Expression Profile Analysis of Circular RNAs in Leishmaniasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Samples
2.2. RNA Extraction
2.3. Library Construction and RNA Sequence Analysis
2.4. Screening od Differentially Expressed circRNAs and miRNAs
2.5. Functional and Pathway Enrichment Analyses
2.6. PPI Network Construction and Regulation ceRNA Network
3. Results
3.1. The Differential Expression of Serum circRNAs and miRNAs
3.2. Functional and Pathway Enrichment Analyses
3.3. PPI Network Module Analysis of Host Genes
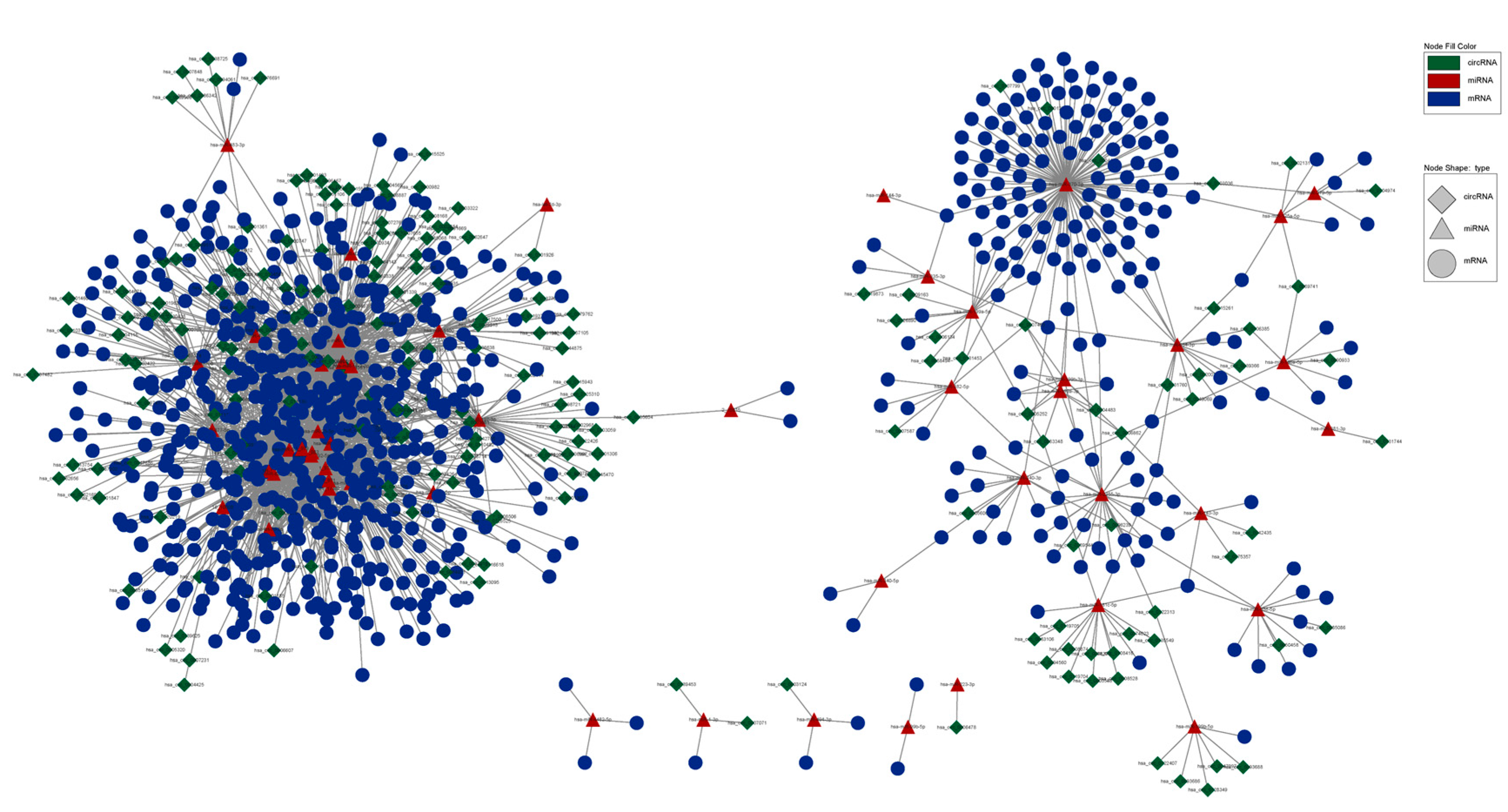
3.4. circRNA–miRNA–mRNA Network
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mersha, T.T.; Mekonnen Wolde, B.; Shumuye, N.A.; Hailu, A.B.; Mohammed, A.H.; Redda, Y.T.; Abera, B.H.; Menghistu, H.T. Prioritization of neglected tropical zoonotic diseases: A one health perspective from Tigray region, Northern Ethiopia. PLoS ONE 2021, 16, e0254071. [Google Scholar] [CrossRef] [PubMed]
- Singh, O.P.; Tiwary, P.; Kushwaha, A.K.; Singh, S.K.; Singh, D.K.; Lawyer, P.; Rowton, E.; Chaubey, R.; Singh, A.K.; Rai, T.K.; et al. Xenodiagnosis to evaluate the infectiousness of humans to sandflies in an area endemic for visceral leishmaniasis in Bihar, India: A transmission-dynamics study. Lancet Microbe 2021, 2, e23–e31. [Google Scholar] [CrossRef]
- Mohammed, A.S.A.; Tian, W.; Zhang, Y.; Peng, P.; Wang, F.; Li, T. Leishmania lipophosphoglycan components: A potent target for synthetic neoglycoproteins as a vaccine candidate for leishmaniasis. Carbohydr. Polym. 2020, 237, 116120. [Google Scholar] [CrossRef] [PubMed]
- de Vlas, S.J.; Stolk, W.A.; le Rutte, E.A.; Hontelez, J.A.; Bakker, R.; Blok, D.J.; Cai, R.; Houweling, T.A.; Kulik, M.C.; Lenk, E.J.; et al. Concerted Efforts to Control or Eliminate Neglected Tropical Diseases: How Much Health Will Be Gained? PLoS Negl. Trop. Dis. 2016, 10, e0004386. [Google Scholar] [CrossRef] [Green Version]
- Babat, S.O.; Cavus, I.; Ozbilgin, A.; Kayalar, H.; Gunduz, C.; Ceylan, S.S.; Girginkardesler, N. Investigation of the Anti-Leishmanial Effects of Prangos ferulacea and Ferula orientalis Extracts Collected from Sirnak Province Against Leishmania tropica Isolated in Turkey. Mikrobiyol. Bul. 2022, 56, 339–348. [Google Scholar] [CrossRef]
- Yasmeen, N.; Jabbar, A.; Shah, T.; Fang, L.X.; Aslam, B.; Naseeb, I.; Shakeel, F.; Ahmad, H.I.; Baloch, Z.; Liu, Y. One Health Paradigm to Confront Zoonotic Health Threats: A Pakistan Prospective. Front. Microbiol. 2021, 12, 719334. [Google Scholar] [CrossRef]
- Rodrigues, V.; Cordeiro-da-Silva, A.; Laforge, M.; Silvestre, R.; Estaquier, J. Regulation of immunity during visceral Leishmania infection. Parasit. Vectors 2016, 9, 118. [Google Scholar] [CrossRef] [Green Version]
- Goyal, V.; Das, V.N.R.; Singh, S.N.; Singh, R.S.; Pandey, K.; Verma, N.; Hightower, A.; Rijal, S.; Das, P.; Alvar, J.; et al. Long-term incidence of relapse and post-kala-azar dermal leishmaniasis after three different visceral leishmaniasis treatment regimens in Bihar, India. PLoS Negl. Trop. Dis. 2020, 14, e0008429. [Google Scholar] [CrossRef]
- Soyal, A.; Gokmen, T.G.; Kayar, B.; Koksal, F. Comparison of convensional and molecular methods used to determine Leishmania species. Trop. Biomed. 2016, 33, 260–267. [Google Scholar]
- Machado de Assis, T.S.; Azeredo-da-Silva, A.L.; Werneck, G.L.; Rabello, A. Cost-effectiveness analysis of diagnostic tests for human visceral leishmaniasis in Brazil. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 464–471. [Google Scholar] [CrossRef]
- Abass, E.; Kang, C.; Martinkovic, F.; Semiao-Santos, S.J.; Sundar, S.; Walden, P.; Piarroux, R.; El Harith, A.; Lohoff, M.; Steinhoff, U. Heterogeneity of Leishmania donovani parasites complicates diagnosis of visceral leishmaniasis: Comparison of different serological tests in three endemic regions. PLoS ONE 2015, 10, e0116408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srividya, G.; Kulshrestha, A.; Singh, R.; Salotra, P. Diagnosis of visceral leishmaniasis: Developments over the last decade. Parasitol. Res. 2012, 110, 1065–1078. [Google Scholar] [CrossRef] [PubMed]
- Faleiro, R.J.; Kumar, R.; Hafner, L.M.; Engwerda, C.R. Immune regulation during chronic visceral leishmaniasis. PLoS Negl. Trop. Dis. 2014, 8, e2914. [Google Scholar] [CrossRef] [Green Version]
- Eger, N.; Schoppe, L.; Schuster, S.; Laufs, U.; Boeckel, J.N. Circular RNA Splicing. Adv. Exp. Med. Biol. 2018, 1087, 41–52. [Google Scholar] [CrossRef]
- Ebbesen, K.K.; Hansen, T.B.; Kjems, J. Insights into circular RNA biology. RNA Biol. 2017, 14, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, A. Different roles of circular RNAs with protein coding potentials. Biochem. Biophys. Res. Commun. 2018, 500, 907–909. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, D.; Pu, W.; Wang, J.; Peng, Y. Circular RNAs in Cancer: Biogenesis, Function, and Clinical Significance. Trends Cancer 2020, 6, 319–336. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, X.; Zhang, M.; Yan, S.; Sun, C.; Xiao, F.; Huang, N.; Yang, X.; Zhao, K.; Zhou, H.; et al. Novel Role of FBXW7 Circular RNA in Repressing Glioma Tumorigenesis. J. Natl. Cancer Inst. 2018, 110, 304–315. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.C.; Hong, H.C.; Yang, C.D.; Lee, W.H.; Huang, H.T.; Huang, H.D. Ouroboros resembling competitive endogenous loop (ORCEL) in circular RNAs revealed through transcriptome sequencing dataset analysis. BMC Genom. 2018, 19, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, W.; Bi, J.; Liu, H.; Yan, D.; He, Q.; Zhou, Q.; Wang, Q.; Xie, R.; Su, Y.; Yang, M.; et al. Circular RNA ACVR2A suppresses bladder cancer cells proliferation and metastasis through miR-626/EYA4 axis. Mol. Cancer 2019, 18, 95. [Google Scholar] [CrossRef] [Green Version]
- Rong, D.; Sun, H.; Li, Z.; Liu, S.; Dong, C.; Fu, K.; Tang, W.; Cao, H. An emerging function of circRNA-miRNAs-mRNA axis in human diseases. Oncotarget 2017, 8, 73271–73281. [Google Scholar] [CrossRef] [Green Version]
- Xiao, M.S.; Wilusz, J.E. An improved method for circular RNA purification using RNase R that efficiently removes linear RNAs containing G-quadruplexes or structured 3’ ends. Nucleic Acids Res. 2019, 47, 8755–8769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Xie, Q.; He, D.; Ling, Y.; Li, Y.; Li, J.; Zhang, H. Circular RNA: New star, new hope in cancer. BMC Cancer 2018, 18, 834. [Google Scholar] [CrossRef]
- Min, X.; Liu, D.L.; Xiong, X.D. Circular RNAs as Competing Endogenous RNAs in Cardiovascular and Cerebrovascular Diseases: Molecular Mechanisms and Clinical Implications. Front. Cardiovasc. Med. 2021, 8, 682357. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zou, Y.; Chen, C.; Tang, Y.; Guo, J. Current Understanding of Circular RNAs in Systemic Lupus Erythematosus. Front. Immunol. 2021, 12, 628872. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.A.; Venkatachalam-Vaz, J.; Drake, J.M. Spillover of zoonotic pathogens: A review of reviews. Zoonoses Public Health 2021, 68, 563–577. [Google Scholar] [CrossRef]
- Li, T.R.; Jia, Y.J.; Wang, Q.; Shao, X.Q.; Lv, R.J. Circular RNA: A new star in neurological diseases. Int. J. Neurosci. 2017, 127, 726–734. [Google Scholar] [CrossRef]
- Rybak-Wolf, A.; Stottmeister, C.; Glazar, P.; Jens, M.; Pino, N.; Giusti, S.; Hanan, M.; Behm, M.; Bartok, O.; Ashwal-Fluss, R.; et al. Circular RNAs in the Mammalian Brain are Highly Abundant, Conserved, and Dynamically Expressed. Mol. Cell 2015, 58, 870–885. [Google Scholar] [CrossRef] [Green Version]
- Suenkel, C.; Cavalli, D.; Massalini, S.; Calegari, F.; Rajewsky, N. A Highly Conserved Circular RNA is Required to Keep Neural Cells in a Progenitor State in the Mammalian Brain. Cell Rep. 2020, 30, 2170–2179. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.; Zhu, N.; Guo, W.; Wang, X.; Li, K.; Yan, J.; Jiang, C.; Han, S.; Xiang, H.; Wu, X.; et al. RNA-Seq Revealed a Circular RNA-microRNA-mRNA Regulatory Network in Hantaan Virus Infection. Front. Cell. Infect. Microbiol. 2020, 10, 97. [Google Scholar] [CrossRef]
- Wesselhoeft, R.A.; Kowalski, P.S.; Parker-Hale, F.C.; Huang, Y.; Bisaria, N.; Anderson, D.G. RNA Circularization Diminishes Immunogenicity and Can Extend Translation Duration In Vivo. Mol. Cell 2019, 74, 508–520. [Google Scholar] [CrossRef]
- Anastasiadou, E.; Faggioni, A.; Trivedi, P.; Slack, F.J. The Nefarious Nexus of Noncoding RNAs in Cancer. Int. J. Mol. Sci. 2018, 19, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, F.; Shi, Z.; Zhao, Y.; Tian, J. CircRNA hsa-circ-0014359 promotes glioma progression by regulating miR-153/PI3K signaling. Biochem. Biophys. Res. Commun. 2019, 510, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, X.; Wei, S.; Chen, Y.; Chen, Y.; Fan, X.; Han, S.; Wu, G. hsa_circ_0013958: A circular RNA and potential novel biomarker for lung adenocarcinoma. FEBS J. 2017, 284, 2170–2182. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Ma, W.; Ke, Z.; Xie, F. CircRNA hsa_circ_100395 regulates miR-1228/TCF21 pathway to inhibit lung cancer progression. Cell Cycle 2018, 17, 2080–2090. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Yang, Y.; Yan, Y.; Li, T.; Li, Y.; Wang, Z.; Shen, Z.; Ye, Y.; Jiang, K.; Wang, S. Analysis of co-expression networks for circular RNAs and mRNAs reveals that circular RNAs hsa_circ_0047905, hsa_circ_0138960 and has-circRNA7690-15 are candidate oncogenes in gastric cancer. Cell Cycle 2017, 16, 2301–2311. [Google Scholar] [CrossRef] [Green Version]
- Rong, D.; Lu, C.; Zhang, B.; Fu, K.; Zhao, S.; Tang, W.; Cao, H. CircPSMC3 suppresses the proliferation and metastasis of gastric cancer by acting as a competitive endogenous RNA through sponging miR-296-5p. Mol. Cancer 2019, 18, 25. [Google Scholar] [CrossRef] [Green Version]
- Pan, G.; Mao, A.; Liu, J.; Lu, J.; Ding, J.; Liu, W. Circular RNA hsa_circ_0061825 (circ-TFF1) contributes to breast cancer progression through targeting miR-326/TFF1 signalling. Cell Prolif. 2020, 53, e12720. [Google Scholar] [CrossRef] [Green Version]
- He, R.; Liu, P.; Xie, X.; Zhou, Y.; Liao, Q.; Xiong, W.; Li, X.; Li, G.; Zeng, Z.; Tang, H. circGFRA1 and GFRA1 act as ceRNAs in triple negative breast cancer by regulating miR-34a. J. Exp. Clin. Cancer Res. 2017, 36, 145. [Google Scholar] [CrossRef] [Green Version]
- Qin, M.; Liu, G.; Huo, X.; Tao, X.; Sun, X.; Ge, Z.; Yang, J.; Fan, J.; Liu, L.; Qin, W. Hsa_circ_0001649: A circular RNA and potential novel biomarker for hepatocellular carcinoma. Cancer Biomark. 2016, 16, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Hu, W.; Huang, X.; Huang, X.; Chen, W.; Hao, L.; Chen, Z.; Wang, J.; Wei, H. Circ_0001178 regulates miR-382/VEGFA axis to facilitate hepatocellular carcinoma progression. Cell Signal. 2020, 72, 109621. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Kong, R.; Wu, C.; Wang, S.; Liu, Z.; Liu, S.; Li, S.; Chen, T.; Mao, C.; Liu, S. Circ-MALAT1 Functions as Both an mRNA Translation Brake and a microRNA Sponge to Promote Self-Renewal of Hepatocellular Cancer Stem Cells. Adv. Sci. 2020, 7, 1900949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, I.; Basak, R.; Mukhopadhyay, A. Hemoglobin Endocytosis and Intracellular Trafficking: A Novel Way of Heme Acquisition by Leishmania. Pathogens 2022, 11, 585. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.A.; Karmakar, J.; Mandal, C.; Chattopadhyay, A. Leishmania donovani Internalizes into Host Cells via Caveolin-mediated Endocytosis. Sci. Rep. 2019, 9, 12636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares-Silva, M.; Diniz, F.F.; Gomes, G.N.; Bahia, D. The Mitogen-Activated Protein Kinase (MAPK) Pathway: Role in Immune Evasion by Trypanosomatids. Front. Microbiol. 2016, 7, 183. [Google Scholar] [CrossRef]
- Cyrino, L.T.; Araujo, A.P.; Joazeiro, P.P.; Vicente, C.P.; Giorgio, S. In vivo and in vitro Leishmania amazonensis infection induces autophagy in macrophages. Tissue Cell 2012, 44, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Franco, L.H.; Fleuri, A.K.A.; Pellison, N.C.; Quirino, G.F.S.; Horta, C.V.; de Carvalho, R.V.H.; Oliveira, S.C.; Zamboni, D.S. Autophagy downstream of endosomal Toll-like receptor signaling in macrophages is a key mechanism for resistance to Leishmania major infection. J. Biol. Chem. 2017, 292, 13087–13096. [Google Scholar] [CrossRef] [Green Version]
- Dias, B.R.S.; de Souza, C.S.; Almeida, N.J.; Lima, J.G.B.; Fukutani, K.F.; Dos Santos, T.B.S.; Franca-Cost, J.; Brodskyn, C.I.; de Menezes, J.P.B.; Colombo, M.I.; et al. Autophagic Induction Greatly Enhances Leishmania major Intracellular Survival Compared to Leishmania amazonensis in CBA/j-Infected Macrophages. Front. Microbiol. 2018, 9, 1890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrakar, P.; Seth, A.; Rani, A.; Dutta, M.; Parmar, N.; Descoteaux, A.; Kar, S. Jagged-Notch-mediated divergence of immune cell crosstalk maintains the anti-inflammatory response in visceral leishmaniasis. J. Cell Sci. 2021, 134, jcs252494. [Google Scholar] [CrossRef] [PubMed]
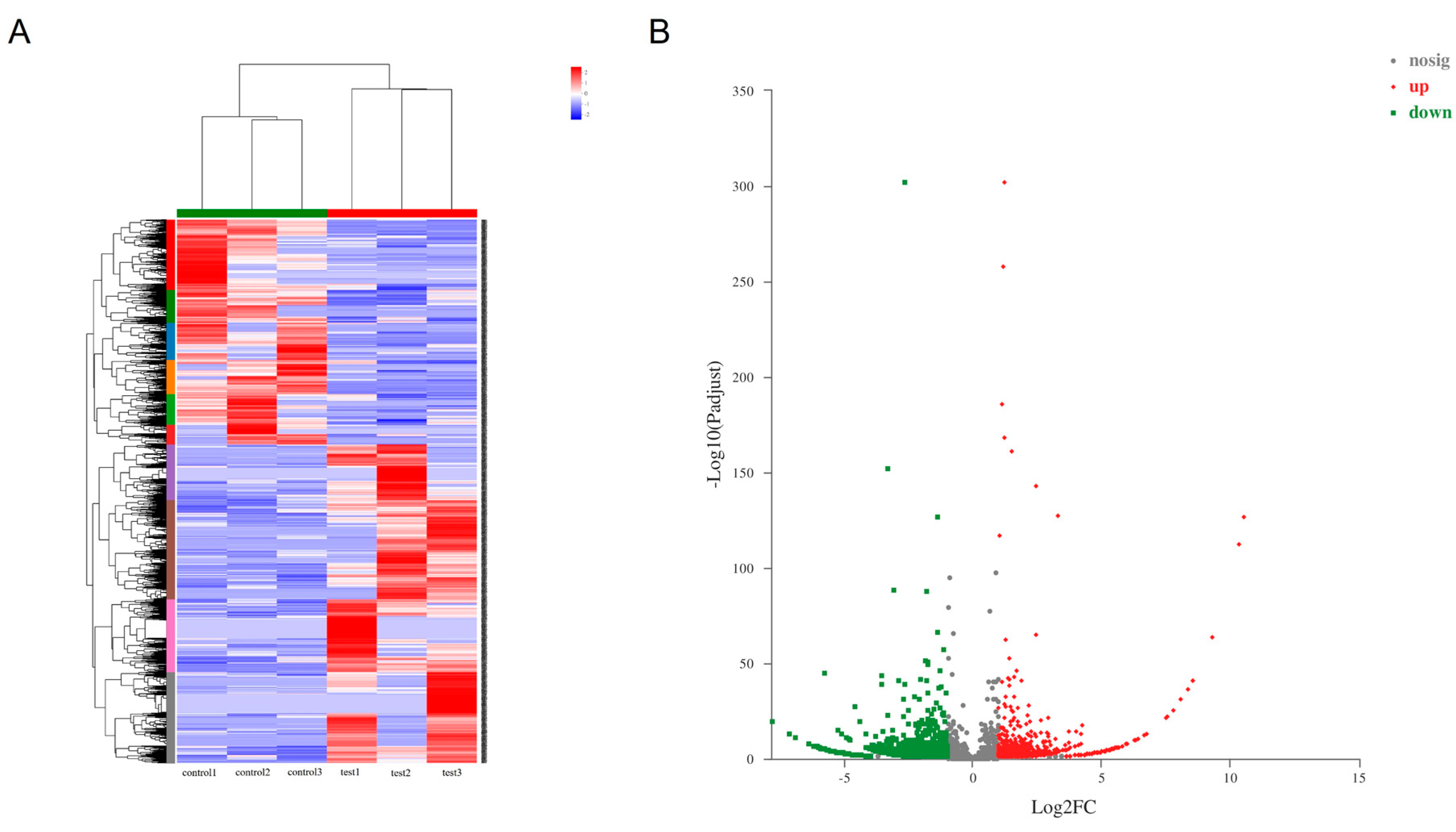



| circRNA ID | logFC | p Value | Regulate | Significant |
|---|---|---|---|---|
| chr13: 30251931_30257867 | 10.54691 | 1.03 × 10−130 | up | yes |
| chr 6: 31271073_31355592 | 10.32427 | 3.57 × 10−116 | up | yes |
| chr 13: 30280063_30283791 | 9.30495 | 4.25 × 10−67 | up | yes |
| chr 6: 29887955_29942626 | 8.557338 | 2.02 × 10−44 | up | yes |
| chr 6: 29829418_29888742 | 8.551388 | 2.84 × 10−44 | up | yes |
| chr13: 30621764_30647057 | 8.339884 | 2.45 × 10−39 | up | yes |
| chr 1: 35850157_35851053 | 8.100131 | 2.25 × 10−34 | up | yes |
| chr1: 244408712_244430099 | 7.772076 | 1.52 × 10−28 | up | yes |
| chr 7: 77083887_77098951 | 7.563262 | 2.43 × 10−25 | up | yes |
| chr 6: 3410188_3438555 | 7.515168 | 1.18 × 10−24 | up | yes |
| circRNA ID | logFC | p Value | Regulate | Significant |
|---|---|---|---|---|
| chr 6: 32521905_32581838 | −7.77687 | 1.49 × 10−22 | down | yes |
| chr 12: 2866393_2872244 | −7.14883 | 7.86 × 10−16 | down | yes |
| chr17: 16034765_16040494 | −7.14883 | 7.86 × 10−16 | down | yes |
| chr 1: 11016844_11020599 | −6.89104 | 1.11 × 10−13 | down | yes |
| chr11: 20407911_20426865 | −6.36747 | 3.68 × 10−10 | down | yes |
| chr17: 63666940_63685578 | −6.36747 | 3.68 × 10−10 | down | yes |
| chr 1: 23971573_23972012 | −6.17483 | 4.15 × 10−9 | down | yes |
| chr2: 171435083_171458075 | −6.12236 | 7.68 × 10−9 | down | yes |
| chr1: 235812971_235833667 | −6.06791 | 1.43 × 10−8 | down | yes |
| chr12: 102269600_102315490 | −6.06791 | 1.43 × 10−8 | down | yes |
| miRNA ID | logFC | p Value | Regulate | Significant |
|---|---|---|---|---|
| hsa-miR-483-3p | 3.906015 | 6.71 × 10−5 | up | yes |
| hsa-let-7b-3p | 3.784429 | 0.000352 | up | yes |
| hsa-miR-486-3p | 3.369297 | 0.000199 | up | yes |
| hsa-miR-16-2-3p | 2.870342 | 1.19 × 10−7 | up | yes |
| hsa-miR-25-5p | 2.467263 | 2.58 × 10−5 | up | yes |
| hsa-let-7d-3p | 2.430329 | 7.36 × 10−15 | up | yes |
| hsa-miR-5100 | 2.400199 | 1.86 × 10−9 | up | yes |
| hsa-miR-6877-5p | 1.977799 | 0.001816 | up | yes |
| hsa-miR-1260b | 1.82933 | 6.81 × 10−6 | up | yes |
| hsa-miR-877-5p | 1.79051 | 2.06 × 10−5 | up | yes |
| miRNA ID | logFC | p Value | Regulate | Significant |
|---|---|---|---|---|
| hsa-miR-4482-5p | −4.00131 | 0.002571 | down | yes |
| hsa-miR-411-5p | −3.16776 | 0.001176 | down | yes |
| hsa-miR-487b-3p | −2.57252 | 0.000307 | down | yes |
| hsa-miR-381-3p | −2.47089 | 0.005327 | down | yes |
| hsa-miR-654-3p | −2.29163 | 0.000147 | down | yes |
| hsa-miR-2355-3p | −2.24553 | 0.00178 | down | yes |
| hsa-miR-382-5p | −2.24167 | 0.001283 | down | yes |
| hsa-miR-494-3p | −2.21251 | 0.000269 | down | yes |
| hsa-miR-1-3p | −2.18407 | 0.000411 | down | yes |
| hsa-miR-146a-5p | −2.1812 | 1.68 × 10−9 | down | yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zeng, W.; Yang, Y.; Zhang, P.; Zhou, Z.; Li, Y.; Guo, Y.; Zhang, Y. Expression Profile Analysis of Circular RNAs in Leishmaniasis. Trop. Med. Infect. Dis. 2022, 7, 176. https://doi.org/10.3390/tropicalmed7080176
Li Z, Zeng W, Yang Y, Zhang P, Zhou Z, Li Y, Guo Y, Zhang Y. Expression Profile Analysis of Circular RNAs in Leishmaniasis. Tropical Medicine and Infectious Disease. 2022; 7(8):176. https://doi.org/10.3390/tropicalmed7080176
Chicago/Turabian StyleLi, Zhongqiu, Wenbo Zeng, Yufeng Yang, Peijun Zhang, Zhengbing Zhou, Yuanyuan Li, Yunhai Guo, and Yi Zhang. 2022. "Expression Profile Analysis of Circular RNAs in Leishmaniasis" Tropical Medicine and Infectious Disease 7, no. 8: 176. https://doi.org/10.3390/tropicalmed7080176
APA StyleLi, Z., Zeng, W., Yang, Y., Zhang, P., Zhou, Z., Li, Y., Guo, Y., & Zhang, Y. (2022). Expression Profile Analysis of Circular RNAs in Leishmaniasis. Tropical Medicine and Infectious Disease, 7(8), 176. https://doi.org/10.3390/tropicalmed7080176







