Immune Responses in Leishmaniasis: An Overview
Abstract
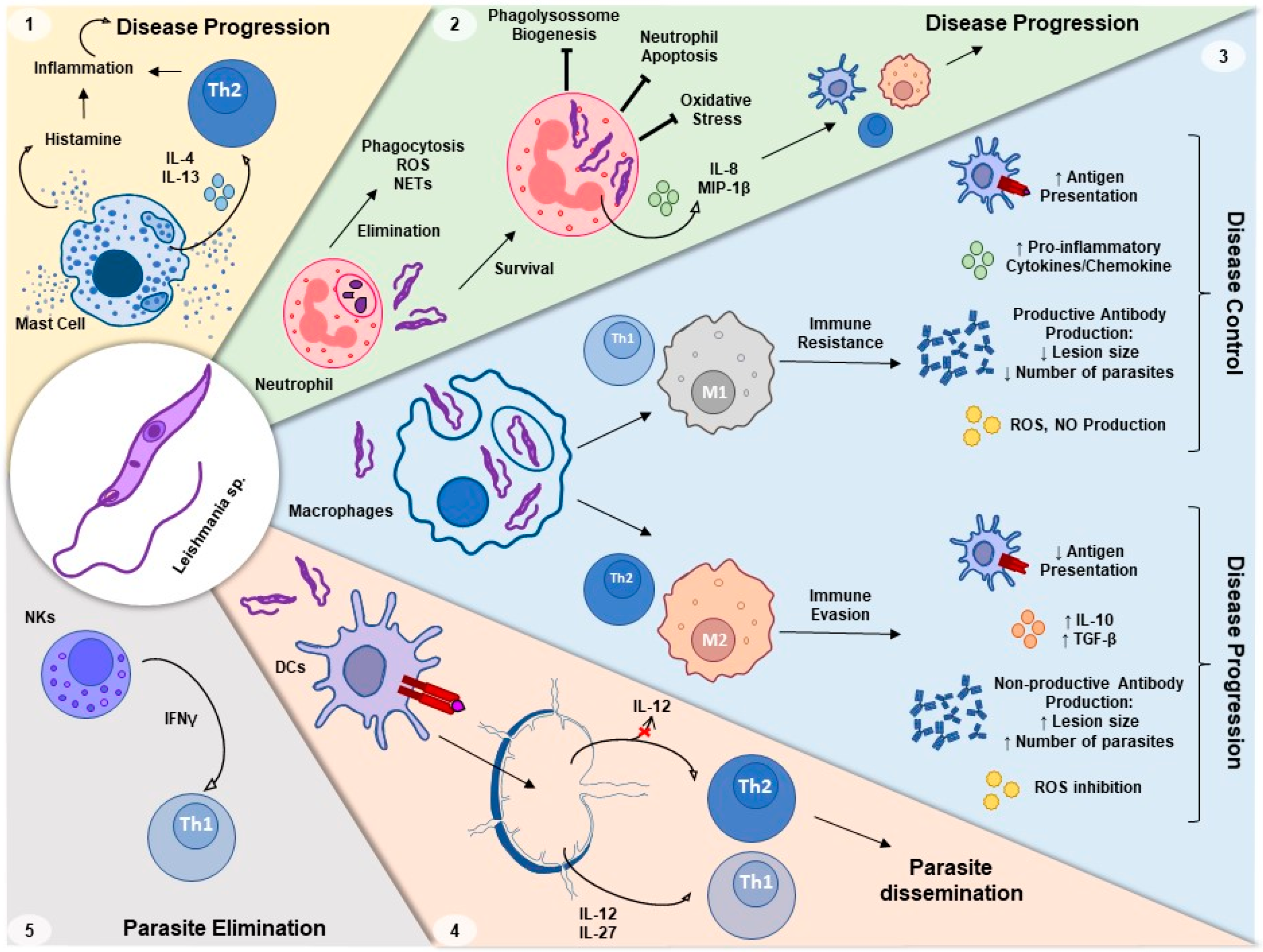
:1. Leishmaniasis
2. Th1 versus Th2 Response to Leishmania Infection
3. Innate Immune Response to Leishmania Infection
3.1. Neutrophils–Leishmania Interaction
3.2. Macrophage–Leishmania Interaction
3.3. Dendritic Cells–Leishmania Interaction
3.4. Leishmania Interaction with Other Innate Immune Cells
4. Leishmania and Host Immune Receptors’ Interaction and Intracellular Processing of Leishmania
5. Changes in Macrophages after Leishmania Infection
6. Leishmania and Oxidative Stress
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, H.L.; Jain, S.; Ruiz Postigo, J.A.; Borisch, B.; Dagne, D.A. The global procurement landscape of leishmaniasis medicines. PLoS Negl. Trop. Dis. 2021, 15, e0009181. [Google Scholar] [CrossRef]
- Benallal, K.E.; Garni, R.; Harrat, Z.; Volf, P.; Dvorak, V. Phlebotomine sand flies (Diptera: Psychodidae) of the Maghreb region: A systematic review of distribution, morphology, and role in the transmission of the pathogens. PLoS Negl. Trop. Dis. 2022, 16, e0009952. [Google Scholar] [CrossRef]
- Daga, M.K.; Rohatgi, I.; Mishra, R. Leishmaniasis. Indian J. Crit. Care Med. 2021, 25, S166–S170. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Lauletta Lindoso, J.A. Cutaneous and mucocutaneous leishmaniasis. Infect. Dis. Clin. N. Am. 2012, 26, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Van Assche, T.; Deschacht, M.; da Luz, R.A.; Maes, L.; Cos, P. Leishmania-macrophage interactions: Insights into the redox biology. Free Radic. Biol. Med. 2011, 51, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, E.; Ghasemian, A.; Abdinasir, A.; Nematollahi Mahani, S.A.; Rawaf, S.; Salehi Vaziri, M.; Gouya, M.M.; Minh Nhu Nguyen, T.; Al Awaidy, S.; Al Ariqi, L.; et al. Emerging and Re-emerging Infectious Diseases in the WHO Eastern Mediterranean Region, 2001–2018. Int. J. Health Policy Manag. 2021. [Google Scholar] [CrossRef] [PubMed]
- Mougneau, E.; Bihl, F.; Glaichenhaus, N. Cell biology and immunology of Leishmania. Immunol. Rev. 2011, 240, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Serafim, T.D.; Coutinho-Abreu, I.V.; Dey, R.; Kissinger, R.; Valenzuela, J.G.; Oliveira, F.; Kamhawi, S. Leishmaniasis: The act of transmission. Trends Parasitol. 2021, 37, 976–987. [Google Scholar] [CrossRef]
- Mirzaei, A.; Maleki, M.; Masoumi, E.; Maspi, N. A historical review of the role of cytokines involved in leishmaniasis. Cytokine 2021, 145, 155297. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, J.M.; Fakiola, M.; Castellucci, L.C. Human genetics of leishmania infections. Hum. Genet. 2020, 139, 813–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filardy, A.A.; Pires, D.R.; DosReis, G.A. Macrophages and neutrophils cooperate in immune responses to Leishmania infection. Cell Mol. Life Sci. 2011, 68, 1863–1870. [Google Scholar] [CrossRef] [PubMed]
- Arcanjo, A.F.; LaRocque-de-Freitas, I.F.; Rocha, J.D.; Zamith, D.; Costa-da-Silva, A.C.; Nunes, M.P.; Mesquita-Santos, F.P.; Morrot, A.; Filardy, A.A.; Mariano, M.; et al. The PGE2/IL-10 Axis Determines Susceptibility of B-1 Cell-Derived Phagocytes (B-1CDP) to Leishmania major Infection. PLoS ONE 2015, 10, e0124888. [Google Scholar] [CrossRef] [Green Version]
- Ehrchen, J.M.; Roth, J.; Roebrock, K.; Varga, G.; Domschke, W.; Newberry, R.; Sorg, C.; Müller-Tidow, C.; Sunderkötter, C.; Kucharzik, T.; et al. The absence of cutaneous lymph nodes results in a Th2 response and increased susceptibility to Leishmania major infection in mice. Infect. Immun. 2008, 76, 4241–4250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Bumb, R.A.; Salotra, P. Evaluation of localized and systemic immune responses in cutaneous leishmaniasis caused by Leishmania tropica: Interleukin-8, monocyte chemotactic protein-1 and nitric oxide are major regulatory factors. Immunology 2010, 130, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Bacellar, O.; Lessa, H.; Schriefer, A.; Machado, P.; Ribeiro de Jesus, A.; Dutra, W.O.; Gollob, K.J.; Carvalho, E.M. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect. Immun. 2002, 70, 6734–6740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurdayal, R.; Nieuwenhuizen, N.E.; Revaz-Breton, M.; Smith, L.; Hoving, J.C.; Parihar, S.P.; Reizis, B.; Brombacher, F. Deletion of IL-4 receptor alpha on dendritic cells renders BALB/c mice hypersusceptible to Leishmania major infection. PLoS Pathog. 2013, 9, e1003699. [Google Scholar] [CrossRef] [Green Version]
- Anderson, C.F.; Stumhofer, J.S.; Hunter, C.A.; Sacks, D. IL-27 regulates IL-10 and IL-17 from CD4+ cells in nonhealing Leishmania major infection. J. Immunol. 2009, 183, 4619–4627. [Google Scholar] [CrossRef] [Green Version]
- Katara, G.K.; Raj, A.; Kumar, R.; Avishek, K.; Kaushal, H.; Ansari, N.A.; Bumb, R.A.; Salotra, P. Analysis of localized immune responses reveals presence of Th17 and Treg cells in cutaneous leishmaniasis due to Leishmania tropica. BMC Immunol. 2013, 14, 52. [Google Scholar] [CrossRef] [Green Version]
- Espir, T.T.; Figueira, L.E.P.; Naiff, M.E.F.; da Costa, A.G.; Ramalho-Ortigão, M.; Malheiro, A.; Franco, A.M. The role of inflammatory, anti-inflammatory, and regulatory cytokines in patients infected with cutaneous leishmaniasis in Amazonas State, Brazil. J. Immunol. Res. 2014, 2014, 481750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietze-Schwonberg, K.; Lopez Kostka, S.; Lorenz, B.; Regen, T.; Waisman, A.; von Stebut, E. IL-17A/F in Leishmania major-resistant C57BL/6 mice. Exp. Derm. 2019, 28, 321–323. [Google Scholar] [CrossRef]
- Gonçalves-de-Albuquerque, S.D.C.; Pessoa-E-Silva, R.; Trajano-Silva, L.A.M.; de Goes, T.C.; de Morais, R.C.S.; da C. Oliveira, C.N.; de Lorena, V.M.B.; de Paiva-Cavalcanti, M. The Equivocal Role of Th17 Cells and Neutrophils on Immunopathogenesis of Leishmaniasis. Front. Immunol. 2017, 8, 1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, A.; Bhattacharya, P.; Joshi, A.B.; Ismail, N.; Dey, R.; Nakhasi, H.L. Role of pro-inflammatory cytokine IL-17 in Leishmania pathogenesis and in protective immunity by Leishmania vaccines. Cell Immunol. 2016, 309, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Von Stebut, E. Cutaneous Leishmania infection: Progress in pathogenesis research and experimental therapy. Exp. Derm. 2007, 16, 340–346. [Google Scholar] [CrossRef]
- Regli, I.B.; Passelli, K.; Hurrell, B.P.; Tacchini-Cottier, F. Survival Mechanisms Used by Some. Front. Immunol. 2017, 8, 1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolářová, I.; Valigurová, A. Hide-and-Seek: A Game Played between Parasitic Protists and Their Hosts. Microorganisms 2021, 9, 2434. [Google Scholar] [CrossRef] [PubMed]
- Filho, A.A.P.; Nascimento, A.A.S.; Saab, N.A.A.; Fugiwara, R.T.; D’Ávila Pessoa, G.C.; Koerich, L.B.; Pereira, M.H.; Araújo, R.N.; Sant’Anna, M.R.V.; Gontijo, N.F. Evasion of the complement system by Leishmania through the uptake of factor H, a complement regulatory protein. Acta Trop. 2021, 224, 106152. [Google Scholar] [CrossRef] [PubMed]
- Hermoso, T.; Fishelson, Z.; Becker, S.I.; Hirschberg, K.; Jaffe, C.L. Leishmanial protein kinases phosphorylate components of the complement system. EMBO J. 1991, 10, 4061–4067. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.C.; Almeida-Campos, F.R.; Horta, M.F.; Ramalho-Pinto, F.J. Leishmania amazonensis promastigotes evade complement killing by interfering with the late steps of the cascade. Parasitology 1997, 115 Pt 6, 601–609. [Google Scholar] [CrossRef]
- Domínguez, M.; Moreno, I.; Aizpurua, C.; Toraño, A. Early mechanisms of Leishmania infection in human blood. Microbes Infect. 2003, 5, 507–513. [Google Scholar] [CrossRef]
- Atayde, V.D.; Aslan, H.; Townsend, S.; Hassani, K.; Kamhawi, S.; Olivier, M. Exosome Secretion by the Parasitic Protozoan Leishmania within the Sand Fly Midgut. Cell Rep. 2015, 13, 957–967. [Google Scholar] [CrossRef] [Green Version]
- Dey, R.; Joshi, A.B.; Oliveira, F.; Pereira, L.; Guimarães-Costa, A.B.; Serafim, T.D.; de Castro, W.; Coutinho-Abreu, I.V.; Bhattacharya, P.; Townsend, S.; et al. Gut Microbes Egested during Bites of Infected Sand Flies Augment Severity of Leishmaniasis via Inflammasome-Derived IL-1β. Cell Host Microbe 2018, 23, 134–143.e136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, B.B.; de Oliveira, C.I.; Brodskyn, C.I.; Barral, A.; Barral-Netto, M. Role of sand fly saliva in human and experimental leishmaniasis: Current insights. Scand. J. Immunol. 2007, 66, 122–127. [Google Scholar] [CrossRef]
- Teixeira, M.J.; Teixeira, C.R.; Andrade, B.B.; Barral-Netto, M.; Barral, A. Chemokines in host-parasite interactions in leishmaniasis. Trends Parasitol. 2006, 22, 32–40. [Google Scholar] [CrossRef]
- Giraud, E.; Lestinova, T.; Derrick, T.; Martin, O.; Dillon, R.J.; Volf, P.; Műller, I.; Bates, P.A.; Rogers, M.E. Leishmania proteophosphoglycans regurgitated from infected sand flies accelerate dermal wound repair and exacerbate leishmaniasis via insulin-like growth factor 1-dependent signalling. PLoS Pathog. 2018, 14, e1006794. [Google Scholar] [CrossRef] [Green Version]
- Pacheco-Fernandez, T.; Volpedo, G.; Verma, C.; Satoskar, A.R. Understanding the immune responses involved in mediating protection or immunopathology during leishmaniasis. Biochem. Soc. Trans. 2021, 49, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Goundry, A.; Romano, A.; Lima, A.P.C.A.; Mottram, J.C.; Myburgh, E. Inhibitor of serine peptidase 2 enhances Leishmania major survival in the skin through control of monocytes and monocyte-derived cells. FASEB J. 2018, 32, 1315–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gwinn, M.R.; Vallyathan, V. Respiratory burst: Role in signal transduction in alveolar macrophages. J. Toxicol. Environ. Health B Crit. Rev. 2006, 9, 27–39. [Google Scholar] [CrossRef]
- Filardy, A.A.; Costa-da-Silva, A.C.; Koeller, C.M.; Guimarães-Pinto, K.; Ribeiro-Gomes, F.L.; Lopes, M.F.; Heise, N.; Freire-de-Lima, C.G.; Nunes, M.P.; DosReis, G.A. Infection with Leishmania major induces a cellular stress response in macrophages. PLoS ONE 2014, 9, e85715. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, B.P.; Regli, I.B.; Tacchini-Cottier, F. Different Leishmania Species Drive Distinct Neutrophil Functions. Trends Parasitol. 2016, 32, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Tacchini-Cottier, F.; Zweifel, C.; Belkaid, Y.; Mukankundiye, C.; Vasei, M.; Launois, P.; Milon, G.; Louis, J.A. An immunomodulatory function for neutrophils during the induction of a CD4+ Th2 response in BALB/c mice infected with Leishmania major. J. Immunol. 2000, 165, 2628–2636. [Google Scholar] [CrossRef] [Green Version]
- Charmoy, M.; Megnekou, R.; Allenbach, C.; Zweifel, C.; Perez, C.; Monnat, K.; Breton, M.; Ronet, C.; Launois, P.; Tacchini-Cottier, F. Leishmania major induces distinct neutrophil phenotypes in mice that are resistant or susceptible to infection. J. Leukoc. Biol. 2007, 82, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Rochael, N.C.; Guimarães-Costa, A.B.; Nascimento, M.T.; DeSouza-Vieira, T.S.; Oliveira, M.P.; Garcia e Souza, L.F.; Oliveira, M.F.; Saraiva, E.M. Classical ROS-dependent and early/rapid ROS-independent release of Neutrophil Extracellular Traps triggered by Leishmania parasites. Sci. Rep. 2015, 5, 18302. [Google Scholar] [CrossRef] [Green Version]
- Moradin, N.; Descoteaux, A. Leishmania promastigotes: Building a safe niche within macrophages. Front. Cell. Infect. Microbiol. 2012, 2, 121. [Google Scholar] [CrossRef] [Green Version]
- Laufs, H.; Müller, K.; Fleischer, J.; Reiling, N.; Jahnke, N.; Jensenius, J.C.; Solbach, W.; Laskay, T. Intracellular survival of Leishmania major in neutrophil granulocytes after uptake in the absence of heat-labile serum factors. Infect. Immun. 2002, 70, 826–835. [Google Scholar] [CrossRef] [Green Version]
- Laskay, T.; van Zandbergen, G.; Solbach, W. Neutrophil granulocytes—Trojan horses for Leishmania major and other intracellular microbes? Trends Microbiol. 2003, 11, 210–214. [Google Scholar] [CrossRef]
- Charmoy, M.; Hurrell, B.P.; Romano, A.; Lee, S.H.; Ribeiro-Gomes, F.; Riteau, N.; Mayer-Barber, K.; Tacchini-Cottier, F.; Sacks, D.L. The Nlrp3 inflammasome, IL-1β, and neutrophil recruitment are required for susceptibility to a nonhealing strain of Leishmania major in C57BL/6 mice. Eur. J. Immunol. 2016, 46, 897–911. [Google Scholar] [CrossRef] [Green Version]
- van Zandbergen, G.; Klinger, M.; Mueller, A.; Dannenberg, S.; Gebert, A.; Solbach, W.; Laskay, T. Cutting edge: Neutrophil granulocyte serves as a vector for Leishmania entry into macrophages. J. Immunol. 2004, 173, 6521–6525. [Google Scholar] [CrossRef] [Green Version]
- Aga, E.; Katschinski, D.M.; van Zandbergen, G.; Laufs, H.; Hansen, B.; Müller, K.; Solbach, W.; Laskay, T. Inhibition of the spontaneous apoptosis of neutrophil granulocytes by the intracellular parasite Leishmania major. J. Immunol. 2002, 169, 898–905. [Google Scholar] [CrossRef] [Green Version]
- Peters, N.C.; Egen, J.G.; Secundino, N.; Debrabant, A.; Kimblin, N.; Kamhawi, S.; Lawyer, P.; Fay, M.P.; Germain, R.N.; Sacks, D. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science 2008, 321, 970–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro-Gomes, F.L.; Otero, A.C.; Gomes, N.A.; Moniz-De-Souza, M.C.; Cysne-Finkelstein, L.; Arnholdt, A.C.; Calich, V.L.; Coutinho, S.G.; Lopes, M.F.; DosReis, G.A. Macrophage interactions with neutrophils regulate Leishmania major infection. J. Immunol. 2004, 172, 4454–4462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afonso, L.; Borges, V.M.; Cruz, H.; Ribeiro-Gomes, F.L.; DosReis, G.A.; Dutra, A.N.; Clarêncio, J.; de Oliveira, C.I.; Barral, A.; Barral-Netto, M.; et al. Interactions with apoptotic but not with necrotic neutrophils increase parasite burden in human macrophages infected with Leishmania amazonensis. J. Leukoc. Biol. 2008, 84, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Gomes, F.L.; Moniz-de-Souza, M.C.; Alexandre-Moreira, M.S.; Dias, W.B.; Lopes, M.F.; Nunes, M.P.; Lungarella, G.; DosReis, G.A. Neutrophils activate macrophages for intracellular killing of Leishmania major through recruitment of TLR4 by neutrophil elastase. J. Immunol. 2007, 179, 3988–3994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horta, M.F.; Mendes, B.P.; Roma, E.H.; Noronha, F.S.; Macêdo, J.P.; Oliveira, L.S.; Duarte, M.M.; Vieira, L.Q. Reactive oxygen species and nitric oxide in cutaneous leishmaniasis. J. Parasitol. Res. 2012, 2012, 203818. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Butcher, B.; Sacks, D.L. Analysis of cytokine production by inflammatory mouse macrophages at the single-cell level: Selective impairment of IL-12 induction in Leishmania-infected cells. Eur. J. Immunol. 1998, 28, 1389–1400. [Google Scholar] [CrossRef]
- Liew, F.Y.; Millott, S.; Parkinson, C.; Palmer, R.M.; Moncada, S. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from L-arginine. J. Immunol. 1990, 144, 4794–4797. [Google Scholar]
- Collin, M.; Bigley, V. Human dendritic cell subsets: An update. Immunology 2018, 154, 3–20. [Google Scholar] [CrossRef]
- Marovich, M.A.; McDowell, M.A.; Thomas, E.K.; Nutman, T.B. IL-12p70 production by Leishmania major-harboring human dendritic cells is a CD40/CD40 ligand-dependent process. J. Immunol. 2000, 164, 5858–5865. [Google Scholar] [CrossRef] [Green Version]
- Jafarzadeh, A.; Nemati, M.; Chauhan, P.; Patidar, A.; Sarkar, A.; Sharifi, I.; Saha, B. Interleukin-27 Functional Duality Balances. Front. Immunol. 2020, 11, 1573. [Google Scholar] [CrossRef]
- Moll, H.; Fuchs, H.; Blank, C.; Röllinghoff, M. Langerhans cells transport Leishmania major from the infected skin to the draining lymph node for presentation to antigen-specific T cells. Eur. J. Immunol. 1993, 23, 1595–1601. [Google Scholar] [CrossRef] [Green Version]
- Lemos, M.P.; Esquivel, F.; Scott, P.; Laufer, T.M. MHC class II expression restricted to CD8alpha+ and CD11b+ dendritic cells is sufficient for control of Leishmania major. J. Exp. Med. 2004, 199, 725–730. [Google Scholar] [CrossRef]
- Kautz-Neu, K.; Noordegraaf, M.; Dinges, S.; Bennett, C.L.; John, D.; Clausen, B.E.; von Stebut, E. Langerhans cells are negative regulators of the anti-Leishmania response. J. Exp. Med. 2011, 208, 885–891. [Google Scholar] [CrossRef] [Green Version]
- Ritter, U.; Meissner, A.; Scheidig, C.; Körner, H. CD8 alpha- and Langerin-negative dendritic cells, but not Langerhans cells, act as principal antigen-presenting cells in leishmaniasis. Eur. J. Immunol. 2004, 34, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- León, B.; López-Bravo, M.; Ardavín, C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity 2007, 26, 519–531. [Google Scholar] [CrossRef] [Green Version]
- Biedermann, T.; Zimmermann, S.; Himmelrich, H.; Gumy, A.; Egeter, O.; Sakrauski, A.K.; Seegmüller, I.; Voigt, H.; Launois, P.; Levine, A.D.; et al. IL-4 instructs TH1 responses and resistance to Leishmania major in susceptible BALB/c mice. Nat. Immunol. 2001, 2, 1054–1060. [Google Scholar] [CrossRef]
- Hurdayal, R.; Nieuwenhuizen, N.E.; Khutlang, R.; Brombacher, F. Inflammatory Dendritic Cells, Regulated by IL-4 Receptor Alpha Signaling, Control Replication, and Dissemination of Leishmania major in mice. Front. Cell. Infect. Microbiol. 2019, 9, 479. [Google Scholar] [CrossRef]
- Bogdan, C. Natural killer cells in experimental and human leishmaniasis. Front. Cell. Infect. Microbiol. 2012, 2, 69. [Google Scholar] [CrossRef] [Green Version]
- Scharton, T.M.; Scott, P. Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J. Exp. Med. 1993, 178, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Messlinger, H.; Sebald, H.; Heger, L.; Dudziak, D.; Bogdan, C.; Schleicher, U. Monocyte-Derived Signals Activate Human Natural Killer Cells in Response to Leishmania Parasites. Front. Immunol. 2018, 9, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naqvi, N.; Srivastava, R.; Selvapandiyan, A.; Puri, N. Host Mast Cells in Leishmaniasis: Friend or Foe? Trends Parasitol. 2020, 36, 952–956. [Google Scholar] [CrossRef]
- Maurer, M.; Lopez Kostka, S.; Siebenhaar, F.; Moelle, K.; Metz, M.; Knop, J.; von Stebut, E. Skin mast cells control T cell-dependent host defense in Leishmania major infections. FASEB J. 2006, 20, 2460–2467. [Google Scholar] [CrossRef]
- Lopez Kostka, S.; Dinges, S.; Griewank, K.; Iwakura, Y.; Udey, M.C.; von Stebut, E. IL-17 promotes progression of cutaneous leishmaniasis in susceptible mice. J. Immunol. 2009, 182, 3039–3046. [Google Scholar] [CrossRef]
- De Oliveira, M.P.; Lima, M.C.; Calheiros, A.S.; Martins, M.A.; Antas, P.R.; De Luca, P.M.; Pirmez, C. Leishmania (Viannia) braziliensis: Human mast cell line activation induced by logarithmic and stationary promastigote derived-lysates. Exp. Parasitol. 2005, 109, 72–79. [Google Scholar] [CrossRef]
- Wershil, B.K.; Theodos, C.M.; Galli, S.J.; Titus, R.G. Mast cells augment lesion size and persistence during experimental Leishmania major infection in the mouse. J. Immunol. 1994, 152, 4563–4571. [Google Scholar] [PubMed]
- Romão, P.R.; Da Costa Santiago, H.; Ramos, C.D.; De Oliveira, C.F.; Monteiro, M.C.; De Queiroz Cunha, F.; Vieira, L.Q. Mast cell degranulation contributes to susceptibility to Leishmania major. Parasite Immunol. 2009, 31, 140–146. [Google Scholar] [CrossRef]
- Ehrchen, J.M.; Roebrock, K.; Foell, D.; Nippe, N.; von Stebut, E.; Weiss, J.M.; Münck, N.A.; Viemann, D.; Varga, G.; Müller-Tidow, C.; et al. Keratinocytes determine Th1 immunity during early experimental leishmaniasis. PLoS Pathog. 2010, 6, e1000871. [Google Scholar] [CrossRef]
- Kane, M.M.; Mosser, D.M. Leishmania parasites and their ploys to disrupt macrophage activation. Curr. Opin. Hematol. 2000, 7, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Aderem, A. Phagocytosis and the inflammatory response. J. Infect. Dis. 2003, 187 (Suppl. S2), S340–S345. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Vlassara, H.; Edelson, P.J.; Cerami, A. Leishmania promastigotes are recognized by the macrophage receptor for advanced glycosylation endproducts. J. Exp. Med. 1987, 165, 140–145. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edelson, P.J. The third component of complement (C3) is responsible for the intracellular survival of Leishmania major. Nature 1987, 327, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Wyler, D.J.; Sypek, J.P.; McDonald, J.A. In vitro parasite-monocyte interactions in human leishmaniasis: Possible role of fibronectin in parasite attachment. Infect. Immun. 1985, 49, 305–311. [Google Scholar] [CrossRef] [Green Version]
- Brittingham, A.; Chen, G.; McGwire, B.S.; Chang, K.P.; Mosser, D.M. Interaction of Leishmania gp63 with cellular receptors for fibronectin. Infect. Immun. 1999, 67, 4477–4484. [Google Scholar] [CrossRef] [Green Version]
- Vannier-Santos, M.A.; Saraiva, E.M.; Martiny, A.; Neves, A.; de Souza, W. Fibronectin shedding by Leishmania may influence the parasite-macrophage interaction. Eur. J. Cell Biol. 1992, 59, 389–397. [Google Scholar] [PubMed]
- Marth, T.; Kelsall, B.L. Regulation of interleukin-12 by complement receptor 3 signaling. J. Exp. Med. 1997, 185, 1987–1995. [Google Scholar] [CrossRef] [Green Version]
- Brittingham, A.; Mosser, D.M. Exploitation of the complement system by Leishmania promastigotes. Parasitol. Today 1996, 12, 444–447. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hoffmann, K.F.; Mendez, S.; Kamhawi, S.; Udey, M.C.; Wynn, T.A.; Sacks, D.L. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J. Exp. Med. 2001, 194, 1497–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belkaid, Y.; Piccirillo, C.A.; Mendez, S.; Shevach, E.M.; Sacks, D.L. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 2002, 420, 502–507. [Google Scholar] [CrossRef]
- Kane, M.M.; Mosser, D.M. The role of IL-10 in promoting disease progression in leishmaniasis. J. Immunol. 2001, 166, 1141–1147. [Google Scholar] [CrossRef] [Green Version]
- Miles, S.A.; Conrad, S.M.; Alves, R.G.; Jeronimo, S.M.; Mosser, D.M. A role for IgG immune complexes during infection with the intracellular pathogen Leishmania. J. Exp. Med. 2005, 201, 747–754. [Google Scholar] [CrossRef]
- Kima, P.E.; Constant, S.L.; Hannum, L.; Colmenares, M.; Lee, K.S.; Haberman, A.M.; Shlomchik, M.J.; McMahon-Pratt, D. Internalization of Leishmania mexicana complex amastigotes via the Fc receptor is required to sustain infection in murine cutaneous leishmaniasis. J. Exp. Med. 2000, 191, 1063–1068. [Google Scholar] [CrossRef] [Green Version]
- Von Stebut, E.; Belkaid, Y.; Jakob, T.; Sacks, D.L.; Udey, M.C. Uptake of Leishmania major amastigotes results in activation and interleukin 12 release from murine skin-derived dendritic cells: Implications for the initiation of anti-Leishmania immunity. J. Exp. Med. 1998, 188, 1547–1552. [Google Scholar] [CrossRef] [Green Version]
- Woelbing, F.; Kostka, S.L.; Moelle, K.; Belkaid, Y.; Sunderkoetter, C.; Verbeek, S.; Waisman, A.; Nigg, A.P.; Knop, J.; Udey, M.C.; et al. Uptake of Leishmania major by dendritic cells is mediated by Fcgamma receptors and facilitates acquisition of protective immunity. J. Exp. Med. 2006, 203, 177–188. [Google Scholar] [CrossRef] [Green Version]
- Ueno, N.; Wilson, M.E. Receptor-mediated phagocytosis of Leishmania: Implications for intracellular survival. Trends Parasitol. 2012, 28, 335–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Uzonna, J.E. The early interaction of Leishmania with macrophages and dendritic cells and its influence on the host immune response. Front. Cell. Infect. Microbiol. 2012, 2, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faria, M.S.; Reis, F.C.; Lima, A.P. Toll-like receptors in leishmania infections: Guardians or promoters? J. Parasitol. Res. 2012, 2012, 930257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallego, C.; Golenbock, D.; Gomez, M.A.; Saravia, N.G. Toll-like receptors participate in macrophage activation and intracellular control of Leishmania (Viannia) panamensis. Infect. Immun. 2011, 79, 2871–2879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komai-Koma, M.; Li, D.; Wang, E.; Vaughan, D.; Xu, D. Anti-Toll-like receptor 2 and 4 antibodies suppress inflammatory response in mice. Immunology 2014, 143, 354–362. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Inchaustegui, D.A.; Tai, W.; Xin, L.; Hogg, A.E.; Corry, D.B.; Soong, L. Distinct roles for MyD88 and Toll-like receptor 2 during Leishmania braziliensis infection in mice. Infect. Immun. 2009, 77, 2948–2956. [Google Scholar] [CrossRef] [Green Version]
- Carneiro, P.P.; Dórea, A.S.; Oliveira, W.N.; Guimarães, L.H.; Brodskyn, C.; Carvalho, E.M.; Bacellar, O. Blockade of TLR2 and TLR4 Attenuates Inflammatory Response and Parasite Load in Cutaneous Leishmaniasis. Front. Immunol. 2021, 12, 706510. [Google Scholar] [CrossRef]
- Abou Fakher, F.H.; Rachinel, N.; Klimczak, M.; Louis, J.; Doyen, N. TLR9-dependent activation of dendritic cells by DNA from Leishmania major favors Th1 cell development and the resolution of lesions. J. Immunol. 2009, 182, 1386–1396. [Google Scholar] [CrossRef] [Green Version]
- Liese, J.; Schleicher, U.; Bogdan, C. TLR9 signaling is essential for the innate NK cell response in murine cutaneous leishmaniasis. Eur. J. Immunol. 2007, 37, 3424–3434. [Google Scholar] [CrossRef]
- Walker, D.M.; Oghumu, S.; Gupta, G.; McGwire, B.S.; Drew, M.E.; Satoskar, A.R. Mechanisms of cellular invasion by intracellular parasites. Cell Mol. Life Sci. 2014, 71, 1245–1263. [Google Scholar] [CrossRef] [Green Version]
- McConville, M.J.; de Souza, D.; Saunders, E.; Likic, V.A.; Naderer, T. Living in a phagolysosome; metabolism of Leishmania amastigotes. Trends Parasitol. 2007, 23, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, G.; Chaudhuri, M.; Pan, A.; Chang, K.P. Surface acid proteinase (gp63) of Leishmania mexicana. A metalloenzyme capable of protecting liposome-encapsulated proteins from phagolysosomal degradation by macrophages. J. Biol. Chem. 1989, 264, 7483–7489. [Google Scholar] [CrossRef]
- Isnard, A.; Shio, M.T.; Olivier, M. Impact of Leishmania metalloprotease GP63 on macrophage signaling. Front. Cell. Infect. Microbiol. 2012, 2, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, N.E.; Chang, H.K.; Wilson, M.E. Novel program of macrophage gene expression induced by phagocytosis of Leishmania chagasi. Infect. Immun. 2004, 72, 2111–2122. [Google Scholar] [CrossRef] [Green Version]
- Pandey, S.P.; Doyen, N.; Mishra, G.C.; Saha, B.; Chandel, H.S. TLR9-deficiency reduces TLR1, TLR2 and TLR3 expressions in Leishmania major-infected macrophages. Exp. Parasitol. 2015, 154, 82–86. [Google Scholar] [CrossRef]
- Buates, S.; Matlashewski, G. General suppression of macrophage gene expression during Leishmania donovani infection. J. Immunol. 2001, 166, 3416–3422. [Google Scholar] [CrossRef] [Green Version]
- Dillon, L.A.; Okrah, K.; Hughitt, V.K.; Suresh, R.; Li, Y.; Fernandes, M.C.; Belew, A.T.; Corrada Bravo, H.; Mosser, D.M.; El-Sayed, N.M. Transcriptomic profiling of gene expression and RNA processing during Leishmania major differentiation. Nucleic Acids Res. 2015, 43, 6799–6813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalavi, K.; Jorjani, O.; Faghihi, M.A.; Mowla, S.J. Cytokine Gene Expression Alterations in Human Macrophages Infected by. Cell J. 2021, 22, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Chaussabel, D.; Semnani, R.T.; McDowell, M.A.; Sacks, D.; Sher, A.; Nutman, T.B. Unique gene expression profiles of human macrophages and dendritic cells to phylogenetically distinct parasites. Blood 2003, 102, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Shadab, M.; Das, S.; Banerjee, A.; Sinha, R.; Asad, M.; Kamran, M.; Maji, M.; Jha, B.; Deepthi, M.; Kumar, M.; et al. RNA-Seq revealed expression of many novel genes associated with Leishmania donovani persistence and clearance in the host macrophage. Front. Cell. Infect. Microbiol. 2019, 9, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osorio y Fortéa, J.; de La Llave, E.; Regnault, B.; Coppée, J.Y.; Milon, G.; Lang, T.; Prina, E. Transcriptional signatures of BALB/c mouse macrophages housing multiplying Leishmania amazonensis amastigotes. BMC Genom. 2009, 10, 119. [Google Scholar] [CrossRef] [Green Version]
- Orikaza, C.M.; Pessoa, C.C.; Paladino, F.V.; Florentino, P.T.V.; Barbiéri, C.L.; Goto, H.; Ramos-Sanchez, E.M.; Franco da Silveira, J.; Rabinovitch, M.; Mortara, R.A.; et al. Dual Host-Intracellular Parasite Transcriptome of Enucleated Cells Hosting Leishmania amazonensis: Control of Half-Life of Host Cell Transcripts by the Parasite. Infect. Immun. 2020, 88, e00261-20. [Google Scholar] [CrossRef] [PubMed]
- Probst, C.M.; Silva, R.A.; Menezes, J.P.; Almeida, T.F.; Gomes, I.N.; Dallabona, A.C.; Ozaki, L.S.; Buck, G.A.; Pavoni, D.P.; Krieger, M.A.; et al. A comparison of two distinct murine macrophage gene expression profiles in response to Leishmania amazonensis infection. BMC Microbiol. 2012, 12, 22. [Google Scholar] [CrossRef] [Green Version]
- Lapara, N.J.; Kelly, B.L. Suppression of LPS-induced inflammatory responses in macrophages infected with Leishmania. J. Inflamm. 2010, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Arango Duque, G.; Descoteaux, A. Leishmania survival in the macrophage: Where the ends justify the means. Curr. Opin. Microbiol. 2015, 26, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Cortez, M.; Huynh, C.; Fernandes, M.C.; Kennedy, K.A.; Aderem, A.; Andrews, N.W. Leishmania promotes its own virulence by inducing expression of the host immune inhibitory ligand CD200. Cell Host Microbe 2011, 9, 463–471. [Google Scholar] [CrossRef] [Green Version]
- Buxbaum, L.U. A detrimental role for IgG and FcgammaR in Leishmania mexicana infection. Immunol. Res. 2008, 42, 197–209. [Google Scholar] [CrossRef]
- Wanasen, N.; Xin, L.; Soong, L. Pathogenic role of B cells and antibodies in murine Leishmania amazonensis infection. Int. J. Parasitol. 2008, 38, 417–429. [Google Scholar] [CrossRef] [Green Version]
- Filardy, A.A.; Pires, D.R.; Nunes, M.P.; Takiya, C.M.; Freire-de-Lima, C.G.; Ribeiro-Gomes, F.L.; DosReis, G.A. Proinflammatory clearance of apoptotic neutrophils induces an IL-12(low)IL-10(high) regulatory phenotype in macrophages. J. Immunol. 2010, 185, 2044–2050. [Google Scholar] [CrossRef] [Green Version]
- Bosque, F.; Saravia, N.G.; Valderrama, L.; Milon, G. Distinct innate and acquired immune responses to Leishmania in putative susceptible and resistant human populations endemically exposed to L. (Viannia) panamensis infection. Scand. J. Immunol. 2000, 51, 533–541. [Google Scholar] [CrossRef] [Green Version]
- Robledo, S.; Wozencraft, A.; Valencia, A.Z.; Saravia, N. Human monocyte infection by Leishmania (Viannia) panamensis. Role of complement receptors and correlation of susceptibility in vitro with clinical phenotype. J. Immunol. 1994, 152, 1265–1276. [Google Scholar]
- Bosque, F.; Milon, G.; Valderrama, L.; Saravia, N.G. Permissiveness of human monocytes and monocyte-derived macrophages to infection by promastigotes of Leishmania (Viannia) panamensis. J. Parasitol. 1998, 84, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Loría-Cervera, E.N.; Andrade-Narvaez, F. The role of monocytes/macrophages in Leishmania infection: A glance at the human response. Acta Trop. 2020, 207, 105456. [Google Scholar] [CrossRef] [PubMed]
- Gantt, K.R.; Goldman, T.L.; McCormick, M.L.; Miller, M.A.; Jeronimo, S.M.; Nascimento, E.T.; Britigan, B.E.; Wilson, M.E. Oxidative responses of human and murine macrophages during phagocytosis of Leishmania chagasi. J. Immunol. 2001, 167, 893–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deschacht, M.; Van Assche, T.; Hendrickx, S.; Bult, H.; Maes, L.; Cos, P. Role of oxidative stress and apoptosis in the cellular response of murine macrophages upon Leishmania infection. Parasitology 2012, 139, 1429–1437. [Google Scholar] [CrossRef]
- Furtado, R.R.; Soares, D.C.; Prado, A.F.; Farias, L.H.S.; Da Silva, B.J.M.; Rodrigues, A.P.D.; Silva, E.O. Constitutive nitric oxide synthase-like enzyme in two species involved in cutaneous and mucocutaneous leishmaniasis. Parasitol. Int. 2021, 83, 102347. [Google Scholar] [CrossRef]
- Coso, S.; Harrison, I.; Harrison, C.B.; Vinh, A.; Sobey, C.G.; Drummond, G.R.; Williams, E.D.; Selemidis, S. NADPH oxidases as regulators of tumor angiogenesis: Current and emerging concepts. Antioxid. Redox Signal. 2012, 16, 1229–1247. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Wang, L.; Du, J.; Li, Y.; Yang, H.; Li, C.; Li, H.; Hu, H. Lipid raft localization of epidermal growth factor receptor alters matrix metalloproteinase-1 expression in SiHa cells via the MAPK/ERK signaling pathway. Oncol. Lett. 2016, 12, 4991–4998. [Google Scholar] [CrossRef] [Green Version]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef] [Green Version]
- Alexandre, J.; Batteux, F.; Nicco, C.; Chéreau, C.; Laurent, A.; Guillevin, L.; Weill, B.; Goldwasser, F. Accumulation of hydrogen peroxide is an early and crucial step for paclitaxel-induced cancer cell death both in vitro and in vivo. Int. J. Cancer 2006, 119, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Conde de la Rosa, L.; Schoemaker, M.H.; Vrenken, T.E.; Buist-Homan, M.; Havinga, R.; Jansen, P.L.; Moshage, H. Superoxide anions and hydrogen peroxide induce hepatocyte death by different mechanisms: Involvement of JNK and ERK MAP kinases. J. Hepatol. 2006, 44, 918–929. [Google Scholar] [CrossRef] [PubMed]
- Rayner, B.S.; Duong, T.T.; Myers, S.J.; Witting, P.K. Protective effect of a synthetic anti-oxidant on neuronal cell apoptosis resulting from experimental hypoxia re-oxygenation injury. J. Neurochem. 2006, 97, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Nacy, C.A.; Groves, M.G. Macrophages in resistance to rickettsial infections: Early host defense mechanisms in experimental scrub typhus. Infect. Immun. 1981, 31, 1239–1250. [Google Scholar] [CrossRef] [Green Version]
- Matheoud, D.; Moradin, N.; Bellemare-Pelletier, A.; Shio, M.T.; Hong, W.J.; Olivier, M.; Gagnon, E.; Desjardins, M.; Descoteaux, A. Leishmania evades host immunity by inhibiting antigen cross-presentation through direct cleavage of the SNARE VAMP8. Cell Host Microbe 2013, 14, 15–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goncalves, R.; Zhang, X.; Cohen, H.; Debrabant, A.; Mosser, D.M. Platelet activation attracts a subpopulation of effector monocytes to sites of Leishmania major infection. J. Exp. Med. 2011, 208, 1253–1265. [Google Scholar] [CrossRef]
- Novais, F.O.; Nguyen, B.T.; Beiting, D.P.; Carvalho, L.P.; Glennie, N.D.; Passos, S.; Carvalho, E.M.; Scott, P. Human classical monocytes control the intracellular stage of Leishmania braziliensis by reactive oxygen species. J. Infect. Dis. 2014, 209, 1288–1296. [Google Scholar] [CrossRef]
- Assreuy, J.; Cunha, F.Q.; Epperlein, M.; Noronha-Dutra, A.; O’Donnell, C.A.; Liew, F.Y.; Moncada, S. Production of nitric oxide and superoxide by activated macrophages and killing of Leishmania major. Eur. J. Immunol. 1994, 24, 672–676. [Google Scholar] [CrossRef]
- Carneiro, P.P.; Conceição, J.; Macedo, M.; Magalhães, V.; Carvalho, E.M.; Bacellar, O. The Role of Nitric Oxide and Reactive Oxygen Species in the Killing of Leishmania braziliensis by Monocytes from Patients with Cutaneous Leishmaniasis. PLoS ONE 2016, 11, e0148084. [Google Scholar] [CrossRef] [Green Version]
- Blos, M.; Schleicher, U.; Soares Rocha, F.J.; Meissner, U.; Röllinghoff, M.; Bogdan, C. Organ-specific and stage-dependent control of Leishmania major infection by inducible nitric oxide synthase and phagocyte NADPH oxidase. Eur. J. Immunol. 2003, 33, 1224–1234. [Google Scholar] [CrossRef]
- Srivastav, S.; Basu Ball, W.; Gupta, P.; Giri, J.; Ukil, A.; Das, P.K. Leishmania donovani prevents oxidative burst-mediated apoptosis of host macrophages through selective induction of suppressors of cytokine signaling (SOCS) proteins. J. Biol. Chem. 2014, 289, 1092–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa-Franco, J.; Araújo-Mendes, E.; Silva-Jardim, I.; L-Santos, J.; Faria, D.R.; Dutra, W.O.; Horta, M.F. Infection-induced respiratory burst in BALB/c macrophages kills Leishmania guyanensis amastigotes through apoptosis: Possible involvement in resistance to cutaneous leishmaniasis. Microbes Infect. 2006, 8, 390–400. [Google Scholar] [CrossRef]
- Linares, E.; Giorgio, S.; Augusto, O. Inhibition of in vivo leishmanicidal mechanisms by tempol: Nitric oxide down-regulation and oxidant scavenging. Free Radic. Biol. Med. 2008, 44, 1668–1676. [Google Scholar] [CrossRef]
- Mukbel, R.M.; Patten, C.; Gibson, K.; Ghosh, M.; Petersen, C.; Jones, D.E. Macrophage killing of Leishmania amazonensis amastigotes requires both nitric oxide and superoxide. Am. J. Trop. Med. Hyg. 2007, 76, 669–675. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa-da-Silva, A.C.; Nascimento, D.d.O.; Ferreira, J.R.M.; Guimarães-Pinto, K.; Freire-de-Lima, L.; Morrot, A.; Decote-Ricardo, D.; Filardy, A.A.; Freire-de-Lima, C.G. Immune Responses in Leishmaniasis: An Overview. Trop. Med. Infect. Dis. 2022, 7, 54. https://doi.org/10.3390/tropicalmed7040054
Costa-da-Silva AC, Nascimento DdO, Ferreira JRM, Guimarães-Pinto K, Freire-de-Lima L, Morrot A, Decote-Ricardo D, Filardy AA, Freire-de-Lima CG. Immune Responses in Leishmaniasis: An Overview. Tropical Medicine and Infectious Disease. 2022; 7(4):54. https://doi.org/10.3390/tropicalmed7040054
Chicago/Turabian StyleCosta-da-Silva, Ana Caroline, Danielle de Oliveira Nascimento, Jesuino R. M. Ferreira, Kamila Guimarães-Pinto, Leonardo Freire-de-Lima, Alexandre Morrot, Debora Decote-Ricardo, Alessandra Almeida Filardy, and Celio Geraldo Freire-de-Lima. 2022. "Immune Responses in Leishmaniasis: An Overview" Tropical Medicine and Infectious Disease 7, no. 4: 54. https://doi.org/10.3390/tropicalmed7040054
APA StyleCosta-da-Silva, A. C., Nascimento, D. d. O., Ferreira, J. R. M., Guimarães-Pinto, K., Freire-de-Lima, L., Morrot, A., Decote-Ricardo, D., Filardy, A. A., & Freire-de-Lima, C. G. (2022). Immune Responses in Leishmaniasis: An Overview. Tropical Medicine and Infectious Disease, 7(4), 54. https://doi.org/10.3390/tropicalmed7040054









