Gaps and Interventions across the Diagnostic Care Cascade of TB Patients at the Level of Patient, Community and Health System: A Qualitative Review of the Literature
Abstract
:1. Introduction
2. Methodology
2.1. Criteria for Considering Studies for This Review
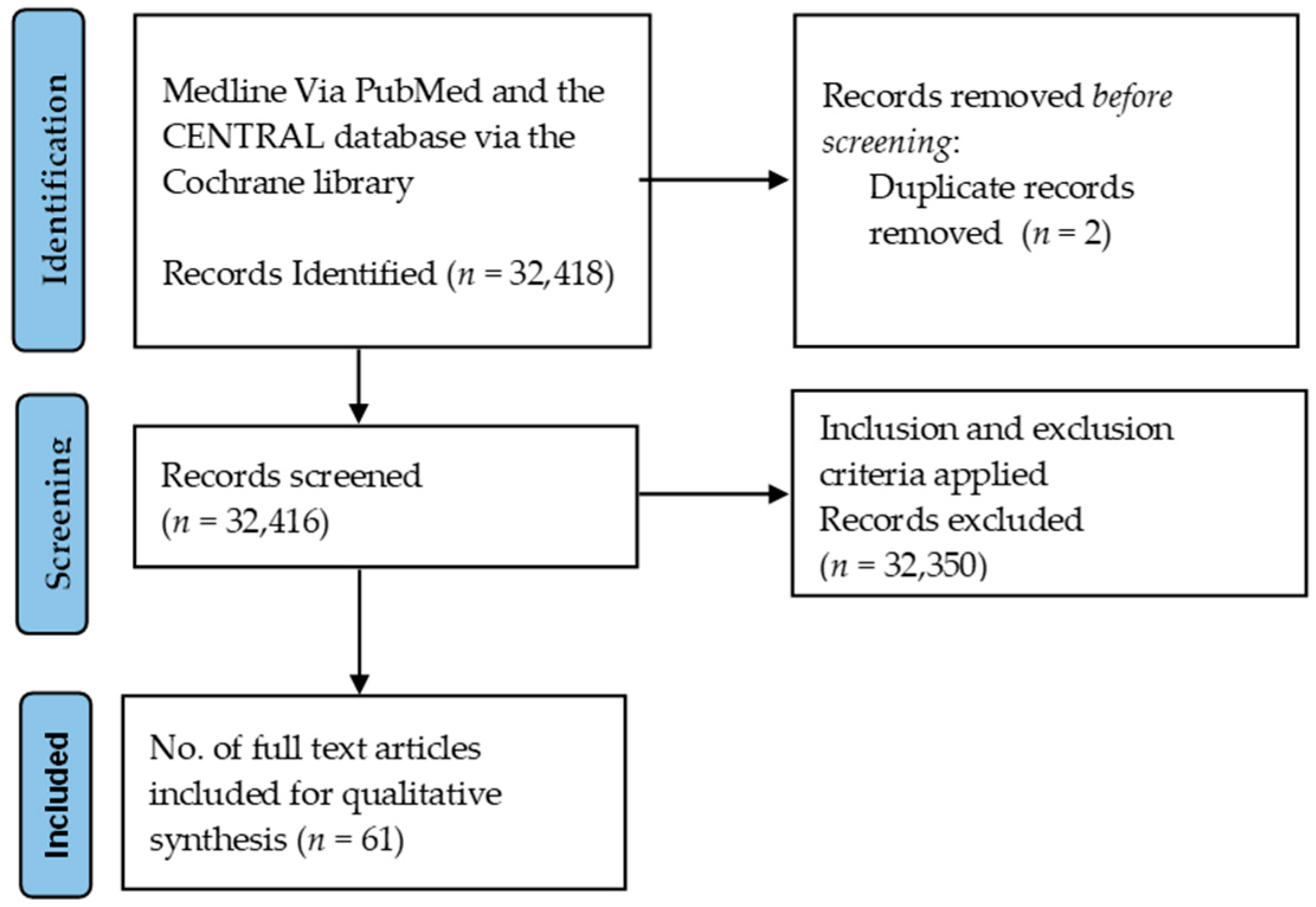
2.2. Search Methods for Identification of Studies
2.3. Selection of Studies and Data Extraction
3. Findings
3.1. General Characteristics of Reviewed Studies (Search Results)
3.2. Diagnostic Gaps and Possible Solutions
- People may not have access to TB diagnostic tests.
- Individuals with a higher risk of TB missed the complete diagnosis algorithm.
- Services are available, but people may not seek care from a diagnostic facility.
- Patients may not get diagnosed with TB, despite reaching health facilities.
3.2.1. Gap: People May Not Have Access to TB Diagnostic Tests
3.2.2. Gap: Individuals with a Higher Risk of TB Missed the Complete Diagnosis Algorithm
3.2.3. Services Are Available, but People May Not Seek Care from a Diagnostic Facility
3.2.4. Gap: Patients Do Not Get Diagnosed Regardless of Reaching Health Facilities
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- WHO. Global Tuberculosis Report 2021. 2021. Available online: https://www.who.int/publications/digital/global-tuberculosis-report-2021 (accessed on 12 May 2022).
- Central TB Division, Ministry of Health and Family Welfare, Government of India. National Strategic Plan 2017–2025. 2017. Available online: https://tbcindia.gov.in/WriteReadData/NSP%20Draft%2020.02.2017%201.pdf (accessed on 12 May 2022).
- Akachi, Y.; Zumla, A.; Atun, R. Investing in improved performance of national tuberculosis programs reduces the tuberculosis burden: Analysis of 22 high-burden countries, 2002–2009. J. Infect. Dis. 2012, 205 (Suppl. S2), S284–S292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.S.; Schwanke Khilji, S.U.; Saw, S.; Coker, R.J. Evidence to inform resource allocation for tuberculosis control in Myanmar: A systematic review based on the SYSRA framework. Health Policy Plan. 2017, 32, 102–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Keshavjee, S.; Atun, R. Health systems performance in managing tuberculosis: Analysis of tuberculosis care cascades among high-burden and non-high-burden countries. J. Glob. Health 2019, 9, 010423. [Google Scholar] [CrossRef] [PubMed]
- Atun, R.; Weil, D.E.; Eang, M.T.; Mwakyusa, D. Health-system strengthening and tuberculosis control. Lancet 2010, 375, 2169–2178. [Google Scholar] [CrossRef]
- Storla, D.G.; Yimer, S.; Bjune, G.A. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health 2008, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Yuen, C.M.; Amanullah, F.; Dharmadhikari, A.; Nardell, E.A.; Seddon, J.A.; Vasilyeva, I.; Zhao, Y.; Keshavjee, S.; Becerra, M.C. Turning off the tap: Stopping tuberculosis transmission through active case-finding and prompt effective treatment. Lancet 2015, 386, 2334–2343. [Google Scholar] [CrossRef]
- Stallworthy, G.; Dias, H.M.; Pai, M. Quality of tuberculosis care in the private health sector. J. Clin. Tuberc. Other Mycobact. Dis. 2020, 20, 100171. [Google Scholar] [CrossRef]
- Bhatnagar, H. User-experience and patient satisfaction with quality of tuberculosis care in India: A mixed-methods literature review. J. Clin. Tuberc. Other Mycobact. Dis. 2019, 17, 100127. [Google Scholar] [CrossRef]
- Alene, M.; Assemie, M.A.; Yismaw, L.; Gedif, G.; Ketema, D.B.; Gietaneh, W.; Chekol, T.D. Patient delay in the diagnosis of tuberculosis in Ethiopia: A systematic review and meta-analysis. BMC Infect. Dis. 2020, 20, 797. [Google Scholar] [CrossRef]
- The End TB Strategy, Global Strategy and Targets for Tuberculosis Prevention, Care and Control after 2015. Available online: https://www.who.int/tb/strategy/End_TB_Strategy.pdf (accessed on 12 May 2022).
- Uplekar, M.; Weil, D.; Lonnroth, K.; Jaramillo, E.; Lienhardt, C.; Dias, H.M.; Falzon, D.; Floyd, K.; Gargioni, G.; Getahun, H.; et al. WHO’s new end TB strategy. Lancet 2015, 385, 1799–1801. [Google Scholar] [CrossRef]
- Houben, R.M.G.J.; Menzies, N.A.; Sumner, T.; Huynh, G.H.; Arinaminpathy, N.; Goldhaber-Fiebert, J.D.; Lin, H.-H.; Wu, C.-Y.; Mandal, S.; Pandey, S.; et al. Feasibility of achieving the 2025 WHO global tuberculosis targets in South Africa, China, and India: A combined analysis of 11 mathematical models. Lancet Glob. Health 2016, 4, e806–e815. [Google Scholar] [CrossRef] [Green Version]
- Reid, M.J.A.; Arinaminpathy, N.; Bloom, A.; Bloom, B.R.; Boehme, C.; Chaisson, R.; Chin, D.P.; Churchyard, G.; Cox, H.; Ditiu, L.; et al. Building a tuberculosis-free world: The Lancet Commission on tuberculosis. Lancet 2019, 393, 1331–1384. [Google Scholar] [CrossRef] [Green Version]
- Agins, B.D.; Ikeda, D.J.; Reid, M.J.; Goosby, E.; Pai, M.; Cattamanchi, A. Improving the cascade of global tuberculosis care: Moving from the “what” to the “how” of quality improvement. Lancet Infect. Dis. 2019, 19, e437–e443. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subbaraman, R.; Nathavitharana, R.R.; Satyanarayana, S.; Pai, M.; Thomas, B.E.; Chadha, V.K.; Rade, K.; Swaminathan, S.; Mayer, K.H. The Tuberculosis Cascade of Care in India’s Public Sector: A Systematic Review and Meta-analysis. PLoS Med. 2016, 13, e1002149. [Google Scholar] [CrossRef] [Green Version]
- Mistry, N.; Lobo, E.; Shah, S.; Rangan, S.; Dholakia, Y. Pulmonary tuberculosis in Patna, India: Durations, delays, and health care seeking behaviour among patients identified through household surveys. JEGH 2017, 7, 241. [Google Scholar] [CrossRef]
- Mistry, N.; Rangan, S.; Dholakia, Y.; Lobo, E.; Shah, S.; Patil, A. Durations and Delays in Care Seeking, Diagnosis and Treatment Initiation in Uncomplicated Pulmonary Tuberculosis Patients in Mumbai, India. PLoS ONE 2016, 11, e0152287. [Google Scholar]
- Sreeramareddy, C.T.; Qin, Z.Z.; Satyanarayana, S.; Subbaraman, R.; Pai, M. Delays in diagnosis and treatment of pulmonary tuberculosis in India: A systematic review. Int. J. Tuberc. Lung Dis. 2014, 18, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Kranzer, K.; Afnan-Holmes, H.; Tomlin, K.; Golub, J.E.; Shapiro, A.E.; Schaap, A.; Corbett, E.L.; Lönnroth, K.; Glynn, J.R. The benefits to communities and individuals of screening for active tuberculosis disease: A systematic review. Int. J. Tuberc. Lung Dis. 2013, 17, 432–446. [Google Scholar] [CrossRef] [Green Version]
- Mundra, A.; Kothekar, P.; Deshmukh, P.R.; Dongre, A. Why tuberculosis patients under revised national tuberculosis control programme delay in healthcare seeking? A mixed-methods research from Wardha District, Maharashtra. Indian J. Public Health 2019, 63, 94–100. [Google Scholar]
- Gianella, C.; Ugarte-Gil, C.; Caro, G.; Aylas, R.; Castro, C.; Lema, C. TB in Vulnerable Populations: The Case of an Indigenous Community in the Peruvian Amazon. Health Hum. Rights 2016, 18, 55–68. [Google Scholar] [PubMed]
- Malacarne, J.; Gava, C.; Escobar, A.L.; Souza-Santos, R.; Basta, P.C. Health service access for tuberculosis diagnosis and treatment among indigenous peoples in Rondônia state, Brazilian Amazon, 2009–2011: A cross-sectional study. Epidemiol. E Serviços De Saúde 2019, 28, e2018231. [Google Scholar]
- Patel, S.; Paulsen, C.; Heffernan, C.; Saunders, D.; Sharma, M.; King, M.; Hoeppner, V.; Orr, P.; Kunimoto, D.; Menzies, D.; et al. Tuberculosis transmission in the Indigenous peoples of the Canadian prairies. PLoS ONE 2017, 12, e0188189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muniyandi, M.; Rao, V.G.; Bhat, J.; Yadav, R. Performance of Revised National Tuberculosis Control Programme (RNTCP) in tribal areas in India. Indian J. Med. Res. 2015, 141, 624–629. [Google Scholar] [PubMed]
- Vesga, J.F.; Hallett, T.B.; Reid, M.J.A.; Sachdeva, K.S.; Rao, R.; Khaparde, S.; Dave, P.; Rade, K.; Kamene, M.; Omesa, E.; et al. Assessing tuberculosis control priorities in high-burden settings: A modeling approach. Lancet Glob. Health 2019, 7, e585–e595. [Google Scholar] [CrossRef] [Green Version]
- Getnet, F.; Demissie, M.; Worku, A.; Gobena, T.; Seyoum, B.; Tschopp, R.; Anderson, C. Determinants of Patient Delay in Diagnosis of Pulmonary Tuberculosis in Somali Pastoralist Setting of Ethiopia: A Matched Case-Control Study. Int. J. Environ. Res. Public Health 2019, 16, 3391. [Google Scholar] [CrossRef] [Green Version]
- Teo, A.K.J.; Ork, C.; Eng, S.; Sok, N.; Tuot, S.; Hsu, L.Y.; Yi, S. Determinants of delayed diagnosis and treatment of tuberculosis in Cambodia: A mixed-methods study. Infect. Dis. Poverty 2020, 9, 49. [Google Scholar] [CrossRef]
- Fekadu, L.; Hanson, C.; Osberg, M.; Makayova, J.; Mingkwan, P.; Chin, D. Increasing Access to Tuberculosis Services in Ethiopia: Findings from a Patient-Pathway Analysis. J. Infect. Dis. 2017, 216 (Suppl. S7), S696–S701. [Google Scholar] [CrossRef] [Green Version]
- Mhimbira, F.A.; Cuevas, L.E.; Dacombe, R.; Mkopi, A.; Sinclair, D. Interventions to increase tuberculosis case detection at primary healthcare or community-level services. Cochrane Database Syst. Rev. 2017, 11, CD011432. [Google Scholar] [CrossRef] [Green Version]
- Sanaie, A.; Mergenthaler, C.; Nasrat, A.; Seddiq, M.K.; Mahmoodi, S.D.; Stevens, R.H.; Creswell, J. An Evaluation of Passive and Active Approaches to Improve Tuberculosis Notifications in Afghanistan. PLoS ONE 2016, 11, e0163813. [Google Scholar]
- Colvin, C.; Mugyabuso, J.; Munuo, G.; Lyimo, J.; Oren, E.; Mkomwa, Z.; Makame, M.; Mwangomale, A.; Mahamba, V.; Mueller, L.; et al. Evaluation of community-based interventions to improve TB case detection in a rural district of Tanzania. Glob. Health Sci. Pract. 2014, 2, 219–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Getnet, F.; Demissie, M.; Assefa, N.; Mengistie, B.; Worku, A. Delay in diagnosis of pulmonary tuberculosis in low-and middle-income settings: Systematic review and meta-analysis. BMC Pulm. Med. 2017, 17, 202. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Basu, S.; Chopra, K.K. Achieving TB elimination in India: The role of latent TB management. Indian J. Tuberc. 2019, 66, 30–33. [Google Scholar] [CrossRef]
- Yassin, M.A.; Datiko, D.G.; Tulloch, O.; Markos, P.; Aschalew, M.; Shargie, E.B.; Dangisso, M.H.; Komatsu, R.; Sahu, S.; Blok, L.; et al. Innovative community-based approaches doubled tuberculosis case notification and improve treatment outcome in Southern Ethiopia. PLoS ONE 2013, 8, e63174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subbaraman, R.; Jhaveri, T.; Nathavitharana, R.R. Closing gaps in the tuberculosis care cascade: An action-oriented research agenda. J. Clin. Tuberc. Other Mycobact. Dis. 2020, 19, 100144. [Google Scholar] [CrossRef]
- Rajeswari, R.; Chandrasekaran, V.; Suhadev, M.; Sivasubramaniam, S.; Sudha, G.; Renu, G. Factors associated with patient and health system delays in the diagnosis of tuberculosis in South India. Int. J. Tuberc. Lung Dis. 2002, 6, 789–795. [Google Scholar]
- Hanson, C.; Osberg, M.; Brown, J.; Durham, G.; Chin, D.P. Finding the Missing Patients with Tuberculosis: Lessons Learned from Patient-Pathway Analyses in 5 Countries. J. Infect. Dis. 2017, 216 (Suppl. S7), S686–S695. [Google Scholar] [CrossRef] [Green Version]
- Garg, T.; Bhardwaj, M.; Deo, S. Role of community health workers in improving cost efficiency in an active case finding tuberculosis programme: An operational research study from rural Bihar, India. BMJ Open 2020, 10, e036625. [Google Scholar] [CrossRef]
- José, B.; Manhiça, I.; Jones, J.; Mutaquiha, C.; Zindoga, P.; Eduardo, I.; Creswell, J.; Qin, Z.Z.; Ramis, O.; Ramiro, I.; et al. Using community health workers for facility and community based TB case finding: An evaluation in central Mozambique. PLoS ONE 2020, 15, e0236262. [Google Scholar] [CrossRef]
- Azman, A.S.; Golub, J.E.; Dowdy, D.W. How much is tuberculosis screening worth? Estimating the value of active case finding for tuberculosis in South Africa, China, and India. BMC Med. 2014, 12, 216. [Google Scholar] [CrossRef]
- Dowdy, D.W.; Lotia, I.; Azman, A.S.; Creswell, J.; Sahu, S.; Khan, A.J. Population-level impact of active tuberculosis case finding in an Asian megacity. PLoS ONE 2013, 8, e77517. [Google Scholar] [CrossRef] [PubMed]
- Daftary, A.; Satyanarayana, S.; Jha, N.; Singh, M.; Mondal, S.; Vadnais, C.; Pai, M. Can community pharmacists improve tuberculosis case finding? A mixed methods intervention study in India. BMJ Glob. Health 2019, 4, e001417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marx, F.M.; Yaesoubi, R.; Menzies, N.A.; Salomon, J.A.; Bilinski, A.; Beyers, N.; Cohen, T. Tuberculosis control interventions targeted to previously treated people in a high-incidence setting: A modelling study. Lancet Glob. Health 2018, 6, e426–e435. [Google Scholar] [CrossRef] [Green Version]
- Walzl, G.; McNerney, R.; du Plessis, N.; Bates, M.; McHugh, T.D.; Chegou, N.N.; Zumla, A. Tuberculosis: Advances and challenges in development of new diagnostics and biomarkers. Lancet Infect. Dis. 2018, 18, e199–e210. [Google Scholar] [CrossRef]
- Kohli, M.; Schiller, I.; Dendukuri, N.; Dheda, K.; Denkinger, C.M.; Schumacher, S.G.; Steingart, K.R. Xpert® MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance. Cochrane Database Syst. Rev. 2018, 8, CD012768. [Google Scholar] [CrossRef] [Green Version]
- WHO. WHO World Health Assembly: Post-2015 Global TB Strategy and Targets (A67/62); WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Fox, G.J.; Nhung, N.V.; Sy, D.N.; Hoa, N.L.; Anh, L.T.; Anh, N.T.; Hoa, N.B.; Dung, N.H.; Buu, T.N.; Loi, N.T.; et al. Household-Contact Investigation for Detection of Tuberculosis in Vietnam. N. Engl. J. Med. 2018, 378, 221–229. [Google Scholar] [CrossRef]
- Fox, G.J.; Dobler, C.C.; Marks, G.B. Active case finding in contacts of people with tuberculosis. Cochrane Database Syst. Rev. 2011, 2011, CD008477. [Google Scholar] [CrossRef]
- Cudahy, P.G.T.; Andrews, J.R.; Bilinski, A.; Dowdy, D.W.; Mathema, B.; Menzies, N.A.; Salomon, J.A.; Shrestha, S.; Cohen, T. Spatially targeted screening to reduce tuberculosis transmission in high-incidence settings. Lancet Infect. Dis. 2019, 19, e89–e95. [Google Scholar] [CrossRef]
- Thomas, B.E.; Suresh, C.; Lavanya, J.; Lindsley, M.M.; Galivanche, A.T.; Sellappan, S.; Ovung, S.; Aravind, A.; Lincy, S.; Raja, A.L.; et al. Understanding pretreatment loss to follow-up of tuberculosis patients: An explanatory qualitative study in Chennai, India. BMJ Glob. Health 2020, 5, e001974. [Google Scholar] [CrossRef] [Green Version]
- Fuge, T.G.; Bawore, S.G.; Solomon, D.W.; Hegana, T.Y. Patient delay in seeking tuberculosis diagnosis and associated factors in Hadiya Zone, Southern Ethiopia. BMC Res. Notes 2018, 11, 115. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.J.; Khowaja, S.; Khan, F.S.; Qazi, F.; Lotia, I.; Habib, A.; Mohammed, S.; Khan, U.; Amanullah, F.; Hussain, H.; et al. Engaging the private sector to increase tuberculosis case detection: An impact evaluation study. Lancet Infect. Dis. 2012, 12, 608–616. [Google Scholar] [CrossRef]
- Oga-Omenka, C.; Boffa, J.; Kuye, J.; Dakum, P.; Menzies, D.; Zarowsky, C. Understanding the gaps in DR-TB care cascade in Nigeria: A sequential mixed-method study. J. Clin. Tuberc. Other Mycobact. Dis. 2020, 21, 100193. [Google Scholar] [CrossRef] [PubMed]
- Corbett, E.L.; Bandason, T.; Duong, T.; Dauya, E.; Makamure, B.; Churchyard, G.J.; Williams, B.G.; Munyati, S.S.; Butterworth, A.E.; Mason, P.R.; et al. Comparison of two active case-finding strategies for community-based diagnosis of symptomatic smear-positive tuberculosis and control of infectious tuberculosis in Harare, Zimbabwe (DETECTB): A cluster-randomised trial. Lancet 2010, 376, 1244–1253. [Google Scholar] [CrossRef] [Green Version]
- Naidoo, P.; Theron, G.; Rangaka, M.X.; Chihota, V.N.; Vaughan, L.O.; Brey, Z.; Pillay, Y. The South African Tuberculosis Care Cascade: Estimated Losses and Methodological Challenges. J. Infect. Dis. 2017, 216 (Suppl. S7), S702–S713. [Google Scholar] [CrossRef]
- Arinaminpathy, N.; Deo, S.; Singh, S.; Khaparde, S.; Rao, R.; Vadera, B.; Kulshrestha, N.; Gupta, D.; Rade, K.; Nair, S.A.; et al. Modelling the impact of effective private provider engagement on tuberculosis control in urban India. Sci. Rep. 2019, 9, 3810. [Google Scholar] [CrossRef]
- Davis, J.L.; Cattamanchi, A.; Cuevas, L.E.; Hopewell, P.C.; Steingart, K.R. Diagnostic accuracy of same-day microscopy versus standard microscopy for pulmonary tuberculosis: A systematic review and meta-analysis. Lancet Infect. Dis. 2013, 13, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Steingart, K.R.; Schiller, I.; Horne, D.J.; Pai, M.; Boehme, C.C.; Dendukuri, N. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst. Rev. 2014, CD009593. Available online: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD009593.pub3/full (accessed on 11 May 2022). [CrossRef]
- Huddart, S.; MacLean, E.; Pai, M. Location, location, location: Tuberculosis services in highest burden countries. Lancet Glob. Health 2016, 4, e907–e908. [Google Scholar] [CrossRef] [Green Version]
- Qin, Z.Z.; Pai, M.; Van Gemert, W.; Sahu, S.; Ghiasi, M.; Creswell, J. How is Xpert MTB/RIF being implemented in 22 high tuberculosis burden countries? Eur. Respir. J. 2015, 45, 549–554. [Google Scholar] [CrossRef] [Green Version]
- Chakravorty, S.; Simmons, A.M.; Rowneki, M.; Parmar, H.; Cao, Y.; Ryan, J.; Banada, P.P.; Deshpande, S.; Shenai, S.; Gall, A.; et al. The New Xpert MTB/RIF Ultra: Improving Detection of Mycobacterium tuberculosis and Resistance to Rifampin in an Assay Suitable for Point-of-Care Testing. MBio 2017, 8, e00812-17. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Fluorescent Light-Emitting Diode (LED) Microscopy for Diagnosis of Tuberculosis: Policy Statement. 2011. Available online: http://whqlibdoc.who.int/publications/2011/9789241501613_eng.pdf (accessed on 4 January 2021).
- Boehme, C.C.; Nicol, M.P.; Nabeta, P.; Michael, J.S.; Gotuzzo, E.; Tahirli, R.; Gler, M.T.; Blakemore, R.; Worodria, W.; Gray, C.; et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: A multicentre implementation study. Lancet 2011, 377, 1495–1505. [Google Scholar] [CrossRef] [Green Version]
- Kik, S.V.; Denkinger, C.M.; Casenghi, M.; Vadnais, C.; Pai, M. Tuberculosis diagnostics: Which target product profiles should be prioritised? Eur. Respir. J. 2014, 44, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.; Schito, M. Tuberculosis diagnostics in 2015: Landscape, priorities, needs, and prospects. J. Infect. Dis. 2015, 211 (Suppl. 2), S21–S28. [Google Scholar] [CrossRef] [PubMed]
- Subbaraman, R.; Nathavitharana, R.R.; Mayer, K.H.; Satyanarayana, S.; Chadha, V.K.; Arinaminpathy, N.; Pai, M. Constructing care cascades for active tuberculosis: A strategy for program monitoring and identifying gaps in quality of care. PLoS Med. 2019, 16, e1002754. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Kwan, A.; Daniels, B.; Satyanarayana, S.; Subbaraman, R.; Bergkvist, S.; Das, R.K.; Das, V.; Pai, M. Use of standardised patients to assess quality of tuberculosis care: A pilot, cross-sectional study. Lancet Infect. Dis. 2015, 15, 1305–1313. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, S.K.; Raman, A.V.; Sachdeva, K.S.; Satyanarayana, S. How did the TB patients reach DOTS services in Delhi? A study of patient treatment seeking behavior. PLoS ONE 2012, 7, e42458. [Google Scholar]
- Bronner Murrison, L.; Ananthakrishnan, R.; Sukumar, S.; Augustine, S.; Krishnan, N.; Pai, M.; Dowdy, D.W. How Do Urban Indian Private Practitioners Diagnose and Treat Tuberculosis? A Cross-Sectional Study in Chennai. PLoS ONE 2016, 11, e0149862. [Google Scholar] [CrossRef] [Green Version]
- Ananthakrishnan, R.; Richardson, M.A.; van den Hof, S.; Rangaswamy, R.; Thiagesan, R.; Auguesteen, S.; Kamp, N. Successfully Engaging Private Providers to Improve Diagnosis, Notification, and Treatment of TB and Drug-Resistant TB: The EQUIP Public-Private Model in Chennai, India. Glob. Health Sci. Pract. 2019, 7, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, R.R.; Oeltmann, J.E.; Ravichandra, C.; Chadda, V.K.; Das, M.; Kumar, A.M.V. Engaging private providers and Ayurvedic practitioners in Bilaspur, India: Did it increase TB case detection? Public Health Action 2016, 6, 154–156. [Google Scholar] [CrossRef] [Green Version]
- Dewan, P.K.; Lal, S.S.; Lonnroth, K.; Wares, F.; Uplekar, M.; Sahu, S.; Granich, R.; Chauhan, L.S. Improving tuberculosis control through public-private collaboration in India: Literature review. BMJ 2006, 332, 574–578. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, N.; Ananthakrishnan, R.; Augustine, S.; Vijayalakshmi, N.K.; Gopi, P.G.; Kumaraswami, V.; Narayanan, P.R. Impact of advocacy on the tuberculosis management practices of private practitioners in Chennai City, India. Int. J. Tuberc. Lung Dis. 2009, 13, 112–118. [Google Scholar] [PubMed]
- Atre, S. A tuberculosis-free world: Is it a delusion? Lancet 2019, 394, 913. [Google Scholar] [CrossRef] [Green Version]
- Albert, H.; Purcell, R.; Wang, Y.Y.; Kao, K.; Mareka, M.; Katz, Z.; Maama, B.L.; Mots’oane, T. Designing an soptimised diagnostic network to improve access to TB diagnosis and treatment in Lesotho. PLoS ONE 2020, 15, e0233620. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Chadha, V.K.; Laxminarayan, R.; Arinaminpathy, N. Counting the lives saved by DOTS in India: A model-based approach. BMC Med. 2017, 15, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NTEP. National Tuberculosis Elimination Program Annual Status Report. India. Available online: https://tbcindia.gov.in/index1.php?lang=1&level=1&sublinkid=4160&lid=2807 (accessed on 12 May 2022).
- Arinaminpathy, N.; Batra, D.; Khaparde, S.; Vualnam, T.; Maheshwari, N.; Sharma, L.; Nair, S.A.; Dewan, P. The number of privately treated tuberculosis cases in India: An estimation from drug sales data. Lancet Infect. Dis. 2016, 16, 1255–1260. [Google Scholar] [CrossRef] [Green Version]
- Satyanarayana, S.; Nair, S.A.; Chadha, S.S.; Shivashankar, R.; Sharma, G.; Yadav, S.; Mohanty, S.; Kamineni, V.; Wilson, N.C.; Harries, A.D.; et al. From where are tuberculosis patients accessing treatment in India? Results from a cross-sectional community based survey of 30 districts. PLoS ONE 2011, 6, e24160. [Google Scholar] [CrossRef] [Green Version]
| Sr. No. | Diagnostic Gaps | Reasons for Identified Gaps | Suggested Interventions |
|---|---|---|---|
| 1 | People may not have access to TB diagnostic tests |
|
|
| 2 | Services are available, but people may not seek care with a diagnostic facility |
|
|
| 3. | Patients do not get a complete diagnosis of TB, despite reaching health facilities |
|
|
| 4. | Individuals with a higher risk of missed diagnosis |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, H.D.; Nazli Khatib, M.; Syed, Z.Q.; Gaidhane, A.M.; Yasobant, S.; Narkhede, K.; Bhavsar, P.; Patel, J.; Sinha, A.; Puwar, T.; et al. Gaps and Interventions across the Diagnostic Care Cascade of TB Patients at the Level of Patient, Community and Health System: A Qualitative Review of the Literature. Trop. Med. Infect. Dis. 2022, 7, 136. https://doi.org/10.3390/tropicalmed7070136
Shah HD, Nazli Khatib M, Syed ZQ, Gaidhane AM, Yasobant S, Narkhede K, Bhavsar P, Patel J, Sinha A, Puwar T, et al. Gaps and Interventions across the Diagnostic Care Cascade of TB Patients at the Level of Patient, Community and Health System: A Qualitative Review of the Literature. Tropical Medicine and Infectious Disease. 2022; 7(7):136. https://doi.org/10.3390/tropicalmed7070136
Chicago/Turabian StyleShah, Harsh D, Mahalaqua Nazli Khatib, Zahiruddin Quazi Syed, Abhay M. Gaidhane, Sandul Yasobant, Kiran Narkhede, Priya Bhavsar, Jay Patel, Anish Sinha, Tapasvi Puwar, and et al. 2022. "Gaps and Interventions across the Diagnostic Care Cascade of TB Patients at the Level of Patient, Community and Health System: A Qualitative Review of the Literature" Tropical Medicine and Infectious Disease 7, no. 7: 136. https://doi.org/10.3390/tropicalmed7070136
APA StyleShah, H. D., Nazli Khatib, M., Syed, Z. Q., Gaidhane, A. M., Yasobant, S., Narkhede, K., Bhavsar, P., Patel, J., Sinha, A., Puwar, T., Saha, S., & Saxena, D. (2022). Gaps and Interventions across the Diagnostic Care Cascade of TB Patients at the Level of Patient, Community and Health System: A Qualitative Review of the Literature. Tropical Medicine and Infectious Disease, 7(7), 136. https://doi.org/10.3390/tropicalmed7070136







