Abstract
Background: Tahyna orthobunyavirus (TAHV) is a neglected mosquito-borne bunyavirus. Although the virus is widespread in continental Europe, TAHV infections are rarely reported. We analyzed the prevalence of TAHV in humans and different animal species as well as mosquitoes collected in urban areas of Zagreb and its surroundings in the period from 2020 to 2022. Methods: The study included 32 patients with neuroinvasive disease (NID), 218 asymptomatic individuals, 98 horses, 94 pet animals (dogs and cats), and 4456 Aedes vexans mosquitoes. Cerebrospinal fluid (CSF) and urine samples of patients with NID were tested for the TAHV RNA using a real-time reverse transcription-polymerase chain reaction (RT-qPCR). Human and animal serum samples were tested for TAHV-neutralizing (NT) antibodies using a virus-neutralization test (VNT). Mosquito pools were tested for TAHV RNA using an RT-qPCR. Results: TAHV NT antibodies were detected in 3/9.4% of patients with NID, 8/3.7% of asymptomatic individuals, 29/29.6% of horses, and 11/11.7% of pet animals. There was no difference in the seroprevalence according to age, sex, and area of residence in asymptomatic individuals. In addition, TAHV seropositivity did not differ according to age and sex in pet animals. None of the tested mosquito pools was TAHV RNA-positive. Conclusions: The presented results highlight the importance of interdisciplinary surveillance (“One Health”) of this neglected viral zoonosis.
1. Introduction
Tahyna orthobunyavirus (TAHV) is an arthropod-borne virus of the family Peribunyaviridae, genus Orthobunyavirus, California encephalitis serogroup [1]. Hares, rabbits, hedgehogs, and rodents are vertebrate hosts for TAHV in a natural cycle, while floodwater mosquitoes Aedes vexans are the primary arthropod vectors. Humans represent only incidental or dead-end hosts for TAHV [2].
In humans, TAHV infections are typically asymptomatic. Symptomatic disease is usually presented as an influenza-like illness occurring in late summer and early autumn, mainly in children [3]. Fever, gastrointestinal disorders, atypical pneumonia, or myocarditis are the most common symptoms of TAHV infection [4]. Despite its association with neurovirulence (meningitis), TAHV infection is still an underdiagnosed disease, with only a few human clinical cases reported in recent decades [5].
TAHV is widespread throughout continental Europe, as evidenced by the virus detection and isolation from mosquitoes as well as the detection of antibodies in humans and animals [6,7,8]. High seroprevalence rates of up to 60–80% have been consistently observed among adult human populations of endemic foci, such as in the Czech Republic [9].
In addition, although transmission is focal and not uniform, seroprevalence studies conducted in wild ungulates in Austria, Hungary, and Romania have revealed that TAHV transmission to animals is widespread in Europe, particularly among wild boars, with a mean seroconversion rate of 15% [8]. In some high-endemic countries, such as the Czech Republic, TAHV antibodies were detected in various animal species with different seroprevalence rates: horses 34.4%, pigs 55%, cattle 5.6%, songbirds 14.0%, cormorants 22.6%, ducks 13.2%, mouflons 33.8%, deer 36.4–40.0%, wild boar 70.0%, and European hares 42.5% [9,10,11,12,13,14].
The presence of TAHV in mosquitoes (virus isolation or RNA detection) was confirmed in many countries including the Czech Republic [15], Slovakia [16], Italy [17], Austria [5,18], Russia [19], and China [20]. After the first isolation in Slovakia in 1958, TAHV was detected in several mosquito species, including Ae. caspius, Ae. cinereus, Ae. dorsalis, Ae. cantans, Anopheles hyrcanus, Ae. punctor, Ae. communis, Ae. flavescens, Ae. excrucians, Oc. detritus and Culex pipiens, Cx. modestus, and Culiseta annulata [5,7,20,21,22].
In Croatia, serologic evidence of TAHV dates back to the 1970s. Very rare seroprevalence studies showed TAHV antibodies in 7.9% of inhabitants of northeast Croatia and 0.2–1.47% of inhabitants of the Croatian littoral [23,24]. Only one study (1984–1988) detected TAHV hemagglutination-inhibiting antibodies in the serum samples of free-ranging European brown bears (Ursus arctos) collected within the Plitvice Lakes and Risnjak National Parks [25]. A more recently conducted Croatian study detected TAHV neutralizing (NT) antibodies in 10.1% of patients with unsolved neuroinvasive disease (NID) tested from 2017 to 2021, who developed symptoms during the arbovirus transmission season. In two patients presenting with meningitis, NT antibodies were also confirmed in the cerebrospinal fluid (CSF), suggesting a recent TAHV infection. The majority of seropositive patients (90.9%) were residents of floodplains along the rivers in continental Croatia; however, sporadic infections were also confirmed in the coastal region [26].
The aim of this study was to analyze the prevalence of TAHV in humans and different animal species as well as the virus detection in mosquitoes collected from urban areas of Zagreb and its surroundings.
2. Materials and Methods
2.1. Study Area
The study was conducted during a three-year period (May 2020–July 2022) and included patients with NID, asymptomatic individuals (seroprevalence investigation), pet animals (dogs, cats), horses, and mosquitoes. The sampling area included Zagreb, the capital of Croatia, and its surroundings. Zagreb is located in the northwest of the country on the banks of the river Sava (GPS coordinates 45°48′55.43″ N, 15°57′59.64″ E) at an elevation of approximately 112 m above sea level. The city is subdivided into 17 districts, of which the majority are located in the River Sava valley, at a low level. The city’s core region is densely built, while the northern part is situated on the Medvednica mountain’s slopes, with forest vegetation and smaller urban settlements. Agricultural lands dominate the eastern, southern, and western parts of the city area. The surface waterways abound in the city’s vicinity. Seven artificial lakes and several artificial watercourses are located in the city area.
Thirty-two mosquito species have been detected in the city of Zagreb. The species Cx. pipiens form molestus predominates in indoor breeding sites. Natural mosquito breeding sites in forested and flooded areas are active only in the spring (March–June), and the most common species are Ae. sticticus, Ae. cantans, Ae. vexans, and Ae. geniculatus. Culex pipiens is the most frequent mosquito species found in streams, while Ae. albopictus and Cx. pipiens are the most common mosquito species in artificial breeding sites [27,28].
The sampling area (human and animal sampling) in northwestern Croatia is presented in Figure 1.
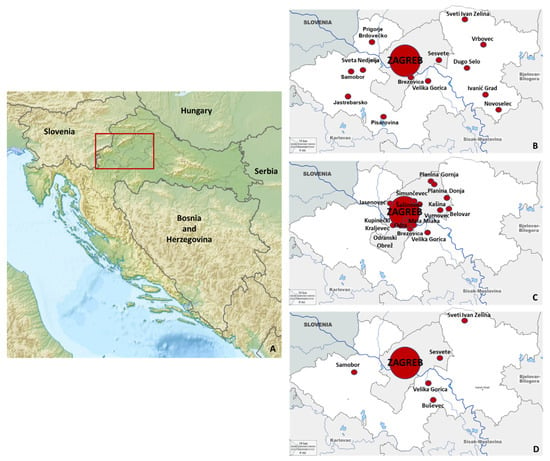
Figure 1.
Sampling area in continental Croatia (A): human (B), horse (C), and pet animals (D) sampling areas.
2.2. Human Sampling and Testing
A total of 32 hospitalized patients presented with NID (febrile headache, meningitis, meningoencephalitis) were tested. Serum, CSF, and urine samples were collected from all patients. In addition, to determine the seroprevalence, a total of 218 serum samples from asymptomatic individuals were tested for the presence of TAHV NT antibodies. Samples were collected from patients during a routine check-up (part of physical examination, prior to surgery, prenatal testing, couples undergoing medically assisted reproduction). No participant reported a recent febrile disease.
CSF and urine samples of patients with NID were tested for the presence of TAHV RNA using a real-time reverse transcription polymerase chain reaction (RT-qPCR). The following primers and probes were used: forward primer: 5′-CCATTCCGTTAGGATCTTCTTCCT-3′, reverse primer 5′-CCTTCCTCTCCGGCTTACG-3′ and probe: FAM-5′-AATGCCGCAAAAGCCAAAGCTGC-3′-TAMRA [29].
TAHV antibodies were detected using a virus-neutralization test (VNT). UVE/TAHV/1958/CS/92 virus strain grown in Vero E6 cells was used as an antigen for the VNT. Virus titer (median tissue culture infectious dose; TCID50) was calculated using the Reed and Muench formula. Serum samples were heat-inactivated (30 min/56 °C) and diluted two-fold starting at 1:5. An equal amount of inactivated serum dilutions and 100 TCID50 of TAHV (25 µL) were mixed and incubated for one hour at 37 °C with CO2. Finally, 50 µL of 2 × 105 Vero E6 cells/mL were added to each well. The plates were incubated at 37 °C with CO2 and inspected for the cytopathic effect after incubation for three days. NT antibody titer was defined as the reciprocal value of the highest serum dilution that showed at least 50% neutralization. Serum samples with neutralizing activity at dilutions ≥ 1:10 were considered seropositive [26].
2.3. Animal Sampling and Testing
Horses and pet animals (cats and dogs) were the animal species included in the study. Animal samples included 98 horse serum samples, 70 dog serum samples, and 24 cat serum samples from Zagreb and its surroundings. Sex and age were available for pet animals, while for horses, data were missing. TAHV VNT was performed as described above.
2.4. Mosquito Sampling and Testing
Mosquitoes were collected by two methods: CDC Mini Light traps (BioQuip, Products, Rancho Dominguez, CA, USA), and aspirator collection (human landing collection). CDC Mini Light traps were equipped with dry ice (CO2) as an attractant and used to collect adult mosquitoes. Traps were placed in the late afternoon before sunset, left overnight, and removed after sunrise (07:00–10:00). Over three years (2020–2022), the traps were set at the same eight collection sites (Figure 2, yellow marks) every 14 days, from May to October in 2020 and 2021 and from May to July in 2022. A total of 248 sampling occasions were gathered (2020: n = 96; 2021: n = 88; 2022: n = 64). Additionally, the sampling occasions using the CDC Mini Light trap were conducted once at three collection sites (Figure 2, green marks). During the same period, mosquito individuals were collected by aspirator as well as using the human landing collection method (Figure 2, blue dots). A total of 30 sampling occasions with Ae. vexans mosquitoes were gathered (2020: n = 18; 2021: n = 11; 2022: n = 1). Various habitats were selected for mosquito sampling: woods and gardens in the urban part of the city and populated areas close to the green belt.
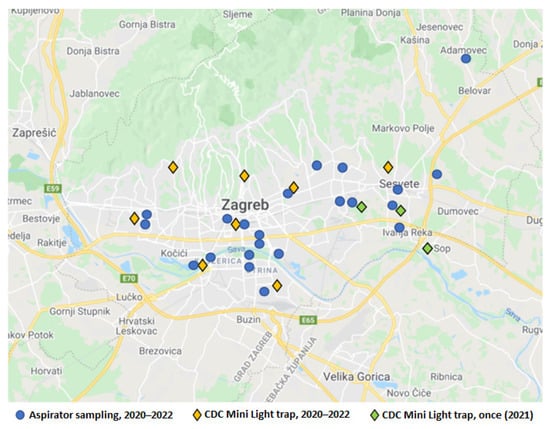
Figure 2.
Distribution of mosquito sampling locations.
The mosquitoes sampled by CDC Mini Light traps were transported to the laboratory in containers with dry ice, transferred to plastic tubes, and stored on dry ice until identification. Mosquitoes collected by the aspirator were transported alive to the laboratory in the aspirator, placed briefly in a freezer at −18 °C, and identified. Female mosquitoes were morphologically identified by species on a chilling surface under a stereomicroscope, using the determination keys by Becker et al. (2010) [30] and Schaffner et al. (2001) [31]. Specimens belonging to the same species collected on the same day and at the same sampling site were pooled, with up to 60 individuals per pool, and stored at −80 °C until virological testing. Only Ae. vexans individuals were tested.
Mosquito pools were tested for the presence of TAHV RNA as described above.
2.5. Statistical Analysis
The differences in seropositivity rates according to sex, age, and area of residence were compared using a chi-square or Fisher’s exact test. The strength of the association between dependent (VNT positivity) and independent variables was assessed by logistic regression. p < 0.05 was considered statistically significant. Statistical analysis was performed using Stata version 16 software.
3. Results
TAHV seroprevalence results are presented in Table 1. In humans, TAHV NT antibodies were detected in 3/32 (9.4%) patients with the neuroinvasive disease and 8/218 (3.7%) asymptomatic individuals. In addition, 29/98 (29.6%) of horses and 11/94 (11.7%) of pet animals were found to be TAHV-seropositive.

Table 1.
Tahyna virus seroprevalence in Zagreb and the surrounding area.
In asymptomatic persons, the seroprevalence rate was highest in 70+ year-olds (7.7%) compared to 2.5–3.8% in other age groups; however, this difference was not significant. No significant difference in seropositivity was found between males and females (3.7% vs. 3.6%) as well as among residents of urban and suburban areas (4.2% vs. 0%) (Table 2).

Table 2.
Tahyna virus seroprevalence in asymptomatic individuals according to demographic characteristics.
Results of the risk analysis in asymptomatic humans showed no association of TAHV seroprevalence with age, sex, and area of residence (Table 3).

Table 3.
Risk analysis for Tahyna virus seropositivity.
In pet animals, there was no difference in the prevalence of TAHV NT antibodies according to age and sex (Table 4).

Table 4.
Seroprevalence of Tahyna virus in pet animals according to sex and age.
The geographic distribution of seropositive humans and animals is presented in Figure 3. All seropositive humans were residents of urban areas. Among seropositive horses, 9 were from Zagreb city, and 20 were from suburban areas of Zagreb surroundings. In a group of pet animals, only one dog was from a suburban area.
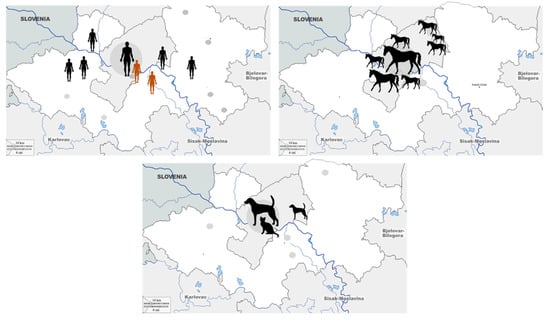
Figure 3.
Geographic distribution of Tahyna virus seropositive humans, horses, and pet animals. (symbol size represents the number of cases).
Comparing the TAHV NT antibody titers, a substantially higher titer was observed in horses (median 80, IQR = 40–160) compared to humans (median 10, IQR = 10–40) and pet animals (median 10, IQR = 10–10) (Figure 4).
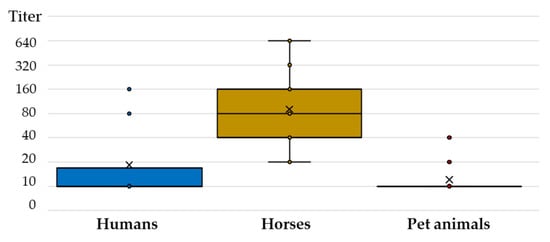
Figure 4.
Tahyna virus-neutralizing antibody titers in humans and animals.
A total of 4456 Ae. vexans mosquitoes were collected and identified in the Zagreb area, of which 4053/90.9% mosquitoes were sampled by traps with CO2, while 403/9.1% were sampled by the aspirator. Mosquito specimens were sorted in 159 pools and tested for the presence of TAHV (Table 5). All tested pools were negative for TAHV.

Table 5.
Number of collected Aedes vexans mosquitoes/pools tested for Tahyna virus.
4. Discussion
Although TAHV is widely distributed in Europe, the number of clinical cases as well as the seroprevalence rates are probably underreported due to the lack of commercially available testing.
The frequency of TAHV NT antibody detection among patients with neuroinvasive disease from Zagreb and its surroundings was similar (9.4%) to a previous Croatian study (10.1%) conducted in patients from both continental and coastal regions. Only one published study, which was from the Sverdlovsk region (Russia) in 1994, analyzed the prevalence of TAHV in patients with encephalitis and found that up to 60% of patients had TAHV antibodies [32]. Therefore, the true prevalence and clinical significance of this neglected virus remain to be determined.
The TAHV seroprevalence rate in asymptomatic Croatian individuals was 3.7%, which was lower than the reported seroprevalence of 7.9% in the eastern region in the 1970s [23]. This difference should be explained at least in part by the studied population. This study included only inhabitants of a restricted urban area in northwest Croatia, while in the 1970s, individuals from a broad geographic area (northeastern continental and middle and south coastal area) were tested. However, the reported seroprevalence in this study was similar to the seroprevalence reported in some urban areas in Europe and Asia. In 2002, residents of an area in the Czech Republic affected by the flood were tested for the presence of antibodies to TAHV. While the highest seroprevalence rates of up to 28% were detected in rural and suburban populations of the forested floodplain along the Labe and Vltava Rivers, low seropositivity of 5% was found in the urban Prague area [33]. Two more recent studies showed lower TAHV seropositivity rates. A seroprevalence of 2% was recorded in Nasiriyah, the capital of the Dhi Qar Governorate in Southern Iraq, in 2012–2013 [34] In addition, TAHV NT antibodies were detected in 0.3% of blood donors from the Alpine Central European region of the Tyrol (North and East Tyrol, Austria, and South Tyrol, northern Italy) [18].
Outside Europe, very high TAHV NT seroprevalence rates ranging from 25% to 52% were recorded in the rural adult population of Cameroon in 2002–2003 [35]. In addition, epidemiological investigations showed high TAHV endemicity in both urban and rural areas in the Lao PDR. Several studies carried out in the communities of the Nakai plateau revealed a TAHV seropositivity of 30.45% in 2007 and 29.06% in 2010 using ELISA [36]. In patients presenting with fever with or without rash, the prevalence of TAHV antibodies was 13% using indirect immunofluorescence in an urban Kashi region, Xinjiang Province, China (2007) [37]. In the Lao PDR, TAHV antibodies were found in 37.7% of young patients under the age of 18 in 2015 [36].
The older age groups had a higher probability of infection and a higher prevalence of antibodies, and such a trend is usually seen in people and animals living in long-term enzootic regions [33]. Interestingly, in a Chinese study conducted at three locations in the Qinghai-Tibet Plateau, all seropositive individuals were under the 30-age group, with seroprevalence ranging from 1.1% to 4.3%. The authors supposed that higher seroprevalence in young age groups may suggest that TAHV was recently imported into the Qinghai-Tibet Plateau [21]. In the present study, no significant difference in seropositivity was found among age groups (2.5–7.7%).
Several studies have shown that TAHV circulates in wildlife in Central and Eastern Europe although seroconversion rates may vary by location and year. Several studies from the Czech Republic showed a declining trend in the TAHV seroprevalence among wild boars. The hemagglutination-inhibiting antibodies were found in 41.7% and 46.7% of wild boars in 1990 and 1993–1997, respectively [13,14]. A lower seropositivity rate of 19.4% was detected in the wild boars sampled at 24 hunting grounds of the Břeclav District (South Moravia) from 2000 to 2002. All these regions are characterized by the presence of wetland/fishpond ecosystems or floodplain forests as well as large mosquito populations [38]. In addition, TAHV NT antibodies were found in 28.9% of examined wild boars in Záhorská Lowland, western Slovakia, in the 1970s [39]. During 2016 and 2017, serum samples from wild boar (Sus scrofa), roe deer (Capreolus capreolus), and red deer (Cervus elaphus) were collected from Austria, Hungary, and Romania. In the wild boar population, TAHV NT seroprevalence rates were reported to be 25.9–27.5% in Austria, 0–55.6% in Romania, and 0–50.0% in Hungary. In addition, the seroprevalence in Austria was highest in wild boar (26.7%) compared to red deer (9.8%), while no roe deer were found seropositive [8].
TAHV seroprevalence in horses from urban areas of Zagreb and its surroundings tested in this study was 29.6%. This high seropositivity rate was similar to those detected in some endemic areas in the Czech Republic. In South Moravia (Břeclav District), a seroprevalence of 34.4% was reported in 1980. In addition, a very high seroprevalence rate of 55% was observed in pigs, while it was low in cattle (5.6%) [9]. In 2007, serum samples were collected from livestock (cows, sheep, and swine) at the abattoirs in Geermu City, Xining City, and Minhe County, China. In addition to IgG seropositivity of 6.7% in cows, 10.0% in sheep, and 3.3% in swine, IgM antibodies were detected in 3.3% of cows, 7.8% of sheep, and 5.0% of swine [21].
In this study, the TAHV seroprevalence rate in dogs and cats was 11.7%, with no difference according to sex and age. To our knowledge, there are no published data on the prevalence of TAHV in pet animals. In addition, there is no research on the clinical impact of TAHV infection in horses and pet animals. The results of this study clearly show that TAHV is circulating in selected animal species. There was additional reasoning for serosurvey of pet animals and horses. Compared to wildlife, serum samples are easier to collect. Moreover, unlike wildlife and other farm animals, these species are living in urban areas. Dogs and cats may live in close proximity to their owners and therefore have shared exposure to household and recreational risk factors. Finally, dogs and cats are scavengers, and pathogens bioaccumulate in them. The listed attributes make animal species selected for this study potential sentinel animals to monitor TAHV activity in cities. In analyzing the NT titers in humans and animals, substantially higher titers were observed in horses compared to humans and pet animals. Although there are no data regarding the longevity of TAHV antibody response in horses, it is still to be determined if higher titer makes them more sensitive tools for TAHV surveillance.
Many studies reported the detection of TAHV in mosquitoes. In 2006, TAHV was isolated from Culex spp. mosquitoes collected in Xinjiang, China [37]. Subsequently, in 2007–2008), TAHV was isolated from Ae. dorsalis and Cx. modestus pools in Inner Mongolia [20]. In 2013, the virus was isolated from An. hyrcanus mosquitoes collected on the fishponds in South Moravia (Czech Republic), a finding that represents the first isolation of TAHV from An. hyrcanus in Europe [7]. In a more recent study (2019), mosquitoes collected at floodplain habitats along three major rivers in eastern Austria, i.e., the Danube River, the Morava River, and the Leitha River, were tested. TAHV RNA was detected in two pools of Ae. vexans collected on the Leitha River. Phylogenetic analysis showed that the sequences obtained were remarkably similar to earlier TAHV isolates from the area, dating back to the initial TAHV isolate in 1958 [5]. In a very recently conducted study (2021), TAHV was detected in Ae. caspius and Cx. pipiens pools collected in the Emilia Romagna Region, Northern Italy. In addition, one isolated strain was obtained from one of the Ae. caspius pool collected in the municipality of Comacchio. Furthermore, TAHV was detected in 10 mosquito pools sampled in 2009, 2010, and 2020, confirming the continuous presence of the virus in this region [22]. In the present study, all tested Ae. vexans pools collected in the Zagreb area were negative for TAHV RNA.
When comparing the seropositivity rates between countries, it is important to keep in mind that the different serological methods (ELISA, IFA, and VNT) used to detect TAHV antibodies may have an impact on the seroprevalence results. In addition, there are some limitations of this study that should be noted. Since a small number of animals were included in the study, the seroprevalence results should be interpreted with caution. In addition, data on the sex and age of the horses were missing.
5. Conclusions
The presented results indicate the circulation of TAHV in northwestern Croatia. Further studies on large samples of humans, animals, and mosquitoes are needed to determine the prevalence of this neglected viral zoonosis.
Author Contributions
Conceptualization, V.S. (Vladimir Stevanovic) and T.V.-C.; methodology, V.S. (Vladimir Stevanovic), V.S. (Vladimir Savic), A.K. and S.K.; formal analysis, A.K., N.d.A.S. and S.K.; investigation, A.K., M.C.P., S.P., M.B., M.S. and V.T.; writing—original draft preparation, V.S., T.V.-C. and A.K.; writing—review and editing, V.S. (Vladimir Stevanovic); visualization, T.V.-C.; supervision, L.B.; funding acquisition, T.V.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Croatian Science Foundation, grant number IP-2016-06-7456: Prevalence and molecular epidemiology of emerging and re-emerging neuroinvasive arboviral infections in Croatia; CRONEUROARBO (to T.V.-C.) and the European Virus Archive goes Global (EVAg) project that has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No. 653316.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committees of the Croatian Institute of Public Health (protocol code 80-1092/1-16, approved on 3 June 2016) and the University Hospital for Infectious Diseases, “Dr. Fran Mihaljevic”, Zagreb (protocol code 01-1347-5-2018, approved on 13 September 2018). The animal study protocol was approved by the Ethics Committee of the Faculty of Veterinary Medicine, University of Zagreb (decision number: 640-01/20-02/12, approved on 18 December 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- ICTV. Genus: Orthobunyavirus. Available online: https://talk.ictvonline.org/ictv-reports/ictv_online_report/negative-sense-rna-viruses/w/peribunyaviridae/1238/genus-orthobunyavirus (accessed on 10 September 2022).
- Hubálek, Z. Mosquito-borne viruses in Europe. Parasitol. Res. 2008, 103 (Suppl. S1), S29–S43. [Google Scholar] [CrossRef] [PubMed]
- Bárdos, V.; Medek, M.; Kania, V.; Hubálek, Z.; Juricova, Z. Das klinische Bild der Tahyna-Virus (California-Gruppe)–Infektionen bei Kindern [The clinical picture in Tahyna virus (California group) infections in children]. Padiatr. Grenzgeb. 1980, 19, 11–23. [Google Scholar] [PubMed]
- Xia, H.; Wang, Y.; Atoni, E.; Zhang, B.; Yuan, Z. Mosquito-Associated Viruses in China. Virol. Sin. 2018, 33, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Camp, J.V.; Kniha, E.; Obwaller, A.; Walochnik, J.; Nowotny, N. The transmission ecology of Tahyna orthobunyavirus in Austria as revealed by longitudinal mosquito sampling and blood meal analysis in floodplain habitats. Parasit. Vectors 2021, 14, 561. [Google Scholar] [CrossRef] [PubMed]
- Hubalek, Z.; Zeman, P.; Halouzka, J.; Juricova, Z.; Bálková, H.; Sikutová, S.; Rudolf, I. Antibodies against mosquito-borne viruses in human population of an area of Central Bohemia affected by the flood of 2002. Epidemiol. Mikrobiol. Imunol. 2004, 53, 112–120. [Google Scholar]
- Hubalek, Z.; Sebesta, O.; Pesko, J.; Betasova, L.; Blazejova, H.; Venclikova, K.; Rudolf, I. Isolation of Tahyna Virus (California Encephalitis Group) From Anopheles hyrcanus (Diptera, Culicidae), a Mosquito Species New to, and Expanding in, Central Europe. J. Med. Entomol. 2014, 51, 1264–1267. [Google Scholar] [CrossRef]
- Camp, J.V.; Haider, R.; Porea, D.; Oslobanu, L.E.; Forgách, P.; Nowotny, N. Serological surveillance for Tahyna virus (California encephalitis orthobunyavirus, Peribunyaviridae) neutralizing antibodies in wild ungulates in Austria, Hungary and Romania. Zoonoses Public Health 2018, 65, 459–463. [Google Scholar] [CrossRef]
- Hubálek, Z. History of Arbovirus Research in the Czech Republic. Viruses 2021, 13, 2334. [Google Scholar] [CrossRef]
- Juricová, Z.; Hubálek, Z.; Halouzka, J.; Sikutová, S. Serological examination of songbirds (Passeriformes) for mosquito-borne viruses Sindbis, Tahyna, and Batai in a south Moravian wetland (Czech Republic). Vector Borne Zoonotic Dis. 2009, 9, 295–299. [Google Scholar] [CrossRef]
- Ernek, E.; Kozuch, O.; Nosek, J.; Hudec, K.; Folk, C. Virus neutralizing antibodies to arboviruses in birds of the order Anseriformes in Czechoslovakia. Acta Virol. 1975, 19, 349–353. [Google Scholar]
- Juricová, Z.; Hubálek, Z.; Halouzka, J.; Machácek, P. Virologic detection of arboviruses in greater cormorants. Vet. Med. 1993, 38, 3759. [Google Scholar]
- Juricová, Z.; Hubálek, Z. Serological surveys for arboviruses in the game animals of southern Moravia (Czech Republic). Folia Zool. 1999, 48, 185–189. [Google Scholar]
- Juricova, Z. Antibodies to arboviruses in game animals in South Moravia (in Czech). Vet. Med. 1992, 37, 633–636. [Google Scholar]
- Hubálek, Z.; Rudolf, I.; Bakonyi, T.; Kazdová, K.; Halouzka, J.; Sebesta, O.; Sikutová, S.; Juricová, Z.; Nowotny, N. Mosquito (Diptera: Culicidae) surveillance for arboviruses in an area endemic for West Nile (Lineage Rabensburg) and Tahyna viruses in Central Europe. J. Med. Entomol. 2010, 47, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Danielová, V.; Málková, D.; Minár, J.; Rehse-Küpper, B.; Hájková, Z.; Halgos, J.; Jedlicka, L. Arbovirus isolations from mosquitoes in South Slovakia. Folia Parasitol. 1978, 25, 187–190. [Google Scholar]
- Calzolari, M.; Bonilauri, P.; Bellini, R.; Caimi, M.; Defilippo, F.; Maioli, G.; Albieri, A.; Medici, A.; Veronesi, R.; Pilani, R.; et al. Arboviral survey of mosquitoes in two northern Italian regions in 2007 and 2008. Vector Borne Zoonotic Dis. 2010, 10, 875–884. [Google Scholar] [CrossRef]
- Sonnleitner, S.T.; Lundström, J.; Baumgartner, R.; Simeoni, J.; Schennach, H.; Zelger, R.; Prader, A.; Schmutzhard, E.; Nowotny, N.; Walder, G. Investigations on California serogroup orthobunyaviruses in the Tyrols: First description of Tahyna virus in the Alps. Vector Borne Zoonotic Dis. 2014, 14, 272–277. [Google Scholar] [CrossRef]
- Nikiforova, M.A.; Kuznetsova, N.A.; Shchetinin, A.M.; Butenko, A.M.; Kozlova, A.A.; Larichev, V.P.; Vakalova, E.V.; Azarian, A.R.; Rubalsky, O.V.; Bashkina, O.A.; et al. Arboviruses in the Astrakhan region of Russia for 2018 season: The development of multiplex PCR assays and analysis of mosquitoes, ticks, and human blood sera. Infect. Genet. Evol. 2021, 88, 104711. [Google Scholar] [CrossRef]
- Cao, Y.; Fu, S.; Tian, Z.; Lu, Z.; He, Y.; Wang, H.; Wang, J.; Guo, W.; Tao, B.; Liang, G. Distribution of mosquitoes and mosquito-borne arboviruses in Inner Mongolia, China. Vector Borne Zoonotic Dis. 2011, 11, 157–781. [Google Scholar] [CrossRef]
- Li, W.J.; Wang, J.L.; Li, M.H.; Fu, S.H.; Wang, H.Y.; Wang, Z.Y.; Jiang, S.Y.; Wang, X.W.; Guo, P.; Zhao, S.C.; et al. Mosquitoes and mosquito-borne arboviruses in the Qinghai-Tibet Plateau--focused on the Qinghai area, China. Am. J. Trop. Med. Hyg. 2010, 82, 705–711. [Google Scholar] [CrossRef]
- Calzolari, M.; Bonilauri, P.; Grisendi, A.; Dalmonte, G.; Vismarra, A.; Lelli, D.; Chiapponi, C.; Bellini, R.; Lavazza, A.; Dottori, M. Arbovirus Screening in Mosquitoes in Emilia-Romagna (Italy, 2021) and Isolation of Tahyna Virus. Microbiol. Spectr. 2022, 27, e0158722. [Google Scholar] [CrossRef] [PubMed]
- Vesenjak-Hirjan, J.; Galinović-Weisglass, M.; Urlić, V.; Bendiš, M.; Miović, P.; Vujošević, N.; Vuksanović, P. Occurrence of arboviruses in the Middle and the South Adriatic (Yugoslavia). In Arboviruses in the Mediterranean Countries; Vesenjak-Hirjan, J., Ed.; Gustav Fischer Verlag: Stuttgart, Germany; New York, NY, USA, 1980; pp. 303–310. [Google Scholar]
- Turković, B.; Brudnjak, Z. Arboviruses in Croatia. Acta Med. Croat. 1998, 52, 87–89. [Google Scholar]
- Madić, J.; Huber, D.; Lugović, B. Serologic survey for selected viral and rickettsial agents of brown bears (Ursus arctos) in Croatia. J. Wildl. Dis. 1993, 29, 572–576. [Google Scholar] [CrossRef]
- Vilibic-Cavlek, T.; Stevanovic, V.; Savic, V.; Markelic, D.; Sabadi, D.; Bogdanic, M.; Kovac, S.; Santini, M.; Tabain, I.; Potocnik-Hunjadi, T.; et al. Detection of Tahyna Orthobunyavirus-Neutralizing Antibodies in Patients with Neuroinvasive Disease in Croatia. Microorganisms 2022, 10, 1443. [Google Scholar] [CrossRef] [PubMed]
- Klobucar, A.; Benic, N.; Krajcar, D.; Kosanovic-Licina, M.L.; Tesic, V.; Merdic, E.; Vrucina, I.; Savic, V.; Barbic, L.; Stevanovic, V.; et al. An overview of mosquitoes and emerging arboviral infections in the Zagreb area, Croatia. J. Infect. Dev. Ctries. 2016, 10, 1286–1293. [Google Scholar] [CrossRef]
- Klobučar, A.; Lipovac, I.; Žagar, N.; Mitrović-Hamzić, S.; Tešić, V.; Vilibić-Čavlek, T.; Merdić, E. First record and spreading of the invasive mosquito Aedes japonicus japonicus (Theobald, 1901) in Croatia. Med. Vet. Entomol. 2019, 33, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cao, Y.X.; He, X.X.; Fu, S.H.; Lyu, Z.; He, Y.; Gao, X.Y.; Liang, G.D.; Wang, H.Y. Real-time RT-PCR Assay for the detection of Tahyna Virus. Biomed. Environ. Sci. 2015, 28, 374–377. [Google Scholar] [CrossRef]
- Becker, N.; Petric, D.; Zgomba, M.; Boase, C.; Dahl, C.; Madon, M.; Kaiser, A. Mosquito and Their Control, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2010; p. 577. [Google Scholar]
- Schaffner, F.; Angel, G.; Geoffroy, B.; Hervy, J.P.; Rhaiem, A.; Brunhes, J. The Mosquitoes of Europe—An Identification and Training Programme; CD Rom, IRD Editions & EID Méditerranée: Montpellier, France, 2001. [Google Scholar]
- Glinskikh, N.P.; Fedotova, T.T.; Pereskokova, I.G.; Melnikov, V.G.; Volkova, L.I. The potentials for the comprehensive diagnosis of viral encephalitis in Sverdlovsk Province. Vopr. Virusol. 1994, 39, 190–191. [Google Scholar]
- Hubálek, Z.; Zeman, P.; Halouzka, J.; Juricová, Z.; Stovicková, E.; Bálková, H.; Sikutová, S.; Rudolf, I. Mosquitoborne viruses, Czech Republic, 2002. Emerg. Infect. Dis. 2005, 11, 116–118. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.M.; Smura, T.; Kuivanen, S.; Huhtamo, E.; Kurkela, S.; Putkuri, N.; Hasony, H.J.; Al-Hello, H.; Vapalahti, O. The Presence and Seroprevalence of Arthropod-Borne Viruses in Nasiriyah Governorate, Southern Iraq: A Cross-Sectional Study. Am. J. Trop. Med. Hyg. 2016, 94, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Kuniholm, M.H.; Wolfe, N.D.; Huang, C.Y.; Mpoudi-Ngole, E.; Tamoufe, U.; LeBreton, M.; Burke, D.S.; Gubler, D.J. Seroprevalence and distribution of Flaviviridae, Togaviridae, and Bunyaviridae arboviral infections in rural Cameroonian adults. Am. J. Trop. Med. Hyg. 2006, 74, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Arbovirus Surveillance Project. Institut Pasteur du Laos. Available online: https://www.pasteur.la/project-carried-on-in-thelab-2016-2017-lao-fr1/research-and-development/ (accessed on 30 July 2022).
- Lu, Z.; Lu, X.J.; Fu, S.H.; Zhang, S.; Li, Z.X.; Yao, X.H.; Feng, Y.P.; Lambert, A.J.; Ni, D.X.; Wang, F.T.; et al. Tahyna virus and human infection, China. Emerg. Infect. Dis. 2009, 15, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Halouzka, J.; Juricova, Z.; Jankova, J.; Hubalek, Z. Serologic survey of wild boars for mosquito-borne viruses in South Moravia (Czech Republic). Vet. Med. 2008, 53, 266–271. [Google Scholar] [CrossRef]
- Kozuch, O.; Nosek, J.; Gresikova, M.; Ernek, E. Surveillance of mosquito-borne natural focus in Zahorska Lowland, 115–118. In 2nd International Arbeitskolloquium Uber Die Naturherde von Infektionskrankheiten in Zentraleuropa; Sixl, W., Ed.; Hygiene Institut der Universitat: Graz, Austria, 1976. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).