |
| Myalgia | 12% | 15–44% |
| Cough | 21.5% | 76% |
| Dyspnoea | 9.5–95.2% | 55% |
| Diarrhoea | 6% | 2–34% |
| Abdominal pain | 17–25% | 2% |
| Vomiting | 30–58% | 4–5% |
| Cutaneous manifestation | Skin flushing that blanch on pressure, petechiae, and convalescent rash | Erythematous rash, urticaria, chickenpox-like vesicles |
| Warning signs: | Persistent vomiting, mucosal bleeding, difficulty in breathing, lethargy/restlessness, postural hypotension, liver enlargement and progressive increases in haematocrit | Difficulty in breathing, persistent pain or pressure in the chest, new confusion, inability to wake or stay awake, bluish lips or face |
| Laboratory Findings | ||
| Thrombocytopenia | 69.51–100% | 12–36.2% |
| Leukopenia | 20–82.2% | 25–29% |
| Lymphopenia | 63% | 63% |
| Raised AST | 63–97% | 31–35% |
| Raised ALT | 45–97% | 24–28% |
| Raised D-dimer | 13–87% | 46.4% |
| Dengue | COVID-19 | |
|---|---|---|
| Viral characteristics | Viral titer correlates with disease severity. There may be strain and serotype differences in pathogenicity. | Relationship between viral titer and severity poorly understood. Certain variants, (via increased transmission, vaccine resistance etc.). |
| Host factors | Age (infant) Women, especially pregnant women. Patients with chronic medical conditions, including diabetes, asthma, obesity and heart disease. Patients with secondary DENV infection. | Age (elderly) Pregnant/recently pregnant women. Comorbidities, such as chronic kidney disease, malignancy, chronic lung disease, dementia, cardiovascular disease, diabetes, immunosuppression, multiple comorbidities. |
| General | Dengue | COVID-19 |
|---|---|---|
| Basic comparisons | Consumptive coagulopathy is common | Consumptive coagulation disorder is seen in limited cases |
| Bleeding in VHF and thrombosis in COVD-19 | Increased permeability in viral haemorrhagic fever also induces coagulation defects that can result in critical bleeding. The systemic viral infection also induces an acute inflammatory and hypercoagulable state causing DIC | COVID-19 is characterised by a high prevalence of thrombotic complications. Infrequently, bleeding can occur, especially in advanced stages of critical illness |
| Pathogenesis | Infected dendritic cells and macrophages lose their ability to regulate type I IFN levels, and lymphocytes undergo cell death. Inappropriate dendritic cell function perturbs the innate immune system and increases vascular permeability. Furthermore, the replicated viruses disseminate throughout the body to systemic reactions such as dysfunction of the visceral parenchymal cells, platelet disability and coagulopathy which lead to DIC resulting in uncontrolled haemorrhage | COVID-19 directly infects macrophages/monocytes, provoking inflammation and thrombosis by releasing proinflammatory cytokines, and expressing TF. Activated neutrophils eject neutrophil extracellular traps and disrupt antithrombogenicity by damaging glycocalyx. Thrombin activates endothelial cells, elicits a proinflammatory reaction, prothrombotic change and activates platelet aggregation. COVID-19 also infects endothelial cells by binding to ACE2 and stimulates the release of factor VIII, VWF and angiopoietin 2, resulting in thrombosis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, A.; Cockcroft, C.; Elshazly, S.; Ahmed, J.; Joyce, K.; Mahfuz, H.; Islam, T.; Rashid, H.; Laher, I. Coagulopathy of Dengue and COVID-19: Clinical Considerations. Trop. Med. Infect. Dis. 2022, 7, 210. https://doi.org/10.3390/tropicalmed7090210
Islam A, Cockcroft C, Elshazly S, Ahmed J, Joyce K, Mahfuz H, Islam T, Rashid H, Laher I. Coagulopathy of Dengue and COVID-19: Clinical Considerations. Tropical Medicine and Infectious Disease. 2022; 7(9):210. https://doi.org/10.3390/tropicalmed7090210
Chicago/Turabian StyleIslam, Amin, Christopher Cockcroft, Shereen Elshazly, Javeed Ahmed, Kevin Joyce, Huque Mahfuz, Tasbirul Islam, Harunor Rashid, and Ismail Laher. 2022. "Coagulopathy of Dengue and COVID-19: Clinical Considerations" Tropical Medicine and Infectious Disease 7, no. 9: 210. https://doi.org/10.3390/tropicalmed7090210
APA StyleIslam, A., Cockcroft, C., Elshazly, S., Ahmed, J., Joyce, K., Mahfuz, H., Islam, T., Rashid, H., & Laher, I. (2022). Coagulopathy of Dengue and COVID-19: Clinical Considerations. Tropical Medicine and Infectious Disease, 7(9), 210. https://doi.org/10.3390/tropicalmed7090210








