Epidemiological Interface of Sylvatic and Dog Rabies in the North West Province of South Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Cohort Used in the Study
2.2. Total RNA Extraction
2.3. Reverse Transcription and Amplification of the Glycoprotein Gene and the Adjacent G-L Intergenic Region
2.4. Reverse Transcription and Amplification of the Partial Nucleoprotein Gene Region
2.5. Sanger Sequencing
2.6. Phylogenetic Analyses
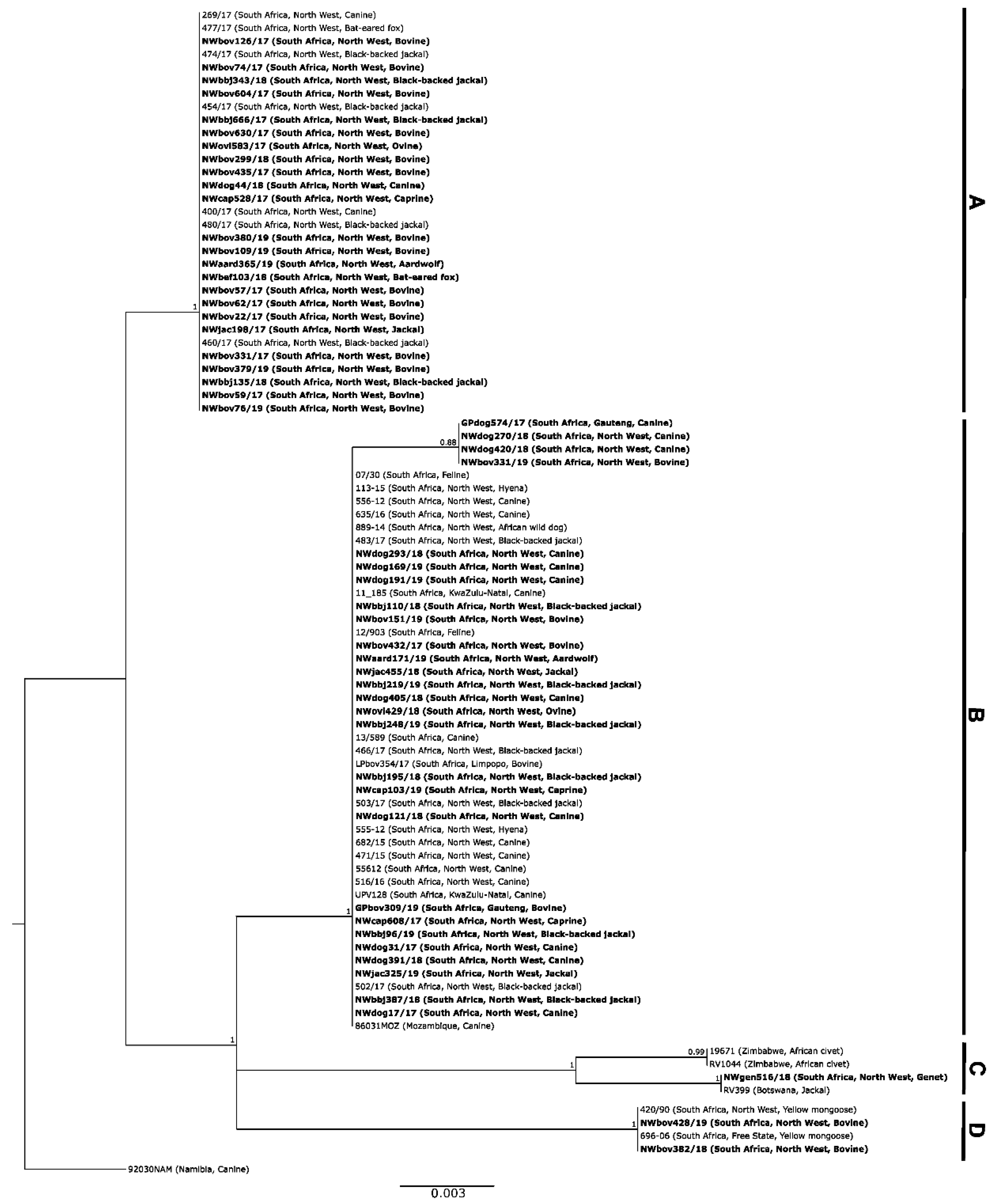
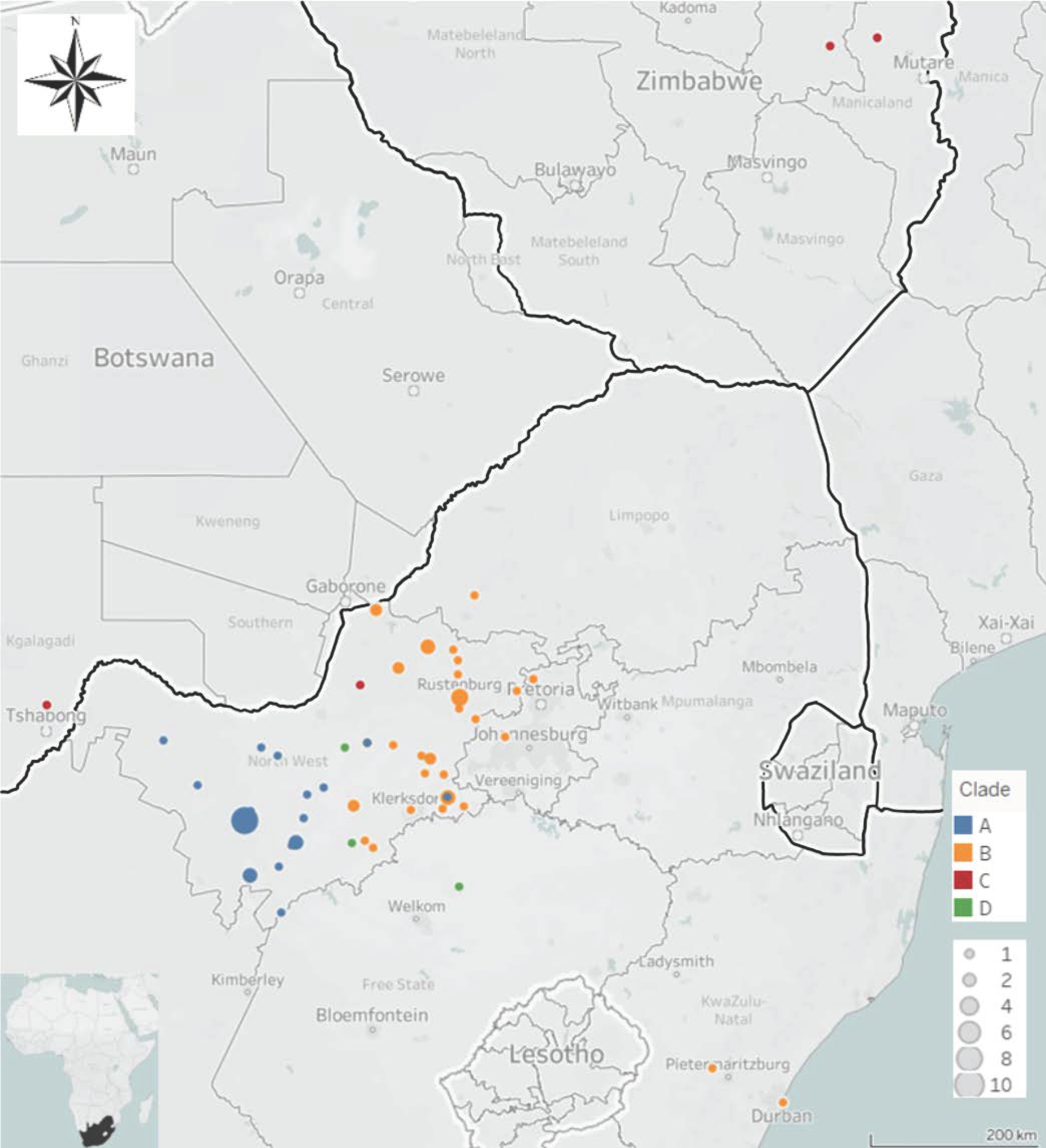
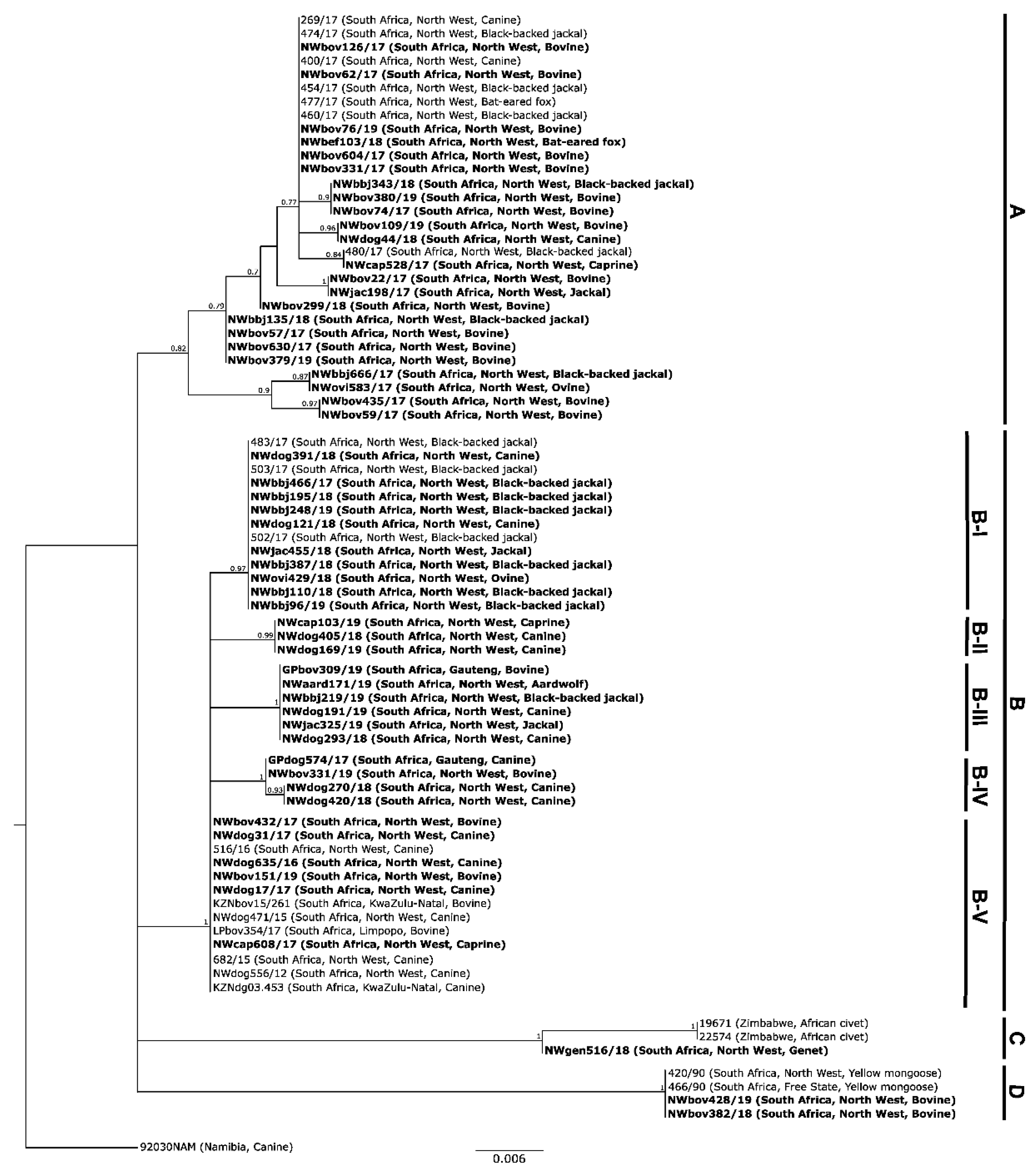
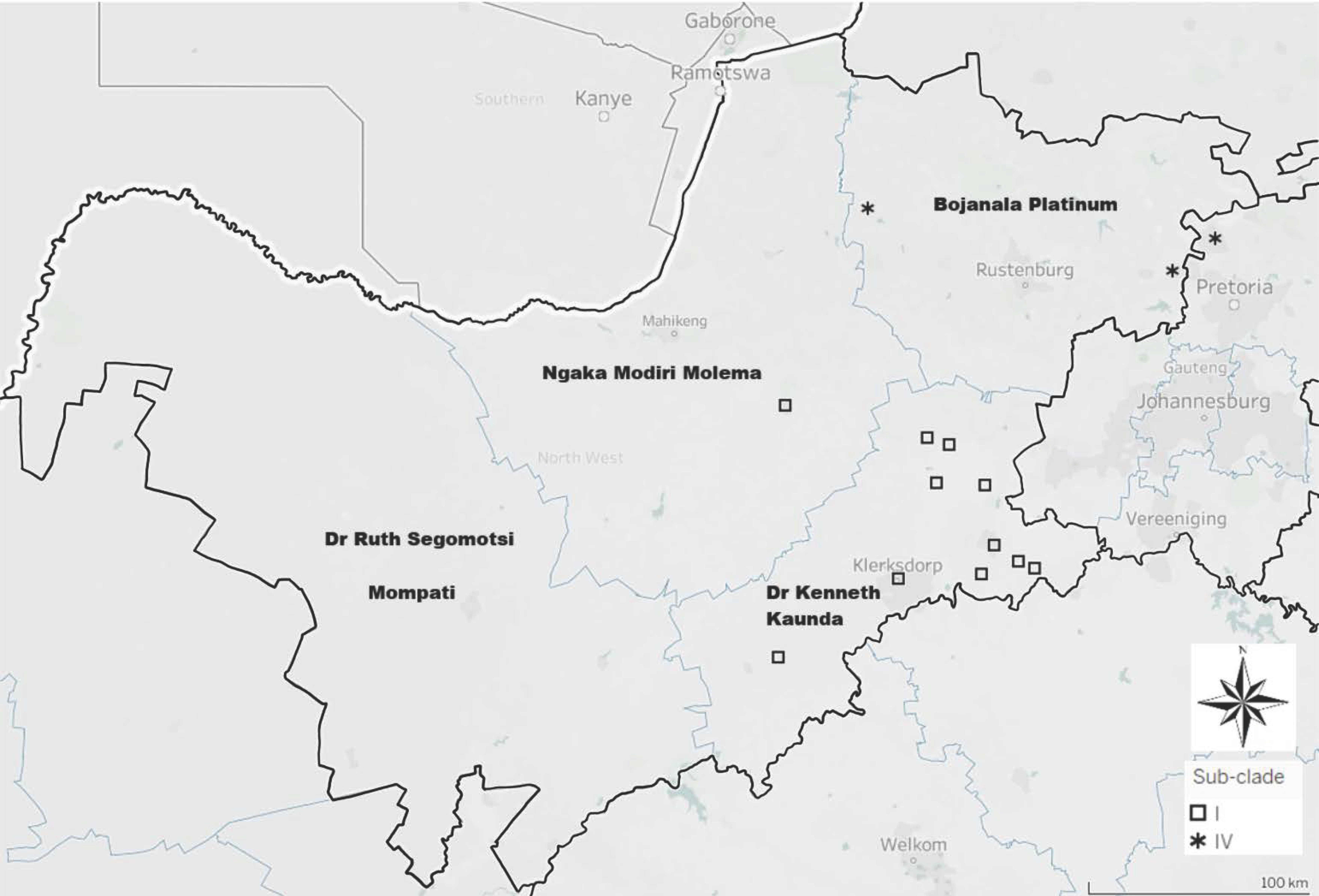
3. Results
3.1. Molecular Epidemiology of RABVs from the North West Province of South Africa
3.1.1. Based on Partial Nucleoprotein Gene Region
3.1.2. Based on the G-L Intergenic Region
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ICTV International Committee on Taxonomy of Viruses (ICTV). Available online: https://talk.ictvonline.org/taxonomy/ (accessed on 9 September 2020).
- WHO. WHO Expert Consultation on Rabies: Third Report; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Lembo, T.; Hampson, K.; Kaare, M.T.; Ernest, E.; Knobel, D.; Kazwala, R.R.; Haydon, D.T.; Cleaveland, S. The Feasibility of Canine Rabies Elimination in Africa: Dispelling Doubts with Data. PLoS Negl. Trop. Dis. 2010, 4, e626. [Google Scholar] [CrossRef] [PubMed]
- Vigilato, M.A.N.; Clavijo, A.; Knobl, T.; Silva, H.M.T.; Cosivi, O.; Schneider, M.C.; Leanes, L.F.; Belotto, A.J.; Espinal, M.A. Progress towards Eliminating Canine Rabies: Policies and Perspectives from Latin America and the Caribbean. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120143. [Google Scholar] [CrossRef] [PubMed]
- Weyer, J. Rabies in South Africa: Where Do We Stand in 2015? South. African J. Infect. Dis. 2015, 30, 40–41. [Google Scholar] [CrossRef][Green Version]
- Hampson, K.; Coudeville, L.; Lembo, T.; Sambo, M.; Kieffer, A.; Attlan, M.; Barrat, J.; Blanton, J.D.; Briggs, D.J.; Cleaveland, S.; et al. Estimating the Global Burden of Endemic Canine Rabies. PLoS Negl. Trop. Dis. 2015, 9, e0003709. [Google Scholar] [CrossRef]
- Swanepoel, R.; Barnard, B.J.H.; Meredith, C.D.; Bishop, G.C.; Bruckner, G.K.; Foggin, C.M.; Hubschle, O.J.B. Rabies in Southern Africa. Onderstepoort J. Vet. Res. 1993, 60, 325. [Google Scholar]
- Cohen, C.; Sartorius, B.; Sabeta, C.; Zulu, G.; Paweska, J.; Mogoswane, M.; Sutton, C.; Nel, L.H.; Swanepoel, R.; Leman, P.A.; et al. Epidemiology and Molecular Virus Characterization of Reemerging Rabies, South Africa. Emerg. Infect. Dis. 2007, 13, 1879–1886. [Google Scholar] [CrossRef]
- Sabeta, C.T.; Mansfield, K.L.; McElhinney, L.M.; Fooks, A.R.; Nel, L.H. Molecular Epidemiology of Rabies in Bat-Eared Foxes (Otocyon Megalotis) in South Africa. Virus Res. 2007, 129, 1–10. [Google Scholar] [CrossRef]
- Ngoepe, C.E.; Sabeta, C.; Nel, L. The Spread of Canine Rabies into Free State Province of South Africa: A Molecular Epidemiological Characterization. Virus Res. 2009, 142, 175–180. [Google Scholar] [CrossRef]
- Mkhize, G.C.; Ngoepe, E.C.; Du Plessis, B.J.A.; Reininghaus, B.; Sabeta, C.T. Re-Emergence of Dog Rabies in Mpumalanga Province, South Africa. Vector-Borne Zoonotic Dis. 2010, 10, 921–926. [Google Scholar] [CrossRef]
- Sabeta, C.T.; Weyer, J.; Geertsma, P.; Mohale, D.; Miyen, J.; Blumberg, L.H.; Leman, P.A.; Phahladira, B.; Shumba, W.; Walters, J. Emergence of Rabies in the Gauteng Province, South Africa: 2010-2011. J. S. Afr. Vet. Assoc. 2013, 84, 1–5. [Google Scholar] [CrossRef]
- Hergert, M.; Le Roux, K.; Nel, L.H. Characteristics of Owned Dogs in Rabies Endemic KwaZulu-Natal Province, South Africa. BMC Vet. Res. 2018, 14, 278. [Google Scholar] [CrossRef]
- Zulu, G.C.; Sabeta, C.T.; Nel, L.H. Molecular Epidemiology of Rabies: Focus on Domestic Dogs (Canis Familiaris) and Black-Backed Jackals (Canis Mesomelas) from Northern South Africa. Virus Res. 2009, 140, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Kissi, B.; Tordo, N.; Bourhy, H. Genetic Polymorphism in the Rabies Virus Nucleoprotein Gene. Virology 1995, 209, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Nel, L.H.; Sabeta, C.T.; von Teichman, B.; Jaftha, J.B.; Rupprecht, C.E.; Bingham, J. Mongoose Rabies in Southern Africa: A Re-Evaluation Based on Molecular Epidemiology. Virus Res. 2005, 109, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Bishop, G.C.; Durrheim, D.N.; Kloeck, P.E.; Godlonton, J.D.; Bingham, J.; Speare, R.; Advisory, R. Guide for the Medical, Veterinary and Allied Professions, 2nd ed.; Government Printing Works: Pretoria, Africa, 2010. [Google Scholar]
- Van Niekerk, H.N. The Cost of Predation on Small Livestock in South Africa by Medium-Sized Predators. Ph.D. Thesis, University of the Free State, Bloemfontein, Africa, 2010. [Google Scholar]
- Cleaveland, S. The Growing Problem of Rabies in Africa. Trans. R. Soc. Trop. Med. Hyg. 1998, 92, 131–134. [Google Scholar] [CrossRef]
- King, A.A.; Fooks, A.R.; Aubert, M.; Wandeler, A.I. Historical Perspective of Rabies in Europe and the Mediterranean Basin; OIE: Paris, France, 2004; ISBN 9290446390. [Google Scholar]
- Sabeta, C.T.; Mkhize, G.C.; Ngoepe, E.C. An Evaluation of Dog Rabies Control in Limpopo Province (South Africa). Epidemiol. Infect. 2011, 139, 1470–1475. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, P.; Nel, L.H. Emerging Epidemic Dog Rabies in Coastal South Africa: A Molecular Epidemiological Analysis. Virus Res. 2007, 126, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Shwiff, S.A.; Hatch, B.; Anderson, A.; Nel, L.H.; Leroux, K.; Stewart, D.; de Scally, M.; Govender, P.; Rupprecht, C.E. Towards Canine Rabies Elimination in KwaZulu-Natal, South Africa: Assessment of Health Economic Data. Transbound. Emerg. Dis. 2016, 63, 408–415. [Google Scholar] [CrossRef]
- Le Roux, K.; Kotze, J.; Perrett, K. Elimination of Dog-Mediated Human Rabies: The Burden of Human Rabies in Africa. Rev Sci Tech Int Off Epizoot 2018, 37, 607–615. [Google Scholar] [CrossRef]
- Grewar, J. Rabies Events in the Western Cape Province 2010 | Agriprobe. Available online: https://journals.co.za/doi/10.10520/EJC19017 (accessed on 26 March 2021).
- Weyer, J.; Szmyd-Potapczuk, A.V.; Blumberg, L.H.; Leman, P.A.; Markotter, W.; Swanepoel, R.; Paweska, J.T.; Nel, L.H. Epidemiology of Human Rabies in South Africa, 1983-2007. Virus Res. 2011, 155, 283–290. [Google Scholar] [CrossRef]
- Markotter, W.; Kuzmin, I.; Rupprecht, C.E.; Randles, J.; Sabeta, C.T.; Wandeler, A.I.; Nel, L.H. Isolation of Lagos Bat Virus from Water Mongoose. Emerg. Infect. Dis. 2006, 12, 1913–1918. [Google Scholar] [CrossRef] [PubMed]
- Sacramento, D.; Bourhy, H.; Tordo, N. PCR Technique as an Alternative Method for Diagnosis and Molecular Epidemiology of Rabies Virus. Mol. Cell. Probes 1991, 5, 229–240. [Google Scholar] [CrossRef]
- von Teichman, B.F.; Thomson, G.R.; Meredith, C.D.; Nel, L.H. Molecular Epidemiology of Rabies Virus in South Africa: Evidence for Two Distinct Virus Groups. J. Gen. Virol. 1995, 76, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Cobb, B.D.; Clarkson, J.M. A Simple Procedure for Optimising the Polymerase Chain Reaction (PCR) Using Modified Taguchi Methods. Nucleic Acids Res. 1994, 22, 3801–3805. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Bouckaert, R.; Vaughan, T.G.; Barido-Sottani, J.; Duchêne, S.; Fourment, M.; Gavryushkina, A.; Heled, J.; Jones, G.; Kühnert, D.; De Maio, N.; et al. BEAST 2.5: An Advanced Software Platform for Bayesian Evolutionary Analysis. PLOS Comput. Biol. 2019, 15, e1006650. [Google Scholar] [CrossRef]
- Sabeta, C.T.; Marston, D.A.; McElhinney, L.M.; Horton, D.L.; Phahladira, B.M.N.; Fooks, A.R. Rabies in the African Civet: An Incidental Host for Lyssaviruses? Viruses 2020, 12, 368. [Google Scholar] [CrossRef]
- Brunker, K.; Marston, D.A.; Horton, D.L.; Cleaveland, S.; Fooks, A.R.; Kazwala, R.; Ngeleja, C.; Lembo, T.; Sambo, M.; Mtema, Z.J.; et al. Elucidating the Phylodynamics of Endemic Rabies Virus in Eastern Africa Using Whole-Genome Sequencing. Virus Evol. 2015, 1, vev011. [Google Scholar] [CrossRef]
- Hayman, D.T.S.; Johnson, N.; Horton, D.L.; Hedge, J.; Wakeley, P.R.; Banyard, A.C.; Zhang, S.; Alhassan, A.; Fooks, A.R. Evolutionary History of Rabies in Ghana. PLoS Negl. Trop. Dis. 2011, 5, e1001. [Google Scholar] [CrossRef]
- StatsSA North West Publication | Statistics South Africa. Available online: http://www.statssa.gov.za/?page_id=1854&PPN=Report-11-02-07&SCH=7908 (accessed on 9 January 2021).
- Badenhorst, C.G. The Economic Cost of Large Stock Predation in the North West Province of South Africa. Ph.D. Thesis, University of the Free State, Bloemfontein, Africa, 2014. [Google Scholar]
- StatsSA Census of Commercial Agriculture. Available online: http://www.statssa.gov.za/publications/Report-11-02-01/CoCA 2017 Fact Sheets.pdf (accessed on 9 January 2021).
- Bingham, J. Canine Rabies Ecology in Southern Africa. Emerg. Infect. Dis. 2005, 11, 1337–1342. [Google Scholar] [CrossRef]
- LeRoux, A.; Balmforth, Z.; Mbatyoti, O.; Bizani, M.; Avenant, N.; Cavallini, P.; Do Linh San, E. A Conservation Assessment of Cynictis Penicillata. In The Red List of Mammals of South Africa, Swaziland and Lesotho; Child, M., Roxburgh, L., Do Linh San, E., Raimondo, D., Davies-Mostert, H., Eds.; South African National Biodiversity Institute and Endangered Wildlife Trust: Midrand, South Africa, 2016. [Google Scholar]
- Bingham, J.; Schumacher, C.L.; Hill, F.W.G.; Aubert, A. Efficacy of SAG-2 Oral Rabies Vaccine in Two Species of Jackal (Canis Adustus and Canis Mesomelas). Vaccine 1999, 17, 551–558. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malan, A.J.; Coetzer, A.; Sabeta, C.T.; Nel, L.H. Epidemiological Interface of Sylvatic and Dog Rabies in the North West Province of South Africa. Trop. Med. Infect. Dis. 2022, 7, 90. https://doi.org/10.3390/tropicalmed7060090
Malan AJ, Coetzer A, Sabeta CT, Nel LH. Epidemiological Interface of Sylvatic and Dog Rabies in the North West Province of South Africa. Tropical Medicine and Infectious Disease. 2022; 7(6):90. https://doi.org/10.3390/tropicalmed7060090
Chicago/Turabian StyleMalan, Ayla J., Andre Coetzer, Claude T. Sabeta, and Louis H. Nel. 2022. "Epidemiological Interface of Sylvatic and Dog Rabies in the North West Province of South Africa" Tropical Medicine and Infectious Disease 7, no. 6: 90. https://doi.org/10.3390/tropicalmed7060090
APA StyleMalan, A. J., Coetzer, A., Sabeta, C. T., & Nel, L. H. (2022). Epidemiological Interface of Sylvatic and Dog Rabies in the North West Province of South Africa. Tropical Medicine and Infectious Disease, 7(6), 90. https://doi.org/10.3390/tropicalmed7060090






