Assessing the Prevalence and Determinants of Exposure-Influenced HIV Testing among a Sample of Pre- and Post-Exposure Prophylaxis-Naïve Young Men Who Have Sex with Men in the United States
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data collection and Measures
2.3. Statistical Analyses
2.4. Ethical Considerations
3. Results
3.1. Participant Characteristics
3.2. Demographic and Psychosocial Correlates of Exposure-Influenced HIV Testing
3.3. Comparison of Behavioral Characteristics
3.4. The Intention of Seeking Future Exposure-Influenced HIV Testing
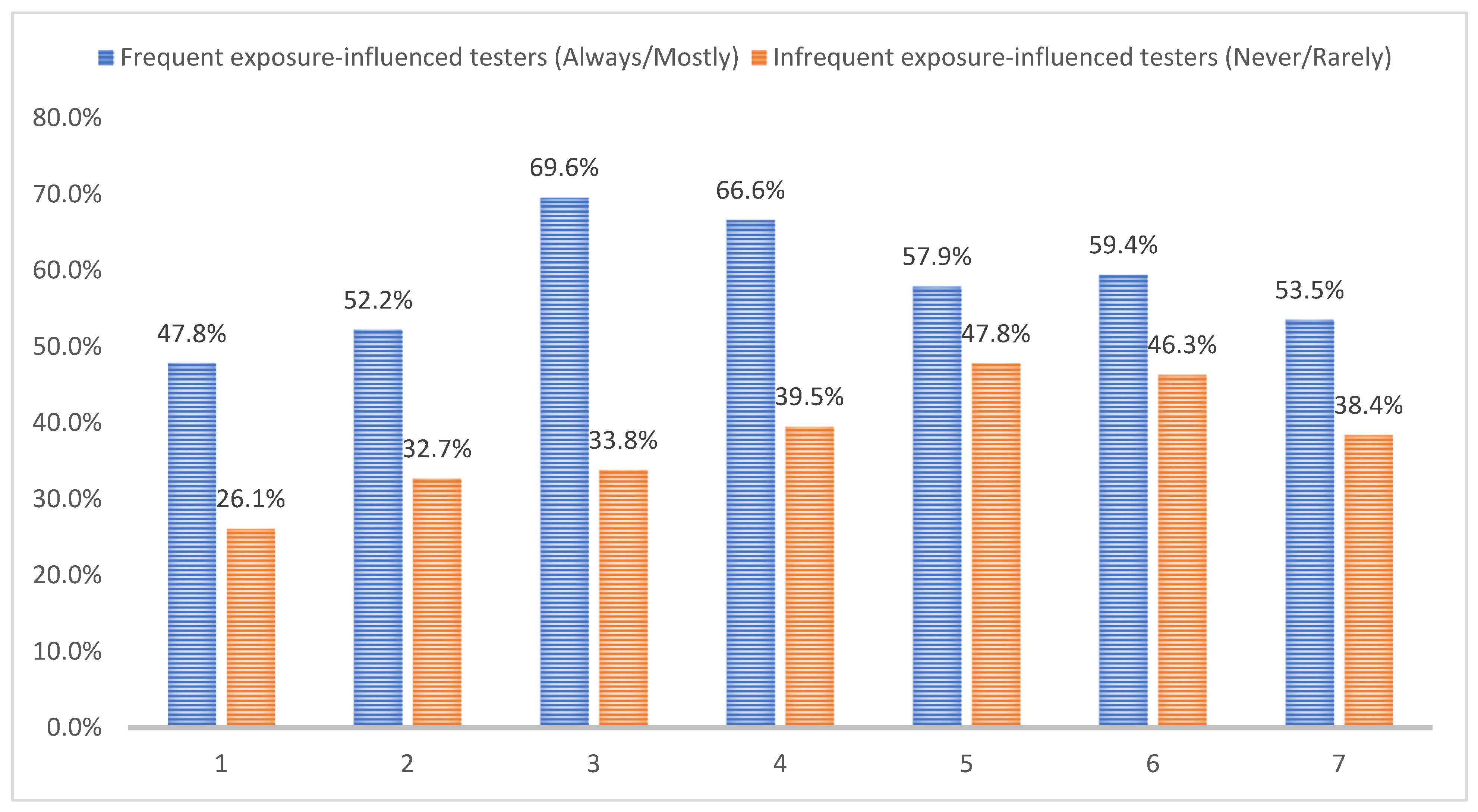
3.5. Barriers and Facilitators of Seeking Exposure-Influenced HIV Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. HIV Surveillance Report; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2019; Volume 32. Available online: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html (accessed on 10 May 2021).
- CDC Fact Sheet. The Nation’s Approach to HIV Prevention for Gay and Bisexual Men. Available online: https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/approach-to-hiv-prevention-508.pdf (accessed on 13 June 2021).
- Marano, M.; Stein, R.; Song, W.; Patel, D.; Taylor-Aidoo, N.; Xu, S.; Scales, L. HIV Testing, Linkage to HIV Medical Care, and Interviews for Partner Services Among Black Men Who Have Sex with Men–Non-Health Care Facilities, 20 Southern U.S. Jurisdictions, 2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 778–781. [Google Scholar] [CrossRef]
- Gardner, E.M.; McLees, M.P.; Steiner, J.F.; Del Rio, C.; Burman, W.J. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin. Infect. Dis. 2011, 52, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Mugavero, M.J.; Amico, K.R.; Westfall, A.O.; Crane, H.M.; Zinski, A.; Willig, J.H.; Dombrowski, J.C.; Norton, W.E.; Raper, J.L.; Kitahata, M.M. Early retention in HIV care and viral load suppression: Implications for a test and treat approach to HIV prevention. J. Acquir. Immune Defic. Syndr. 2012, 59, 86. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Cohen, S.; Follansbee, S.; Cohan, D.; Weber, S.; Sachdev, D.; Buchbinder, S. Early experiences implementing pre-exposure prophylaxis (PrEP) for HIV prevention in San Francisco. PLoS Med. 2014, 11, e1001613. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Campbell, C.K.; Lippman, S.A.; Moss, N.; Lightfoot, M. Strategies to increase HIV testing among MSM: A synthesis of the literature. AIDS Behav. 2018, 22, 2387–2412. [Google Scholar] [CrossRef]
- Liu, A.; Colfax, G.; Cohen, S.; Bacon, O.; Kolber, M.; Amico, K.; Mugavero, M.; Grant, R.; Buchbinder, S. The spectrum of engagement in HIV prevention: Proposal for a PrEP cascade. In Proceedings of the 7th International Conference on HIV Treatment and Prevention Adherence, Miami, FL, USA, 3 June 2012; pp. 3–5. [Google Scholar]
- Branson, B.M.; Handsfield, H.H.; Lampe, M.A.; Janssen, R.S.; Taylor, A.W.; Lyss, S.B.; Clark, J.E. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in healthcare settings. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2006, 55, 1-CE-4. [Google Scholar]
- Beckwith, C.G.; Flanigan, T.P.; Del Rio, C.; Simmons, E.; Wing, E.J.; Carpenter, C.C.; Bartlett, J.G. It is time to implement routine, not risk-based, HIV testing. Clin. Infect. Dis. 2005, 40, 1037–1040. [Google Scholar] [CrossRef]
- Moyer, V.A. Screening for HIV: US preventive services task force recommendation statement. Ann. Intern. Med. 2013, 159, 51–60. [Google Scholar] [CrossRef]
- Vodstrcil, L.A.; Fairley, C.K.; Chen, M.Y.; Denham, I. Risk-based HIV testing of men who have sex with men would result in missed HIV diagnoses. Sex. Transm. Dis. 2012, 39, 492. [Google Scholar] [CrossRef]
- Sanchez, T.H.; Zlotorzynska, M.; Sineath, R.C.; Kahle, E.; Tregear, S.; Sullivan, P.S. National trends in sexual behavior, substance use and HIV testing among United States men who have sex with men recruited online, 2013 through 2017. AIDS Behav. 2018, 22, 2413–2425. [Google Scholar] [CrossRef]
- Frye, V.; Wilton, L.; Hirshfield, S.; Chiasson, M.A.; Lucy, D.; Usher, D.; McCrossin, J.; Greene, E.; Koblin, B.; Team, A.A.M.S. Preferences for HIV test characteristics among young, Black Men Who Have Sex With Men (MSM) and transgender women: Implications for consistent HIV testing. PLoS ONE 2018, 13, e0192936. [Google Scholar] [CrossRef] [PubMed]
- Noble, M.; Jones, A.M.; Bowles, K.; DiNenno, E.A.; Tregear, S.J. HIV testing among internet-using MSM in the United States: Systematic review. AIDS Behav. 2017, 21, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Silenzio, V.M.; Nash, R.; Luther, P.; Bauermeister, J.; Vermund, S.H.; Zhang, C. Suboptimal Recent and Regular HIV Testing Among Black Men Who Have Sex With Men in the United States: Implications From a Meta-Analysis. JAIDS J. Acquir. Immune Defic. Syndr. 2019, 81, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Kanny, D.; Jeffries IV, W.L.; Chapin-Bardales, J.; Denning, P.; Cha, S.; Finlayson, T.; Wejnert, C.; Abrego, M.; Al-Tayyib, A.; Anderson, B. Racial/ethnic disparities in HIV preexposure prophylaxis among men who have sex with men—23 urban areas, 2017. Morb. Mortal. Wkly. Rep. 2019, 68, 801. [Google Scholar] [CrossRef]
- Oster, A.M.; Johnson, C.H.; Le, B.C.; Balaji, A.B.; Finlayson, T.J.; Lansky, A.; Mermin, J.; Valleroy, L.; MacKellar, D.; Behel, S. Trends in HIV prevalence and HIV testing among young MSM: Five United States cities, 1994–2011. AIDS Behav. 2014, 18, 237–247. [Google Scholar] [CrossRef]
- Control, C.f.D.; Prevention. HIV testing among men who have sex with men--21 cities, United States, 2008. MMWR. Morb. Mortal. Wkly. Rep. 2011, 60, 694–699. [Google Scholar]
- Paz-Bailey, G.; Hall, H.I.; Wolitski, R.J.; Prejean, J.; Van Handel, M.M.; Le, B.; LaFlam, M.; Koenig, L.J.; Mendoza, M.C.B.; Rose, C. HIV testing and risk behaviors among gay, bisexual, and other men who have sex with men—United States. MMWR. Morb. Mortal. Wkly. Rep. 2013, 62, 958. [Google Scholar]
- Sarno, E.L.; Bettin, E.; Jozsa, K.; Newcomb, M.E. Sexual health of rural and urban young male couples in the United States: Differences in HIV testing, pre-exposure prophylaxis use, and condom use. AIDS Behav. 2021, 25, 191–202. [Google Scholar] [CrossRef]
- Creasy, S.L.; Henderson, E.R.; Bukowski, L.A.; Matthews, D.D.; Stall, R.D.; Hawk, M.E. Hiv testing and art adherence among unstably housed black men who have sex with men in the United States. AIDS Behav. 2019, 23, 3044–3051. [Google Scholar] [CrossRef]
- Liu, Y.; Qian, H.-Z.; Ruan, Y.; Wu, P.; Osborn, C.Y.; Jia, Y.; Yin, L.; Lu, H.; He, X.; Shao, Y. Frequent HIV testing: Impact on HIV risk among Chinese men who have sex with men. J. Acquir. Immune Defic. Syndr. 2016, 72, 452. [Google Scholar] [CrossRef]
- O’Byrne, P. HIV self-testing: A review and analysis to guide HIV prevention policy. Public Health Nurs. 2021, 38, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, O.W.; Salerno, J.P. Facilitators, barriers, and outcomes of self-initiated HIV testing: An integrative literature Review. Res. Theory Nurs. Pract. 2019, 33, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, O.W.; Williams, J.R.; Garcia, A. “The right place and the right time”: A qualitative study of the decision-making process of self-initiated HIV testing among young adults. Res. Nurs. Health 2020, 43, 186–194. [Google Scholar] [CrossRef]
- Liu, Y.; Brown, L.; Przybyla, S.; Bleasdale, J.; Mitchell, J.; Zhang, C. Characterizing racial differences of mental health burdens, psychosocial determinants, and impacts on HIV prevention outcomes among young men who have sex with men: A community-based study in two US cities. J. Racial Ethn. Health Disparities 2021, 9, 1114–1124. [Google Scholar] [CrossRef]
- Liu, Y.; Bleasdale, J.; Przybyla, S.; Higgins, M.C.; Zhang, C. Racial Variations in Psychosocial Vulnerabilities Linked to Differential Poppers Use and Associated HIV-Related Outcomes among Young Men Who Have Sex with Men: A Study in Two US Metropolitan Areas. Subst. Use Misuse 2022, 57, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Russ, S.; Mitchell, J.; Przybyla, S.; Zhang, C. Assessing the Determinants of Quality of Life and the Impact on HIV Prevention Measures among HIV-Negative and Status-Unknown Young Men Who Have Sex with Men: A Study in Two US Metropolitan Areas. Int. J. Environ. Res. Public Health 2022, 19, 726. [Google Scholar] [CrossRef]
- Granich, R.M.; Gilks, C.F.; Dye, C.; De Cock, K.M.; Williams, B.G. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: A mathematical model. Lancet 2009, 373, 48–57. [Google Scholar] [CrossRef]
- Coates, T.J.; Richter, L.; Caceres, C. Behavioural strategies to reduce HIV transmission: How to make them work better. Lancet 2008, 372, 669–684. [Google Scholar] [CrossRef]
- Furegato, M.; Mitchell, H.; Ogaz, D.; Woodhall, S.; Connor, N.; Hughes, G.; Nardone, A.; Mohammed, H. The role of frequent HIV testing in diagnosing HIV in men who have sex with men. HIV Med. 2018, 19, 118–122. [Google Scholar] [CrossRef]
- Mitchell, J.; Torres, M.B.; Asmar, L.; Danh, T.; Horvath, K.J. Developing sustainable and impactful mobile phone HIV testing interventions for spanish-speaking men who have sex with men in the United States: Lessons learned from informative interviews. JMIR Public Health Surveill. 2018, 4, e8992. [Google Scholar] [CrossRef]
- Mustanski, B.S.; Newcomb, M.E.; Du Bois, S.N.; Garcia, S.C.; Grov, C. HIV in young men who have sex with men: A review of epidemiology, risk and protective factors, and interventions. J. Sex Res. 2011, 48, 218–253. [Google Scholar] [CrossRef] [PubMed]
- Kubicek, K.; Arauz-Cuadra, C.; Kipke, M.D. Attitudes and perceptions of biomedical HIV prevention methods: Voices from young men who have sex with men. Arch. Sex. Behav. 2015, 44, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Eaton, L.A.; Driffin, D.D.; Bauermeister, J.; Smith, H.; Conway-Washington, C. Minimal awareness and stalled uptake of pre-exposure prophylaxis (PrEP) among at risk, HIV-negative, black men who have sex with men. AIDS Patient Care STDs 2015, 29, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, N.; Pivik, K. Age and gender differences in risky driving: The roles of positive affect and risk perception. Accid. Anal. Prev. 2011, 43, 923–931. [Google Scholar] [CrossRef]
- Benotsch, E.G.; Snipes, D.J.; Martin, A.M.; Bull, S.S. Sexting, substance use, and sexual risk behavior in young adults. J. Adolesc. Health 2013, 52, 307–313. [Google Scholar] [CrossRef]
- Fields, E.L.; Bogart, L.M.; Smith, K.C.; Malebranche, D.J.; Ellen, J.; Schuster, M.A. “I always felt I had to prove my manhood”: Homosexuality, masculinity, gender role strain, and HIV risk among young Black men who have sex with men. Am. J. Public Health 2015, 105, 122–131. [Google Scholar] [CrossRef]
- Storholm, E.D.; Volk, J.E.; Marcus, J.L.; Silverberg, M.J.; Satre, D.D. Risk perception, sexual behaviors, and PrEP adherence among substance-using men who have sex with men: A qualitative study. Prev. Sci. 2017, 18, 737–747. [Google Scholar] [CrossRef]
- Clifton, S.; Nardone, A.; Field, N.; Mercer, C.H.; Tanton, C.; Macdowall, W.; Johnson, A.M.; Sonnenberg, P. HIV testing, risk perception, and behaviour in the British population. AIDS 2016, 30, 943. [Google Scholar] [CrossRef]
- Scheim, A.I.; Travers, R. Barriers and facilitators to HIV and sexually transmitted infections testing for gay, bisexual, and other transgender men who have sex with men. AIDS Care 2017, 29, 990–995. [Google Scholar] [CrossRef]
- Elkington, K.S.; Bauermeister, J.A.; Zimmerman, M.A. Psychological distress, substance use, and HIV/STI risk behaviors among youth. J. Youth Adolesc. 2010, 39, 514–527. [Google Scholar] [CrossRef]
- Bauermeister, J.A.; Muessig, K.E.; Flores, D.D.; LeGrand, S.; Choi, S.; Dong, W.; Harper, G.W.; Hightow-Weidman, L.B. Stigma diminishes the protective effect of social support on psychological distress among young black men who have sex with men. AIDS Educ. Prev. 2018, 30, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Golub, S.A.; Gamarel, K.E. The impact of anticipated HIV stigma on delays in HIV testing behaviors: Findings from a community-based sample of men who have sex with men and transgender women in New York City. AIDS Patient Care STDs 2013, 27, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Mannheimer, S.; Wang, L.; Wilton, L.; Tieu, H.; Del Rio, C.; Buchbinder, S.; Fields, S.; Glick, S.; Cummings, V.; Eshleman, S. Infrequent HIV testing and late HIV diagnosis are common among a cohort of Black men who have sex with men (BMSM) in six US cities. J. Acquir. Immune Defic. Syndr. 2014, 67, 438. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.E.; Wilton, L.; Phillips, G.; Glick, S.N.; Kuo, I.; Brewer, R.A.; Elliott, A.; Watson, C.; Magnus, M. Understanding structural barriers to accessing HIV testing and prevention services among black men who have sex with men (BMSM) in the United States. AIDS Behav. 2014, 18, 972–996. [Google Scholar] [CrossRef] [PubMed]
- Eaton, L.A.; Driffin, D.D.; Kegler, C.; Smith, H.; Conway-Washington, C.; White, D.; Cherry, C. The role of stigma and medical mistrust in the routine health care engagement of black men who have sex with men. Am. J. Public Health 2015, 105, e75–e82. [Google Scholar] [CrossRef]
- Brown, L.L.; Martin, E.G.; Knudsen, H.K.; Gotham, H.J.; Garner, B.R. Resilience-Focused HIV Care to Promote Psychological Well-Being During COVID-19 and Other Catastrophes. Front. Public Health 2021, 9, 1130. [Google Scholar] [CrossRef]
- McGoy, S.L.; Pettit, A.C.; Morrison, M.; Alexander, L.R.; Johnson, P.; Williams, B.; Banister, D.; Young, M.K.; Wester, C.; Rebeiro, P.F. Use of social network strategy among young black men who have sex with men for HIV testing, linkage to care, and reengagement in care, Tennessee, 2013–2016. Public Health Rep. 2018, 133, 43S–51S. [Google Scholar] [CrossRef]
- Barnes, S.L.; Hollingsworth, C. Spirituality and social media: The search for support among Black men who have sex with men in Tennessee. J. Homosex. 2020, 67, 79–103. [Google Scholar] [CrossRef]
- Gebru, N.M.; Benvenuti, M.C.; Rowland, B.H.; Kalkat, M.; Chauca, P.G.; Leeman, R.F. Relationships among Substance Use, Sociodemographics, Pre-Exposure Prophylaxis (PrEP) Awareness and Related Attitudes among Young Adult Men Who Have Sex with Men. Subst. Use Misuse 2022, 57, 786–798. [Google Scholar] [CrossRef]
- Stults, C.B.; Javdani, S.; Greenbaum, C.A.; Kapadia, F.; Halkitis, P.N. Intimate partner violence and substance use risk among young men who have sex with men: The P18 cohort study. Drug Alcohol Depend. 2015, 154, 54–62. [Google Scholar] [CrossRef]
- Rivera, A.V.; Harriman, G.; Carrillo, S.A.; Braunstein, S.L. Trends in methamphetamine use among men who have sex with men in New York City, 2004–2017. AIDS Behav. 2021, 25, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Grov, C. HIV risk and substance use in men who have sex with men surveyed in bathhouses, bars/clubs, and on Craigslist. org: Venue of recruitment matters. AIDS Behav. 2012, 16, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Vosburgh, H.; Mansergh, G.; Sullivan, P.S.; Purcell, D.W. A review of the literature on event-level substance use and sexual risk behavior among men who have sex with men. AIDS Behav. 2012, 16, 1394–1410. [Google Scholar] [CrossRef] [PubMed]
- Maulsby, C.; Millett, G.; Lindsey, K.; Kelley, R.; Johnson, K.; Montoya, D.; Holtgrave, D. HIV among black men who have sex with men (MSM) in the United States: A review of the literature. AIDS Behav. 2014, 18, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Bandura, A.; Freeman, W.H.; Lightsey, R. Self-Efficacy: The Exercise of Control. J. Cogn. Psychother. 1999, 13, 158–166. [Google Scholar] [CrossRef]

| Characteristics | Exposure-Influenced HIV Testing a | ||||||
|---|---|---|---|---|---|---|---|
| Total (N = 261) | Never/Rarely (N = 192) | Always/Mostly (N = 69) | p-Value | aPR (95% CI) b | |||
| n (%) or Median (IQR) | n (%) or Median (IQR) | n (%) or Median (IQR) | |||||
| Age (years) | 0.005 | ||||||
| 18–24 | 123 (47.5) | 101 | (52.6) | 23 | (33.3) | Reference | |
| 25–35 | 137 (52.5) | 91 | (47.4) | 46 | (66.7) | 2.35 (1.12–4.97) | |
| Age of first condomless anal sex (years) | 0.049 | ||||||
| <18 | 132 (50.6) | 104 | (54.2) | 28 | (40.6) | ||
| ≥18 | 129 (49.4) | 88 | (45.8) | 41 | (59.4) | ||
| Race | 0.583 | ||||||
| Non-Hispanic white | 61 (23.4) | 48 | (25.0) | 13 | (18.8) | ||
| Non-Hispanic black | 179 (68.6) | 129 | (67.2) | 50 | (72.5) | ||
| Other † | 21 (8.0) | 15 | (7.8) | 6 | (8.7) | ||
| Employment | 0.077 | ||||||
| Currently employed | 173 (66.3) | 124 | (64.6) | 49 | (71.0) | ||
| Currently unemployed | 43 (16.5) | 29 | (15.1) | 14 | (20.3) | ||
| Currently a student | 45 (17.2) | 39 | (20.3) | 6 | (8.7) | ||
| Education | 0.141 | ||||||
| High school or lower | 65 (24.9) | 52 | (27.1) | 13 | (18.9) | ||
| Some college | 110 (42.2) | 83 | (43.2) | 27 | (39.1) | ||
| College and above | 86 (32.9) | 57 | (29.7) | 29 | (42.0) | ||
| Annual personal income (US dollars) | 0.424 | ||||||
| <USD 20,000 | 127 (48.7) | 98 | (51.0) | 29 | (42.0) | ||
| USD 20,000–40,000 | 85 (32.6) | 59 | (30.7) | 26 | (37.7) | ||
| >USD 40,000 | 49 (18.7) | 35 | (18.3) | 14 | (20.3) | ||
| Sexual orientation | 0.117 | ||||||
| Homosexual/gay | 187 (71.6) | 131 | (68.2) | 56 | (81.2) | ||
| Heterosexual | 32 (12.3) | 27 | (14.1) | 5 | (7.2) | ||
| Bisexual | 42 (16.1) | 34 | (17.7) | 8 | (11.6) | ||
| Sexual orientation disclosure to healthcare professionals | 0.006 | ||||||
| No | 75 (28.7) | 64 | (33.3) | 11 | (15.9) | Reference | |
| Yes | 186 (71.3) | 128 | (66.7) | 58 | (84.1) | 2.63 (1.29–5.36) | |
| Venues for finding sex partners | 0.045 | ||||||
| Gay-frequented venues (bars, clubs, etc.) | 47 (18.0) | 35 | (18.2) | 12 | (17.4) | ||
| Internet (Facebook, Reddit, etc.) | 43 (16.5) | 38 | (19.8) | 5 | (7.2) | ||
| Geosocial networking app (Grindr, etc.) | 171 (65.5) | 119 | (62.0) | 52 | (75.4) | ||
| Perception of HIV risk | 0.064 | ||||||
| No/low risk | 198 (75.9) | 140 | (72.9) | 58 | (84.1) | Reference | |
| Moderate/high risk | 63 (24.1) | 52 | (27.1) | 11 | (15.9) | 0.29 (0.13,0.67) | |
| Health insurance | 0.924 | ||||||
| No | 54 (20.7) | 40 | (20.8) | 14 | (20.3) | ||
| Yes | 207 (79.3) | 152 | (79.2) | 55 | (79.7) | ||
| Housing stability (1–10), median (IQR) | 9 (6–10) | 9 | (5–10) | 9 | (7–10) | 0.617 | |
| Food security (0–6), median (IQR) | 1 (0–4) | 1 | (0–4) | 0 | (0–2) | 0.098 | |
| Perceived HIV stigma (12–48), median (IQR) | 30 (24–36) | 32 | (29–36) | 29 | (24–32) | 0.031 | 0.89 (0.84–0.96) |
| Internalized homophobia (4–20), median (IQR) | 5 (4–12) | 6 | (4–12) | 4 | (4–8) | 0.038 | 0.91 (0.82–0.98) |
| Perceived social support (8–32), median (IQR) | 74 (57–90) | 73 | (57–87) | 76 | (62–95) | 0.042 | 1.02 (1.01–1.03) |
| Subjective loneliness (19–95), median (IQR) | 19 (15–23) | 19 | (16–23) | 18 | (14–23) | 0.472 | |
| General resilience (0–40), median (IQR) | 29 (23–36) | 28 | (22–35) | 30 | (26–36) | 0.061 | 1.03 (1.01–1.07) |
| Characteristics | Exposure-Influenced HIV Testing a | |||||
|---|---|---|---|---|---|---|
| Total (N = 261) | Never/Rarely (N = 192) | Always/Mostly (N = 69) | p-Value | |||
| n (%) or Median (IQR) | n (%) or Median (IQR) | n (%) or Median (IQR) | ||||
| Ever used any tobacco product ‡. | 0.534 | |||||
| No | 61 (23.4) | 43 | (22.4) | 18 | (26.1) | |
| Yes | 200 (76.6) | 149 | (77.6) | 51 | (73.9) | |
| Ever used any recreational drug † | 0.947 | |||||
| No | 56 (21.5) | 41 | (21.3) | 15 | (21.7) | |
| Yes | 205 (78.5) | 151 | (78.7) | 54 | (78.3) | |
| Recreational drug use before sex in the past 6 months | 0.048 | |||||
| No | 162 (62.1) | 114 | (59.4) | 48 | (69.6) | |
| Yes | 99 (37.9) | 78 | (40.6) | 21 | (30.4) | |
| Ever drank alcohol | 0.478 | |||||
| No | 41 (15.7) | 32 | (16.7) | 9 | (13.0) | |
| Yes | 220 (84.3) | 160 | (83.3) | 60 | (87.0) | |
| Hazardous alcohol drinking in the past 6 months | 0.439 | |||||
| No (AUDIT-C < 4) | 154 (59.0) | 116 | (60.4) | 38 | (55.1) | |
| Yes (AUDIT-C ≥ 4) | 107 (41.0) | 76 | (39.6) | 31 | (44.9) | |
| Binge drinking in the past 6 months ※ | 0.002 | |||||
| No | 73 (28.0) | 41 | (21.4) | 32 | (46.4) | |
| Yes | 188 (72.0) | 151 | (78.6) | 37 | (53.6) | |
| Alcohol use before sex | 0.259 | |||||
| No | 121 (46.4) | 85 | (44.3) | 36 | (52.2) | |
| Yes | 140 (53.6) | 107 | (55.7) | 33 | (47.8) | |
| Lifetime male sex partner | 0.863 | |||||
| <10 | 137 (53.5) | 100 | (53.2) | 37 | (54.4) | |
| ≥10 | 119 (46.5) | 88 | (46.8) | 31 | (45.6) | |
| Had group sex in the past 6 months | 0.036 | |||||
| No | 194 (76.4) | 135 | (72.9) | 59 | (85.5) | |
| Yes | 60 (23.6) | 50 | (27.1) | 10 | (14.5) | |
| Had condomless insertive anal sex with men in the past 6 months | 0.553 | |||||
| No | 114 (45.1) | 85 | (46.2) | 29 | (42.0) | |
| Yes | 139 (54.9) | 99 | (53.8) | 40 | (58.0) | |
| Had condomless receptive anal sex with men in the past 6 months | 0.404 | |||||
| No | 125 (49.2) | 94 | (50.8) | 31 | (44.9) | |
| Yes | 129 (50.8) | 91 | (49.2) | 38 | (55.1) | |
| Had anal and/or oral sex with known HIV-positive partners in the past 6 months | 0.321 | |||||
| No | 200 (81.3) | 142 | (79.8) | 58 | (85.3) | |
| Yes | 46 (18.7) | 36 | (20.2) | 10 | (14.7) | |
| Ever had sexually transmitted disease testing | 0.003 | |||||
| No | 40 (15.3) | 37 | (19.3) | 3 | (4.4) | |
| Yes | 221 (84.7) | 155 | (80.7) | 66 | (95.6) | |
| History of sexually transmitted infections § | 0.049 | |||||
| No | 179 (68.6) | 137 | (71.3) | 42 | (60.9) | |
| Yes | 82 (31.4) | 55 | (28.7) | 27 | (39.1) | |
| HIV pre-exposure awareness | 0.006 | |||||
| No | 66 (25.3) | 57 | (29.7) | 9 | (13.0) | |
| Yes | 195 (74.7) | 135 | (70.3) | 60 | (87.0) | |
| Lifetime number of HIV testing, median (IQR) | 4 (2–8) | 3 | (1–6) | 10 | (5–17) | <0.001 |
| Condom use self-efficacy (5–35), median (IQR) | 30 (26–34) | 29 | (25–34) | 33 | (28–35) | 0.013 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Hawkins, M.; Osman, A.; Zhang, C. Assessing the Prevalence and Determinants of Exposure-Influenced HIV Testing among a Sample of Pre- and Post-Exposure Prophylaxis-Naïve Young Men Who Have Sex with Men in the United States. Trop. Med. Infect. Dis. 2022, 7, 146. https://doi.org/10.3390/tropicalmed7080146
Liu Y, Hawkins M, Osman A, Zhang C. Assessing the Prevalence and Determinants of Exposure-Influenced HIV Testing among a Sample of Pre- and Post-Exposure Prophylaxis-Naïve Young Men Who Have Sex with Men in the United States. Tropical Medicine and Infectious Disease. 2022; 7(8):146. https://doi.org/10.3390/tropicalmed7080146
Chicago/Turabian StyleLiu, Yu, Mary Hawkins, Amna Osman, and Chen Zhang. 2022. "Assessing the Prevalence and Determinants of Exposure-Influenced HIV Testing among a Sample of Pre- and Post-Exposure Prophylaxis-Naïve Young Men Who Have Sex with Men in the United States" Tropical Medicine and Infectious Disease 7, no. 8: 146. https://doi.org/10.3390/tropicalmed7080146
APA StyleLiu, Y., Hawkins, M., Osman, A., & Zhang, C. (2022). Assessing the Prevalence and Determinants of Exposure-Influenced HIV Testing among a Sample of Pre- and Post-Exposure Prophylaxis-Naïve Young Men Who Have Sex with Men in the United States. Tropical Medicine and Infectious Disease, 7(8), 146. https://doi.org/10.3390/tropicalmed7080146








