Dengue Meteorological Determinants during Epidemic and Non-Epidemic Periods in Taiwan
Abstract
1. Introduction
2. Materials and Methods
2.1. Surveillance Data
2.2. Meteorological Data
2.3. Statistics Analysis
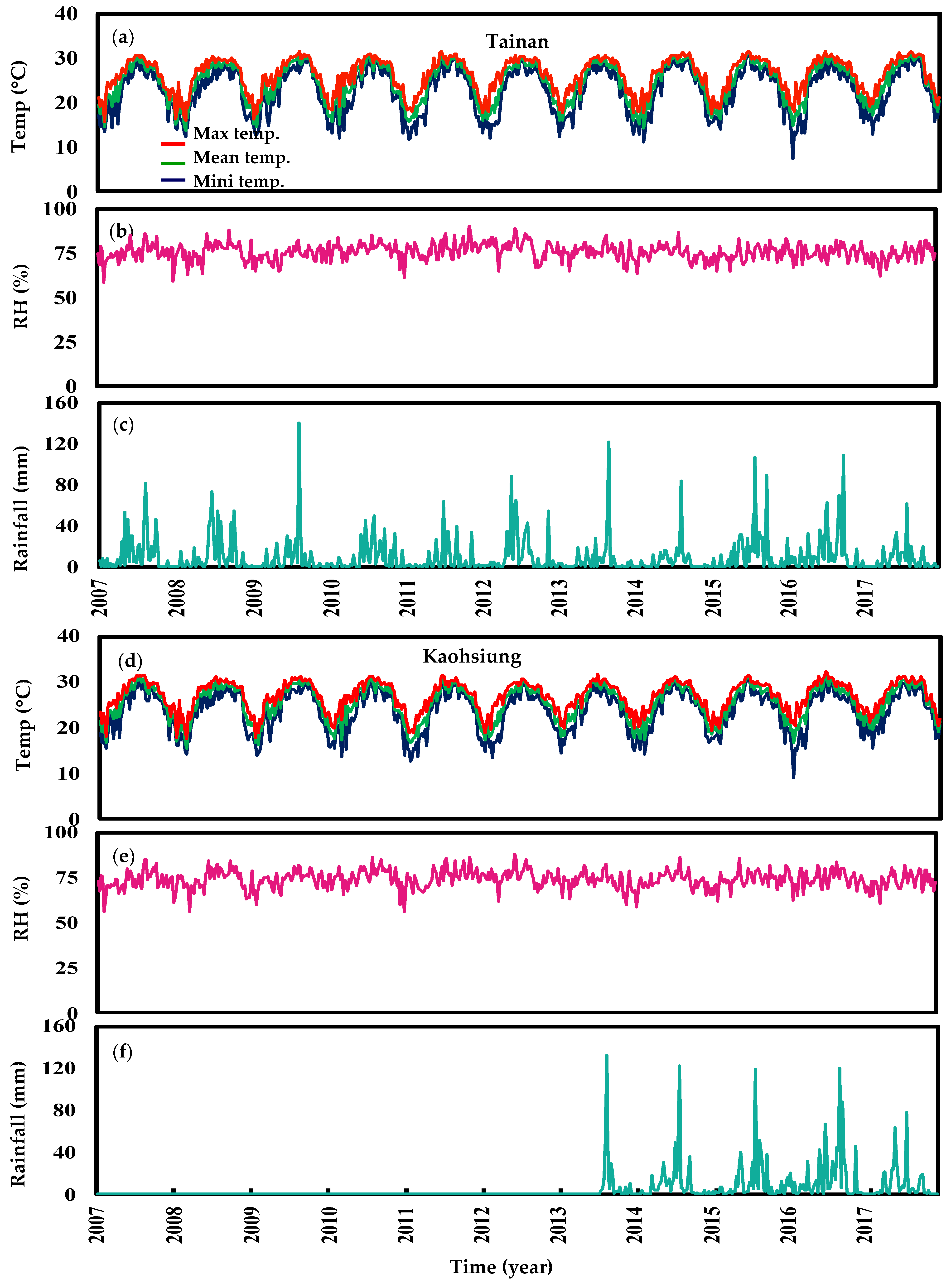
3. Results
3.1. Data Description
3.2. Trend Analysis
3.3. Lagged-Time Poisson Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Stanaway, J.D.; Shepard, D.S.; Undurraga, E.A.; Halasa, Y.A.; Coffeng, L.E.; Brady, O.J.; Hay, S.I.; Bedi, N.; Bensenor, I.M.; Castañeda-Orjuela, C.A.; et al. The global burden of dengue: An analysis from the Global Burden of Disease Study 2013. Lancet Infect. Dis. 2016, 16, 712–723. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Dengue and Severe Dengue; World Health Organization (WHO): Geneva, Switzerland, 2022; Available online: https://www.who.int/health-topics/dengue-and-severe-dengue#tab=tab_1 (accessed on 9 July 2021).
- Li, Y.; Dou, Q.; Lu, Y.; Xiang, H.; Yu, X.; Liu, S. Effects of ambient temperature and precipitation on the risk of dengue fever: A systematic review and updated meta-analysis. Environ. Res. 2020, 191, 110043. [Google Scholar] [CrossRef] [PubMed]
- Akter, R.; Hu, W.; Gatton, M.; Bambrick, H.; Naish, S.; Tong, S. Different responses of dengue to weather variability across climate zones in Queensland, Australia. Environ. Res. 2020, 184, 109222. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, T.; Lin, H.; Zhu, G.; Zeng, W.; Li, X.; Zhang, B.; Song, T.; Deng, A.; Zhang, M.; et al. Weather variables and the El Niño Southern Oscillation may drive the epidemics of dengue in Guangdong Province, China. Sci. Total Environ. 2018, 624, 926–934. [Google Scholar] [CrossRef]
- Chuang, T.W.; Chaves, L.F.; Chen, P.J. Effects of local and regional climatic fluctuations on dengue outbreaks in southern Taiwan. PLoS ONE 2017, 12, e0178698. [Google Scholar] [CrossRef]
- Lee, H.S.; Nguyen-Viet, H.; Nam, V.S.; Lee, M.; Won, S.; Duc, P.P.; Grace, D. Seasonal patterns of dengue fever and associated climate factors in 4 provinces in Vietnam from 1994 to 2013. BMC Infect. Dis. 2017, 17, 218. [Google Scholar] [CrossRef]
- Guzman, M.G.; Harris, E. Dengue. Lancet 2015, 385, 453–465. [Google Scholar] [CrossRef]
- Morin, C.W.; Comrie, A.C.; Ernst, K. Climate and dengue transmission: Evidence and implications. Environ. Health Perspect. 2013, 121, 1264–1272. [Google Scholar] [CrossRef]
- Liu, X.; Liu, K.; Yue, Y.; Wu, H.; Yang, S.; Guo, Y.; Ren, D.; Zhao, N.; Yang, J.; Liu, Q. Determination of factors affecting dengue occurrence in representative areas of China: A principal component regression analysis. Front. Public Health 2021, 8, 603872. [Google Scholar] [CrossRef]
- Tuladhar, R.; Singh, A.; Varma, A.; Choudhary, D.K. Climatic factors influencing dengue incidence in an epidemic area of Nepal. BMC Res. Notes 2019, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Focks, D.A.; Barrera, R. Dengue Transmission Dynamics: Assessment and Implications for Control; World Health Organization on behalf of the Special Programme for Research and Training in Tropical Diseases: Geneva, Switzerland, 2007. [Google Scholar]
- Zhao, X.; Chen, F.; Feng, Z.; Li, X.; Zhou, X.H. Characterizing the effect of temperature fluctuation on the incidence of malaria: An epidemiological study in south-west China using the varying coefficient distributed lag non-linear model. Malar. J. 2014, 13, 192. [Google Scholar] [CrossRef]
- Lambrechts, L.; Paaijmans, K.P.; Fansiri, T.; Carrington, L.B.; Kramer, L.D.; Thomas, M.B.; Scott, T.W. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc. Natl. Acad. Sci. USA 2011, 108, 7460–7465. [Google Scholar] [CrossRef] [PubMed]
- Paaijmans, K.P.; Read, A.F.; Thomas, M.B. Understanding the link between malaria risk and climate. Proc. Natl. Acad. Sci. USA 2009, 106, 13844–13849. [Google Scholar] [CrossRef]
- Colón-González, F.J.; Fezzi, C.; Lake, I.R.; Hunter, P.R. The effects of weather and climate change on dengue. PLoS Negl. Trop. Dis. 2013, 7, e2503. [Google Scholar] [CrossRef]
- Wu, P.C.; Guo, H.R.; Lung, S.C.; Lin, C.Y.; Su, H.J. Weather as an effective predictor for occurrence of dengue fever in Taiwan. Acta Trop. 2007, 103, 50–57. [Google Scholar] [CrossRef]
- Yang, C.F.; Hou, J.N.; Chen, T.H.; Chen, W.J. Discriminable roles of Aedes aegypti and Aedes albopictus in establishment of dengue outbreaks in Taiwan. Acta Trop. 2014, 130, 17–23. [Google Scholar] [CrossRef]
- Taiwan Center for Disease Control. Taiwan National Infectious Disease Statistics System for Dengue Virus; Taiwan Center for Disease Control: Taipei, Taiwan, 2015. Available online: https://nidss.cdc.gov.tw/nndss/DiseaseMap?id=061 (accessed on 9 July 2021).
- Lee, J.C.; Cia, C.T.; Lee, N.Y.; Ko, N.Y.; Chen, P.L.; Ko, W.C. Causes of death among dengue patients causes of death among hospitalized adults with dengue fever in Tainan, 2015: Emphasis on cardiac events and bacterial infections. J. Microbiol. Immunol. Infect. 2022, 55, 207–214. [Google Scholar] [CrossRef]
- Wang, S.F.; Chang, K.; Loh, E.W.; Wang, W.H.; Tseng, S.P.; Lu, P.L.; Chen, Y.H.; Chen, Y.M.A. Consecutive large dengue outbreaks in Taiwan in 2014–2015. Emerg. Microbes Infect. 2016, 5, e123. [Google Scholar] [CrossRef]
- Wang, S.F.; Wang, W.H.; Chang, K.; Chen, Y.H.; Tseng, S.P.; Yen, C.H.; Wu, D.C.; Chen, Y.M.A. Severe Dengue Fever Outbreak in Taiwan. Am. J. Trop. Med. Hyg. 2016, 94, 193. [Google Scholar] [CrossRef]
- Wang, W.-H.; Lin, C.-Y.; Chang, K.; Urbina, A.N.; Assavalapsakul, W.; Thitithanyanont, A.; Lu, P.-L.; Chen, Y.-H.; Wang, S.-F. A clinical and epidemiological survey of the largest dengue outbreak in Southern Taiwan in 2015. Int. J. Infect. Dis. 2019, 88, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.-J.; Liu, C.-K.; Tsai, W.-Y.; Liu, L.-T.; Tyson, J.; Tsai, C.-Y.; Lin, P.-C.; Wang, W.-K. Seroprevalence of dengue virus in two districts of Kaohsiung City after the largest dengue outbreak in Taiwan since World War II. PLoS Negl. Trop. Dis. 2018, 12, e0006879. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.C.; Hsieh, C.L.; Lu, C.Y. Trend and geographic analysis of the prevalence of dengue in Taiwan, 2010–2015. Int. J. Infect. Dis. 2017, 54, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Hsieh, Y.C.; Chen, C.J.; Lin, T.Y.; Huang, Y.C. Retrospective Seroepidemiology study of dengue virus infection in Taiwan. BMC Infect. Dis. 2021, 21, 96. [Google Scholar] [CrossRef]
- Chen, S.C.; Liao, C.M.; Chio, C.P.; Chou, H.H.; You, S.H.; Cheng, Y.H. Lagged temperature effect with mosquito transmission potential explains dengue variability in southern Taiwan: Insights from a statistical analysis. Sci. Total Environ. 2010, 408, 4069–4075. [Google Scholar] [CrossRef]
- Chien, L.C.; Yu, H.L. Impact of meteorological factors on the spatiotemporal patterns of dengue fever incidence. Environ. Int. 2014, 73, 46–56. [Google Scholar] [CrossRef]
- Jain, R.; Sontisirikit, S.; Iamsirithaworn, S.; Prendinger, H. Pridection of dengue outbreaks based on disease surveillance, meteorological nad socio-economic data. BMC Infect. Dis. 2019, 19, 272. [Google Scholar] [CrossRef]
- Yuan, H.Y.; Liang, J.; Lin, P.S.; Sucipto, K.; Tsegaye, M.M.; Wen, T.H.; Pfeiffer, S.; Pfeiffer, D. The effects of seasonal climate variability on dengue annual incidence in Hong Kong: A modelling study. Sci. Rep. 2020, 10, 4297. [Google Scholar] [CrossRef]
- Taiwan Center of Disease Control. Available online: https://nidss.cdc.gov.tw/Home/Index?op=4 (accessed on 9 July 2021).
- Taiwan Environmental Protection Agency. Available online: https://www.epa.gov.tw/ (accessed on 9 July 2021).
- Central Weather Bureau Typhoon Database. Available online: https://rdc28.cwb.gov.tw/TDB/public/warning_typhoon_list/ (accessed on 9 July 2021).
- Lu, L.; Lin, H.; Tian, L.; Yang, W.; Sun, J.; Liu, Q. Time series analysis of dengue fever and weather in Guangzhou, China. BMC Public Health 2009, 9, 395. [Google Scholar] [CrossRef]
- Pan, W. Akaike’s information criterion in generalized estimating equations. Biometrics 2001, 57, 120–125. [Google Scholar] [CrossRef]
- Promprou, S.; Jaroensutasinee, M.; Jaroensutasinee, K. Climatic factors affecting dengue haemorrhagic fever incidence in southern Thailand. Dengue Bull. 2005, 29, 41–48. [Google Scholar]
- Wiwanitkit, V. An observation on correlation between rainfall and the prevalence of clinical cases of dengue in Thailand. J. Vector Borne Dis. 2006, 43, 73–76. [Google Scholar]
- Pham, H.V.; Doan, H.T.; Phan, T.T.; Minh, N.N. Ecological factors associated with dengue fever in a Central Highlands province, Vietnam. BMC Infect. Dis. 2011, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Lin, C.Y.; Yang, C.Y.; Chan, T.C.; Chiang, P.H.; Chen, Y.H. Relationship between the incidence of dengue virus transmission in traditional market and climatic conditions in Kaohsiung city. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 9916642. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.Y.; Wen, T.H.; Kung, Y.H.; Tsou, H.H.; Chen, C.H.; Chen, L.W.; Lin, P.S. Prediction of annual dengue incidence by hydro-climatic extremes for southern Taiwan. Int. J. Biometeorol. 2019, 63, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.F.; Chang, K.; Lu, R.W.; Wang, W.H.; Chen, Y.H.; Chen, M.; Wu, D.C.; Chen, Y.M.A. Large Dengue virus type 1 outbreak in Taiwan. Emerg. Microbes Infect. 2015, 4, e46. [Google Scholar] [CrossRef] [PubMed]
- Lwin, M.O.; Vijaykumar, S.; Fernando, O.N.N.; Cheong, S.A.; Rathnayake, V.S.; Lim, G.; Theng, Y.-L.; Chaudhuri, S.; Foo, S. A 21st century approach to tackling dengue: Crowdsourced surveillance, predictive mapping and tailored communication. Acta Trop. 2014, 130, 100–107. [Google Scholar] [CrossRef]
- Lai, L.W. Influence of environmental conditions on asynchronous outbreaks of dengue disease and increasing vector population in Kaohsiung, Taiwan. Int. J. Environ. Health Res. 2011, 21, 133–146. [Google Scholar] [CrossRef]
- National Science and Technology Center for Disaster Reduction. 2021. Available online: https://den.ncdr.nat.gov.tw/1132/1188/ (accessed on 9 July 2021).
- Reiter, P.; Lathrop, S.; Bunning, M.; Biggerstaff, B.; Singer, D.; Tiwari, T.; Baber, L.; Amador, M.; Thirion, J.; Hayes, J.; et al. Texas lifestyle limits transmission of dengue virus. Emerg. Infect. Dis. 2003, 9, 86–89. [Google Scholar] [CrossRef]
- Gubler, D.J. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 1998, 11, 480–496. [Google Scholar] [CrossRef]



| Non-Epidemic Periods | Epidemic Periods | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time-Lag (Months) | Mean Temp. (°C) | Max. Temp. (°C) | Min. Temp. (°C) | Rainfall (mm) | RH (%) | Time-Lag (Months) | Mean Temp. (°C) | Max. Temp. (°C) | Min. Temp. (°C) | Rainfall (mm) | RH (%) |
| Tainan | |||||||||||
| 0 | 0.07 | 0.05 | 0.05 | 4 × 10−5 | 0.08 | 0.16 | 0.16 | 0.17 | 0.08 | 0.09 | |
| 1 | 0.30 | 0.26 | 0.28 | 0.15 | 0.23 | 1 | 0.21 | 0.22 | 0.20 | 0.46 | 0.19 |
| 2 | 0.49 | 0.44 | 0.49 | 0.48 | 0.41 | 2 | 0.24 | 0.22 | 0.25 | 0.21 | 0.07 |
| 3 | 0.55 | 0.52 | 0.56 | 0.56 | 0.34 | 3 | 0.25 | 0.22 | 0.29 | 0.005 | 0.02 |
| 4 | 0.56 | 0.54 | 0.56 | 0.41 | 0.17 | 4 | 0.16 | 0.15 | 0.16 | 0.02 | 0.17 |
| 5 | 0.44 | 0.45 | 0.44 | 0.20 | 5 | 0.05 | 0.07 | 0.03 | |||
| 6 | 0.21 | 0.24 | 0.21 | 6 | 0.01 | 0.0002 | 0.04 | ||||
| Kaohsiung | |||||||||||
| 0 | 0.0027 | 0.01 | 1 × 10−5 | 0.04 | 0.04 | 0.0016 | 0.0002 | 0.004 | 0.04 | 0.0002 | |
| 1 | 0.14 | 0.11 | 0.17 | 0.01 | 0.12 | 1 | 0.06 | 0.04 | 0.07 | 0.001 | 0.02 |
| 2 | 0.39 | 0.34 | 0.41 | 0.20 | 0.30 | 2 | 0.20 | 0.19 | 0.20 | 0.14 | 0.16 |
| 3 | 0.53 | 0.48 | 0.54 | 0.51 | 0.47 | 3 | 0.31 | 0.30 | 0.31 | 0.55 | 0.35 |
| 4 | 0.63 | 0.59 | 0.64 | 0.53 | 0.28 | 4 | 0.48 | 0.44 | 0.49 | 0.28 | 0.30 |
| 5 | 0.64 | 0.62 | 0.64 | 0.40 | 5 | 0.58 | 0.54 | 0.61 | |||
| 6 | 0.51 | 0.53 | 0.49 | 6 | 0.42 | 0.42 | 0.40 | ||||
| Tainan | Non-Epidemic Periods | Tainan | Epidemic Periods | ||
|---|---|---|---|---|---|
| β | p | β | p | ||
| Mini temp. (Lag 3) b | 0.1737 | *** | Mini temp. (Lag 4) | 0.2474 | ** |
| Mean temp. (Lag 5) | 0.1041 | ** | Mini temp. (Lag 5) | −0.2164 | *** |
| Max. temp. | 0.1396 | *** | Max. temp. (Lag 2) | 0.8400 | *** |
| RH | 0.1099 | * | Max. temp. (Lag 3) | 0.8289 | *** |
| RH (Lag 2) | −0.0699 | *** | RH | −0.0822 | *** |
| Rainfall | −0.0928 | *** | RH (Lag 1) | 0.1360 | *** |
| Rainfall (Lag 2) | 0.0542 | *** | RH (Lag 4) | −0.1221 | *** |
| Rainfall (Lag 1) | 0.0335 | *** | |||
| Rainfall (Lag 3) | −0.0404 | *** | |||
| QICu | 63.17 | QICu | −8.49 × 10−3 | ||
| Kaohsiung | Non-Epidemic Periods | Kaohsiung | Epidemic Periods | ||
| β | p | β | p | ||
| Mini temp. (Lag 1) | 0.6896 | *** | Mini temp. (Lag 5) | 0.3832 | *** |
| Mini temp. (Lag 2) | −0.8118 | *** | RH (Lag 1) | −0.0723 | *** |
| Mean temp. (Lag 1) | −0.7499 | *** | Rainfall (Lag 1) | 0.0127 | *** |
| Mean temp. (Lag 2) | 2.6743 | *** | Rainfall (Lag 2) | 0.0139 | *** |
| Mean temp. (Lag 4) | 0.1532 | * | |||
| Mean temp. (Lag 5) | −0.1665 | ** | |||
| Mean temp. (Lag 6) | 0.1678 | *** | |||
| Max. temp. (Lag 2) | −1.8695 | *** | |||
| Rainfall (Lag 3) | 0.0122 | * | |||
| QICu | 69.24 | QICu | −3116.30 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, S.-H.; Chen, S.-C.; Huang, Y.-H.; Tsai, H.-C. Dengue Meteorological Determinants during Epidemic and Non-Epidemic Periods in Taiwan. Trop. Med. Infect. Dis. 2022, 7, 408. https://doi.org/10.3390/tropicalmed7120408
You S-H, Chen S-C, Huang Y-H, Tsai H-C. Dengue Meteorological Determinants during Epidemic and Non-Epidemic Periods in Taiwan. Tropical Medicine and Infectious Disease. 2022; 7(12):408. https://doi.org/10.3390/tropicalmed7120408
Chicago/Turabian StyleYou, Shu-Han, Szu-Chieh Chen, Yi-Han Huang, and Hsin-Chieh Tsai. 2022. "Dengue Meteorological Determinants during Epidemic and Non-Epidemic Periods in Taiwan" Tropical Medicine and Infectious Disease 7, no. 12: 408. https://doi.org/10.3390/tropicalmed7120408
APA StyleYou, S.-H., Chen, S.-C., Huang, Y.-H., & Tsai, H.-C. (2022). Dengue Meteorological Determinants during Epidemic and Non-Epidemic Periods in Taiwan. Tropical Medicine and Infectious Disease, 7(12), 408. https://doi.org/10.3390/tropicalmed7120408






