Epidemiological Characteristics and the Dynamic Transmission Model of Dengue Fever in Zhanjiang City, Guangdong Province in 2018
Abstract
:1. Introduction
2. Methods
2.1. Materials
2.2. Study Site
2.3. Data Collection
2.4. Case Definitions
2.5. Vector Surveillance
2.5.1. Surveillance Area
2.5.2. Monitoring Methods
2.5.3. The Frequency of Monitoring
2.5.4. Data Analysis and Feedback
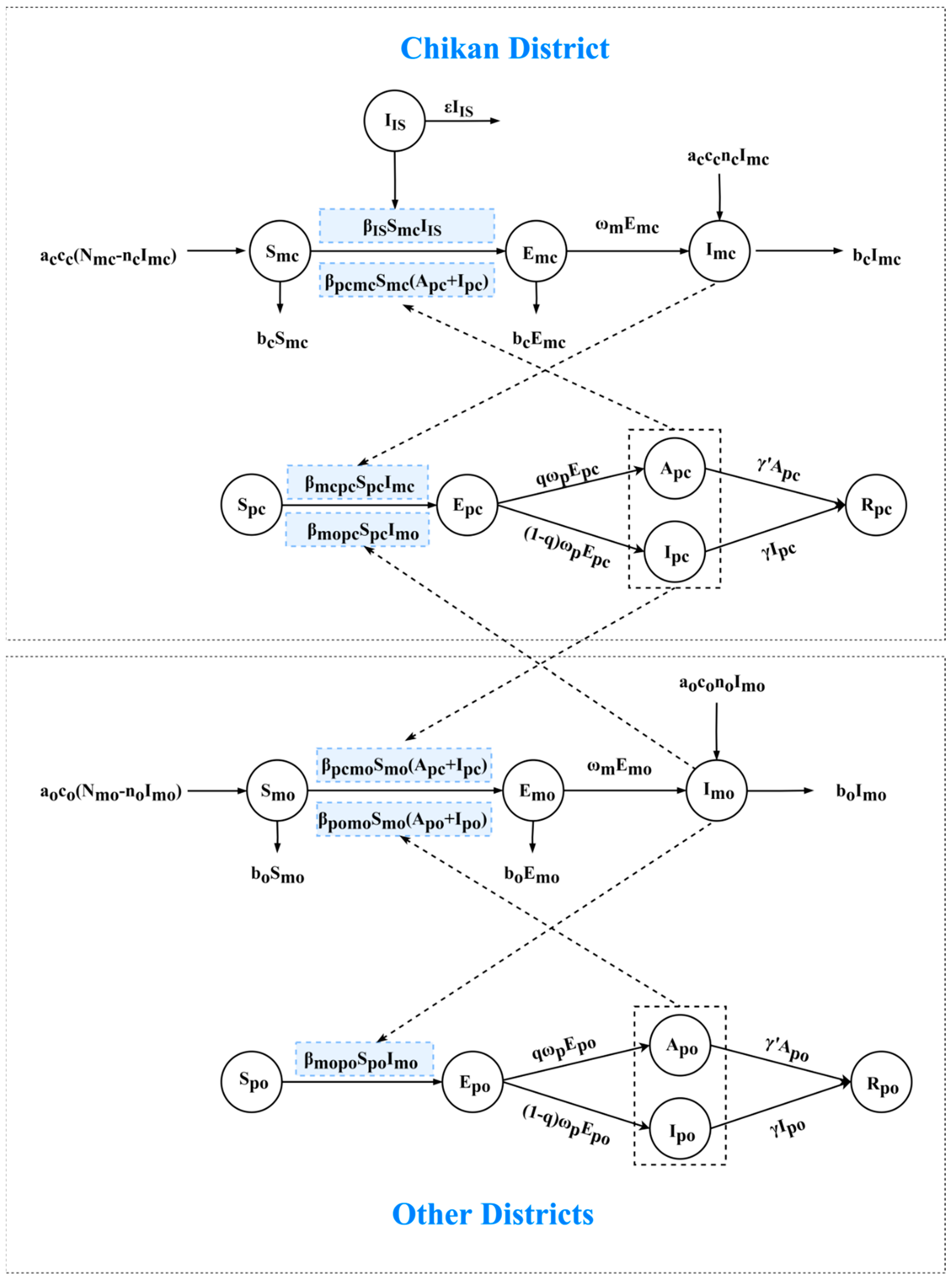
2.6. Transmission Model
2.7. Parameter Estimation
2.8. Scenarios
2.9. Simulation Method
2.10. Normalization
3. Results
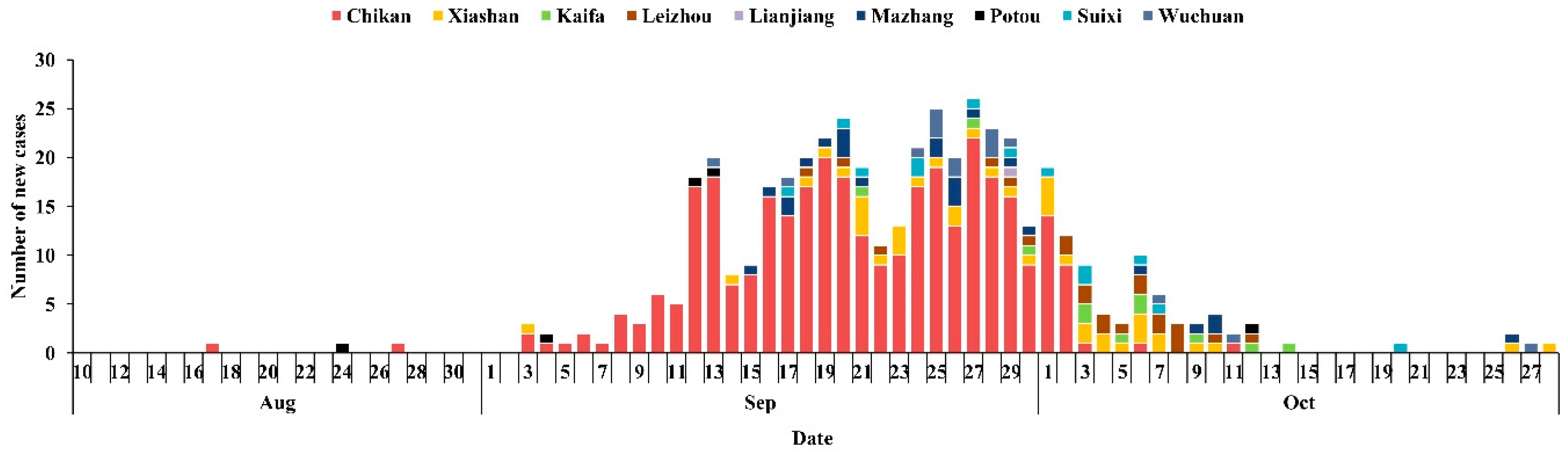
3.1. Reported Dengue Fever Cases in Zhanjiang City in 2018
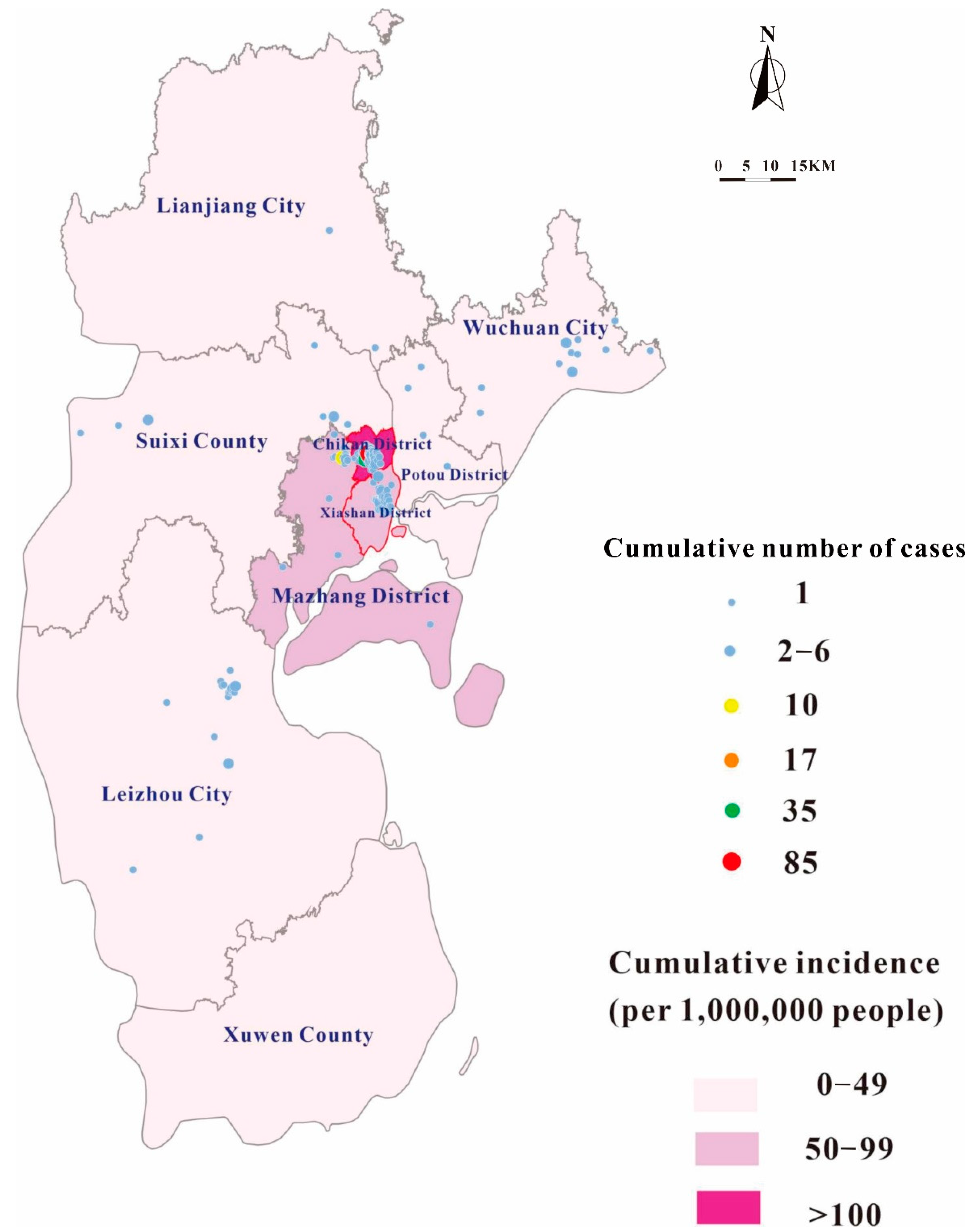
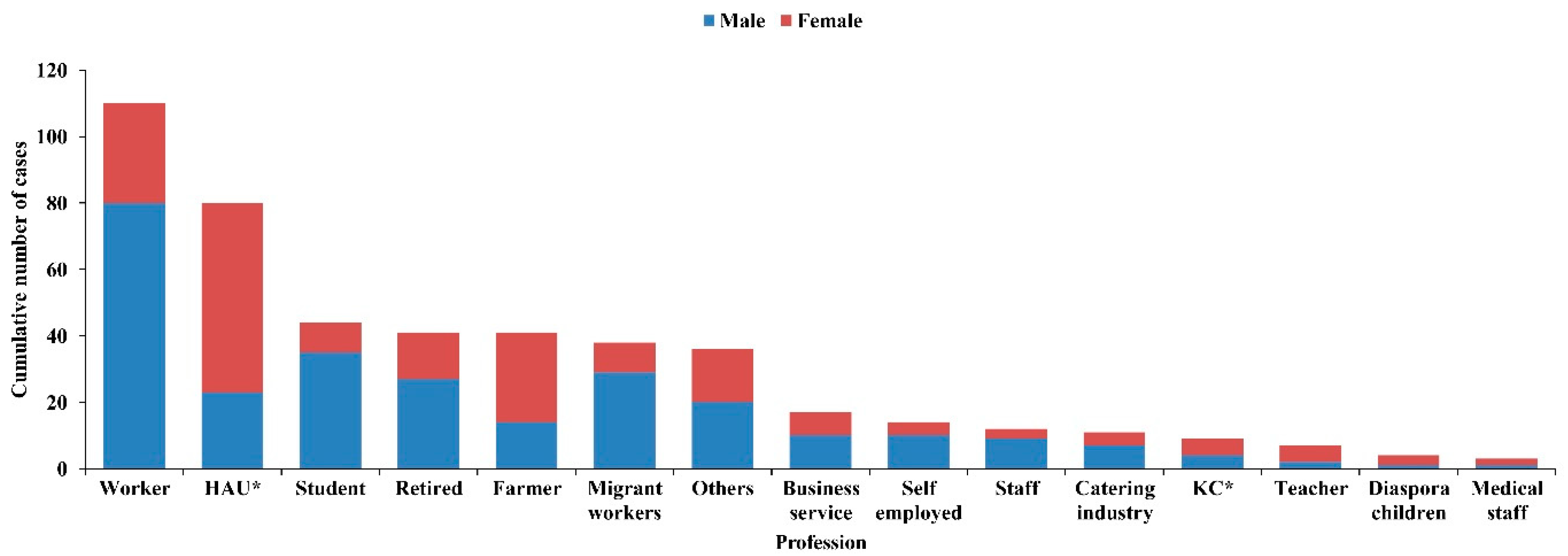
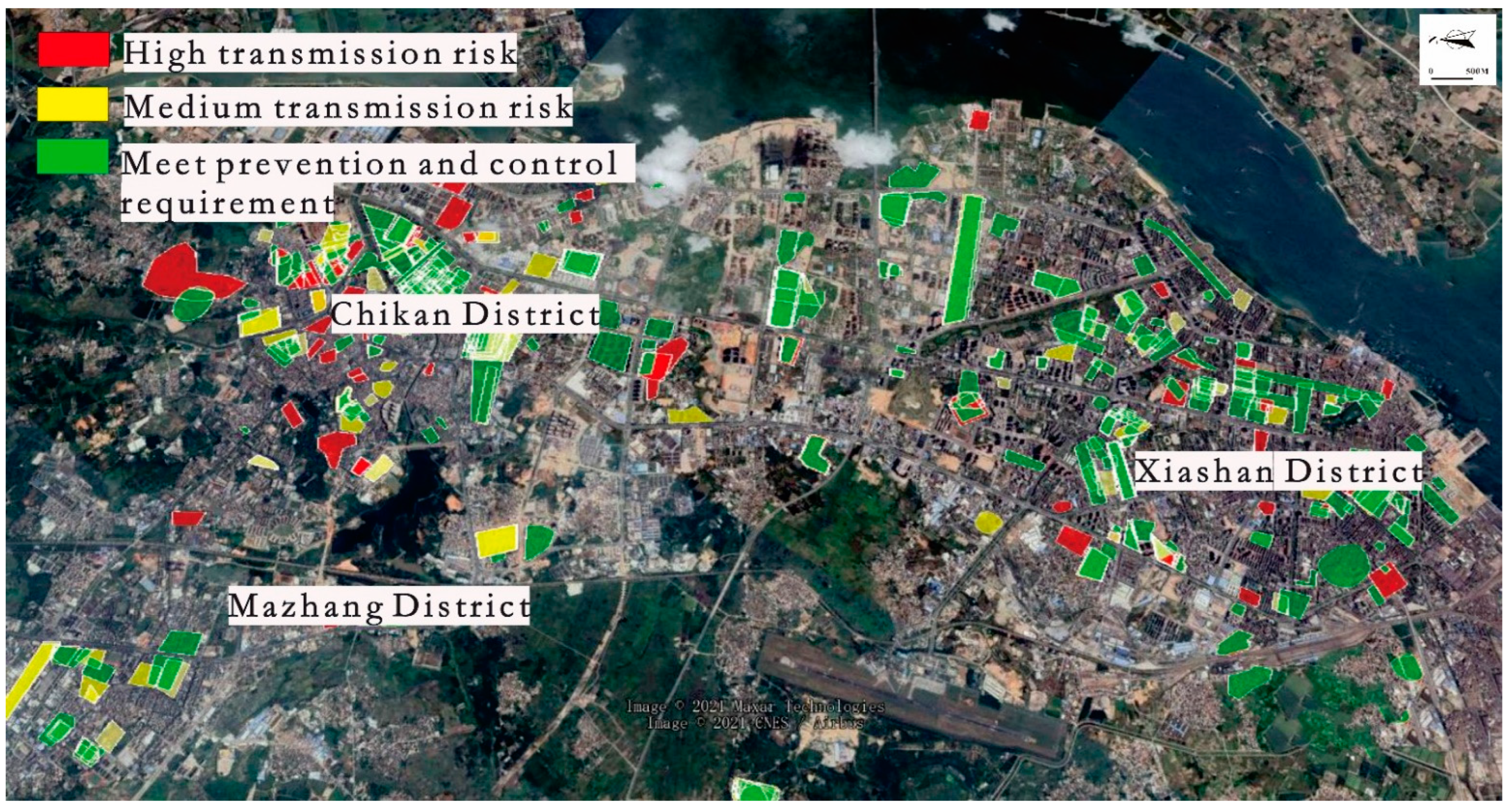
3.2. Disease Distribution
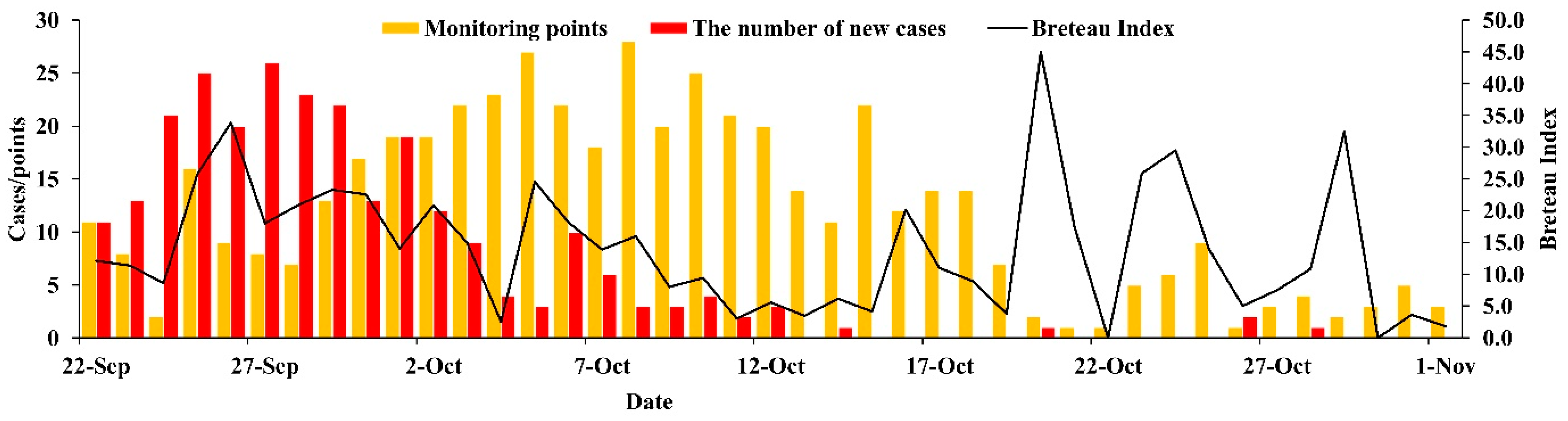
3.3. Vector Surveillance
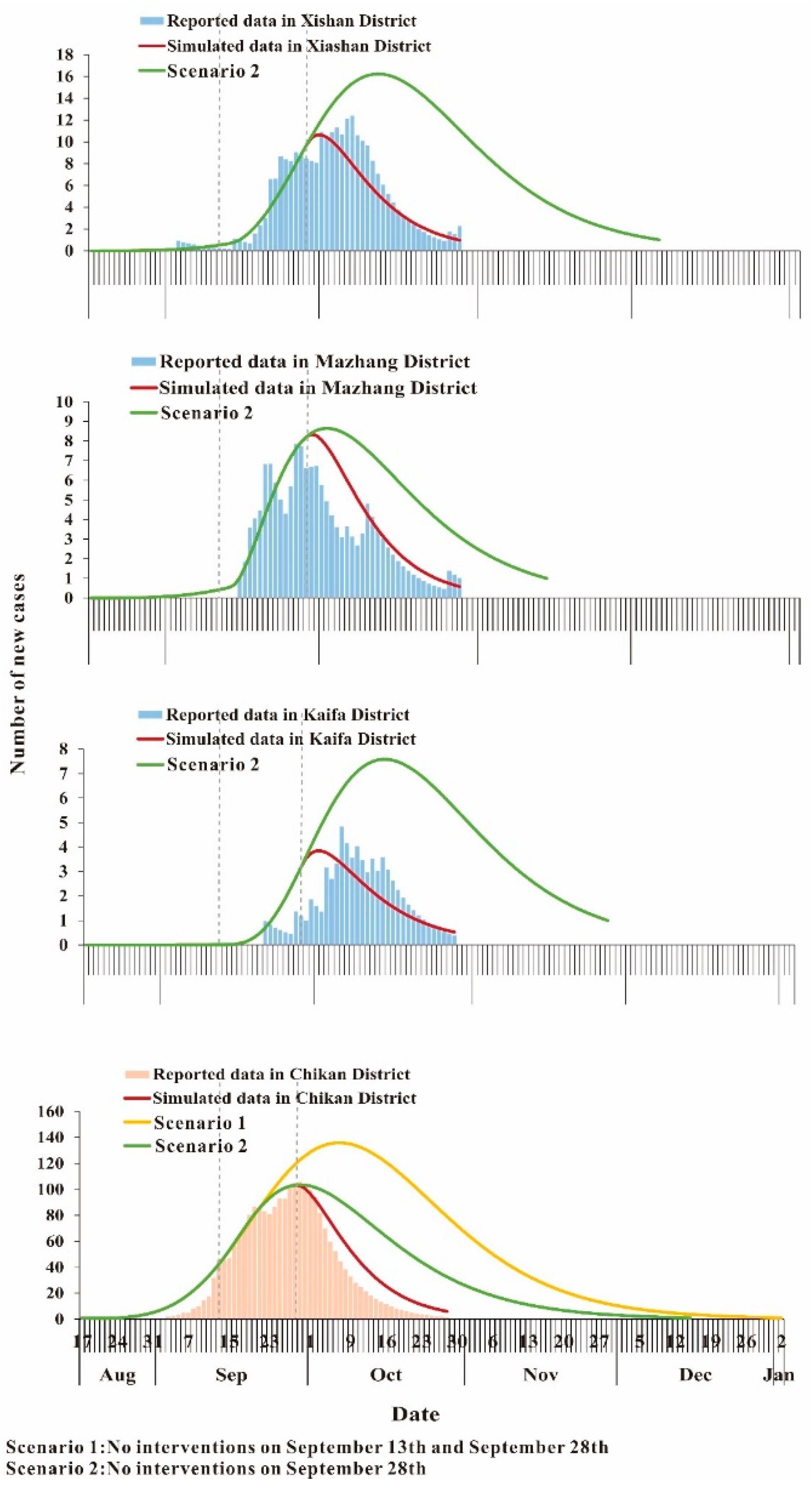
3.4. Curve Fitting
3.5. Effectiveness of the Interventions
3.6. Comparison of Transmission Relative Rate
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, K.; Hou, X.; Wang, Y.; Sun, J.; Xiao, J.; Li, R.; Lu, L.; Xu, L.; Sang, S.; Hu, J.; et al. The driver of dengue fever incidence in two high-risk areas of China: A comparative study. Sci. Rep. 2019, 9, 19510. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Huang, Z.; Zhou, H.; Anders, K.L.; Perkins, T.A.; Yin, W.; Li, Y.; Mu, D.; Chen, Q.; Zhang, Z.; et al. The changing epidemiology of dengue in China, 1990–2014: A descriptive analysis of 25 years of nationwide surveillance data. BMC Med. 2015, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Dengue and Severe Dengue: World Health Organization. 2020. Available online: https://www.who.int/health-topics/dengue-and-severe-dengue#tab=tab_1 (accessed on 1 December 2020).
- Kraemer, M.U.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Bortel, W.V.; et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife 2015, 4, e08347. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, L.; Wang, H.; Cao, Z.; Zha, L.; Li, Z.; Ye, Z.; Zhang, J.; Song, H.; Sun, Y. Prediction model for dengue fever based on interactive effects between multiple meteorological factors in Guangdong, China (2008–2016). PLoS ONE 2019, 14, e0225811. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Y.; Yan, H.; Zhang, P.; Xu, X.; Tang, B.; Zhao, P.; Ren, R. A survey of the 2014 dengue fever epidemic in Guangzhou, China. Emerg. Microbes Infect. 2015, 4, e57. [Google Scholar] [CrossRef]
- Fan, J.; Lin, H.; Wang, C.; Bai, L.; Yang, S.; Chu, C.; Yang, W.; Liu, Q. Identifying the high-risk areas and associated meteorological factors of dengue transmission in Guangdong Province, China from 2005 to 2011. Epidemiol. Infect. 2014, 142, 634–643. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Cheng, Q.; Zhang, Y.; Ye, G.; Huang, X.; Zhao, Z.; Rui, J.; Hu, Q.; Frutos, R.; et al. Dengue fever transmission between a construction site and its surrounding communities in China. Parasit. Vectors 2021, 14, 22. [Google Scholar] [CrossRef]
- Sang, S.; Chen, B.; Wu, H.; Yang, Z.; Di, B.; Wang, L.; Tao, X.; Liu, X.; Liu, Q. Dengue is still an imported disease in China: A case study in Guangzhou. Infect. Genet. Evol. 2015, 32, 178–190. [Google Scholar] [CrossRef]
- Zhao, S.; Musa, S.S.; Meng, J.; Qin, J.; He, D. The long-term changing dynamics of dengue infectivity in Guangdong, China, from 2008–2018: A modelling analysis. Trans. R. Soc. Trop. Med. Hyg. 2020, 114, 62–71. [Google Scholar] [CrossRef]
- Wu, J.Y.; Lun, Z.R.; James, A.A.; Chen, X.G. Dengue Fever in mainland China. Am. J. Trop. Med. Hyg. 2010, 83, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Luo, X.; Shao, J.; Yan, H.; Qiu, Y.; Ke, P.; Zheng, W.; Xu, B.; Li, W.; Sun, D.; et al. Epidemiology and characteristics of the dengue outbreak in Guangdong, Southern China, in 2014. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Chen, J.; Feng, X.M.; Ruan, S.G. Analysis of a Dengue Model with Vertical Transmission and Application to the 2014 Dengue Outbreak in Guangdong Province, China. Bull. Math. Biol. 2018, 80, 2633–2651. [Google Scholar] [CrossRef]
- Yang, H.M.; Boldrini, J.L.; Fassoni, A.C.; Freitas, L.F.; Gomez, M.C.; de Lima, K.K.; Andrade, V.R.; Freitas, A.R.R. Fitting the Incidence Data from the City of Campinas, Brazil, Based on Dengue Transmission Modellings Considering Time-Dependent Entomological Parameters. PLoS ONE 2016, 11, e0152186. [Google Scholar] [CrossRef] [PubMed]
- Chavez, J.P.; Gotz, T.; Siegmund, S.; Wijaya, K.P. An SIR-Dengue transmission model with seasonal effects and impulsive control. Math. Biosci. 2017, 289, 29–39. [Google Scholar] [CrossRef]
- Bhuju, G.; Phaijoo, G.R.; Gurung, D.B. Fuzzy Approach Analyzing SEIR-SEI Dengue Dynamics. Biomed. Res. Int. 2020, 2020, 1508613. [Google Scholar] [CrossRef] [PubMed]
- Grunnill, M. An exploration of the role of asymptomatic infections in the epidemiology of dengue viruses through susceptible, asymptomatic, infected and recovered (SAIR) models. J. Theor. Biol. 2018, 439, 195–204. [Google Scholar] [CrossRef]
- Xu, Z.; Bambrick, H.; Pongsumpun, P.; Ming Tang, I.; Yakob, L.; Devine, G.; Frentiu, F.D.; Williams, G.; Hu, W. Does Bangkok have a central role in the dengue dynamics of Thailand? Parasit. Vectors 2020, 13, 22. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, H.; Tan, Q.; Zhou, H.; Guan, D.; Zhang, X.; Duan, J.; Cai, S.; Peng, Z.; He, J.; et al. The epidemiological characteristics and molecular phylogeny of the dengue virus in Guangdong, China, 2015. Sci. Rep. 2018, 8, 9976. [Google Scholar] [CrossRef]
- Yi, B.; Chen, Y.; Ma, X.; Rui, J.; Cui, J.A.; Wang, H.; Li, J.; Chan, S.F.; Wang, R.; Ding, K.; et al. Incidence dynamics and investigation of key interventions in a dengue outbreak in Ningbo City, China. PLoS Negl. Trop. Dis. 2019, 13, e0007659. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.M.; Macoris, M.L.; Galvani, K.C.; Andrighetti, M.T.; Wanderley, D.M. Assessing the effects of temperature on the population of Aedes aegypti, the vector of dengue. Epidemiol. Infect. 2009, 137, 1188–1202. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.; Johansson, M.A. The incubation periods of Dengue viruses. PLoS ONE 2012, 7, e50972. [Google Scholar] [CrossRef] [PubMed]
- Andraud, M.; Hens, N.; Marais, C.; Beutels, P. Dynamic epidemiological models for dengue transmission: A systematic review of structural approaches. PLoS ONE 2012, 7, e49085. [Google Scholar] [CrossRef]
- Wang, T.; Wang, M.; Shu, B.; Chen, X.Q.; Luo, L.; Wang, J.Y.; Cen, Y.Z.; Anderson, B.D.; Merrill, M.M.; Merrill, H.R.; et al. Evaluation of inapparent dengue infections during an outbreak in Southern China. PLoS Negl. Trop. Dis. 2015, 9, e0003677. [Google Scholar] [CrossRef] [PubMed]
- Grunnill, M.; Boots, M. How Important is Vertical Transmission of Dengue Viruses by Mosquitoes (Diptera: Culicidae)? J. Med. Entomol. 2016, 53, 1–19. [Google Scholar] [CrossRef]
- Sang, S.; Yin, W.; Bi, P.; Zhang, H.; Wang, C.; Liu, X.; Chen, B.; Yang, W.; Liu, Q. Predicting local dengue transmission in Guangzhou, China, through the influence of imported cases, mosquito density and climate variability. PLoS ONE 2014, 9, e102755. [Google Scholar] [CrossRef]
- Liang, L.; Hualiang, L.; Linwei, T.; Weizhong, Y.; Jimin, S.; Qiyong, L. Time series analysis of dengue fever and weather in Guangzhou, China. BMC Public Health 2009, 9, 395. [Google Scholar] [CrossRef]
- Zonetti, L.F.C.; Coutinho, M.C.; de Araujo, A.S. Molecular Aspects of the Dengue Virus Infection Process: A Review. Protein Pept. Lett. 2018, 25, 712–719. [Google Scholar] [CrossRef]
- Gubler, D.J. Dengue, Urbanization and Globalization: The Unholy Trinity of the 21(st) Century. Trop. Med. Health 2011, 39 (Suppl. S4), 3–11. [Google Scholar] [CrossRef]
- Hales, S.; de Wet, N.; Maindonald, J.; Woodward, A. Potential effect of population and climate changes on global distribution of dengue fever: An empirical model. Lancet 2002, 360, 830–834. [Google Scholar] [CrossRef]
- Stoddard, S.T.; Forshey, B.M.; Morrison, A.C.; Paz-Soldan, V.A.; Vazquez-Prokopec, G.M.; Astete, H.; Reiner, R.C., Jr.; Vilcarromero, S.; Elder, J.P.; Halsey, E.S.; et al. House-to-house human movement drives dengue virus transmission. Proc. Natl. Acad. Sci. USA 2013, 110, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Karl, S.; Halder, N.; Kelso, J.K.; Ritchie, S.A.; Milne, G.J. A spatial simulation model for dengue virus infection in urban areas. BMC Infect. Dis. 2014, 14, 447. [Google Scholar] [CrossRef] [PubMed]
- Nevai, A.L.; Soewono, E. A model for the spatial transmission of dengue with daily movement between villages and a city. Math. Med. Biol. 2014, 31, 150–178. [Google Scholar] [CrossRef] [PubMed]
- Mincham, G.; Baldock, K.L.; Rozilawati, H.; Williams, C.R. Development of a mechanistic dengue simulation model for Guangzhou. Epidemiol. Infect. 2019, 147, e125. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Parameter | Description | Unit | Value | Range | Method |
|---|---|---|---|---|---|
| ac/ao | Daily birth rate of mosquitoes in Chikan district or other districts *1 | day−1 | 0.0714 | 0.0200–0.2500 | References |
| cc/co | Seasonality parameter of the mosquitoes’ population in Chikan district or other districts | 1 | See Equation (2) *2 | 0–1 | Curve fitting |
| nc/no | Proportion of transovarial transmission in Chikan District or other districts | 1 | 0.1 | 0.0140–0.1740 | Reference |
| bc/bo | Daily death rate of mosquitoes in Chikan District or other districts | day−1 | 0.0714 | 0.0200–0.2500 | Reference |
| ωm/ωp | Relative incubation rate of mosquito infection or human infection | day−1 | 0.1000/0.1667 | 0.0833–0.1250/ | Reference |
| 0.1250–0.2500 | |||||
| q | Proportion of asymptomatic infections | 1 | 0.6875 | 0–1 | Reference |
| γ | Relative removal rate of infectious individuals | day−1 | 0.1429 | 0.0714–0.3333 | Reference |
| γ’ | Relative removal rate of asymptomatic individuals | day−1 | 0.1429 | 0.0714–0.3333 | Reference |
| τ | Simulation delay of the initial time in the whole season | day | 279 | ≥0 | Analysis of the reported data |
| T | Duration of the cycle | day | 365 | ≥0 | Analysis of the reported data |
| βIS | Relative transmission rate from imported mosquitoes to mosquitoes in Chikan District | 1 | See Table 2 | ≥0 | Curve fitting |
| βmcpc | Relative transmission rate from mosquitoes in Chikan District to humans in Chikan District | 1 | See Table 2 | ≥0 | Curve fitting |
| βmopc | Relative transmission rate from mosquitoes in other districts to humans in Chikan District | 1 | See Table 2 | ≥0 | Curve fitting |
| βmopo | Relative transmission rate from mosquitoes in other districts to humans in other districts | 1 | See Table 2 | ≥0 | Curve fitting |
| βpcmc | Relative transmission rate from humans in Chikan District to mosquitoes in Chikan District | 1 | See Table 2 | ≥0 | Curve fitting |
| βpcmo | Relative transmission rate from humans in Chikan District to mosquitoes in other districts | 1 | See Table 2 | ≥0 | Curve fitting |
| βpomo | Relative transmission rate from humans in other districts to humans in other districts | 1 | See Table 2 | ≥0 | Curve fitting |
| Variable | Male | Female | Total | Duration (Days) |
|---|---|---|---|---|
| District | ||||
| Chikan | 200 | 133 | 333 | 56 |
| Xiashan | 22 | 18 | 40 | 56 |
| Leizhou | 10 | 12 | 22 | 25 |
| Mzhang | 13 | 10 | 23 | 42 |
| Wuchuan | 6 | 9 | 15 | 45 |
| Suixi | 5 | 8 | 13 | 34 |
| Kaifa | 8 | 3 | 11 | 23 |
| Potou | 4 | 1 | 5 | 50 |
| Lianjiang | 0 | 1 | 1 | 1 |
| Total | 268 | 195 | 463 | - |
| Parameter | Chikan–Kaifa | Chikan–Mazhang | Chikan–Xiashan |
|---|---|---|---|
| βIS | 1.73 × 10−9 | 2.23 × 10−9 | 1.53 × 10−9 |
| βpcmc | 3.19 × 10−8 | 4.13 × 10−8 | 3.10 × 10−8 |
| βmcpc | 1.86 × 10−5 | 1.49 × 10−5 | 1.94 × 10−5 |
| βmopc | 7.88 × 10−10 | 1.18 × 10−9 | 7.31 × 10−10 |
| βpomo | 5.16 × 10−16 | 6.72 × 10−16 | 5.30 × 10−16 |
| βpcmo | 5.21 × 10−8 | 2.55 × 10−8 | 2.57 × 10−8 |
| βmopo | 5.67 × 10−7 | 1.66 × 10−6 | 1.70 × 10−6 |
| District | Fit | Scenario 1 | Scenario 2 | |||||
|---|---|---|---|---|---|---|---|---|
| Number of New Cases | Duration | Number of New Cases | Proportion * | Duration | Number of New Cases | Proportion * | Duration | |
| Chikan | 410 | 56 | 974 | 137.56% | 114 | 581 | 41.71% | 95 |
| Kaifa | 12 | 23 | - | - | - | 43 | 258.33% | 37 |
| Chikan | 406 | 56 | 932 | 129.56% | 114 | 579 | 42.61% | 95 |
| Xiashan | 34 | 55 | - | - | - | 97 | 185.29% | 54 |
| Chikan | 406 | 56 | 917 | 125.86% | 113 | 586 | 44.33% | 96 |
| Mazhang | 27 | 41 | - | - | - | 43 | 59.26% | 33 |
| Relative Transmission | Chikan–Kaifa District | Chikan–Xiashan District | Chikan–Mazhang District |
|---|---|---|---|
| NβIS | 0.441441 | 0.463294 | 0.389146 |
| Nβpcmc | 0.449167 | 0.464421 | 0.39408 |
| Nβmcpc | 1 | 1 | 1 |
| Nβmopc | 0.441441 | 0.463294 | 0.389146 |
| Nβpomo | 0.441441 | 0.463294 | 0.389146 |
| Nβpcmo | 0.064007 | 0.211337 | 0.389146 |
| Nβpcmo | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Huang, J.-F.; Kang, M.; Liu, X.-C.; Lin, H.-Y.; Zhao, Z.-Y.; Ye, G.-Q.; Lin, S.-N.; Rui, J.; Xu, J.-W.; et al. Epidemiological Characteristics and the Dynamic Transmission Model of Dengue Fever in Zhanjiang City, Guangdong Province in 2018. Trop. Med. Infect. Dis. 2022, 7, 209. https://doi.org/10.3390/tropicalmed7090209
Zhang M, Huang J-F, Kang M, Liu X-C, Lin H-Y, Zhao Z-Y, Ye G-Q, Lin S-N, Rui J, Xu J-W, et al. Epidemiological Characteristics and the Dynamic Transmission Model of Dengue Fever in Zhanjiang City, Guangdong Province in 2018. Tropical Medicine and Infectious Disease. 2022; 7(9):209. https://doi.org/10.3390/tropicalmed7090209
Chicago/Turabian StyleZhang, Meng, Jie-Feng Huang, Min Kang, Xing-Chun Liu, Hong-Yan Lin, Ze-Yu Zhao, Guo-Qiang Ye, Sheng-Nan Lin, Jia Rui, Jing-Wen Xu, and et al. 2022. "Epidemiological Characteristics and the Dynamic Transmission Model of Dengue Fever in Zhanjiang City, Guangdong Province in 2018" Tropical Medicine and Infectious Disease 7, no. 9: 209. https://doi.org/10.3390/tropicalmed7090209
APA StyleZhang, M., Huang, J.-F., Kang, M., Liu, X.-C., Lin, H.-Y., Zhao, Z.-Y., Ye, G.-Q., Lin, S.-N., Rui, J., Xu, J.-W., Zhu, Y.-Z., Wang, Y., Yang, M., Tang, S.-X., Cheng, Q., & Chen, T.-M. (2022). Epidemiological Characteristics and the Dynamic Transmission Model of Dengue Fever in Zhanjiang City, Guangdong Province in 2018. Tropical Medicine and Infectious Disease, 7(9), 209. https://doi.org/10.3390/tropicalmed7090209






