Visceral Leishmaniasis after Anti-Interleukin 17A (IL-17A) Therapy in a Patient Affected by Psoriatic Arthritis
Abstract
1. Introduction
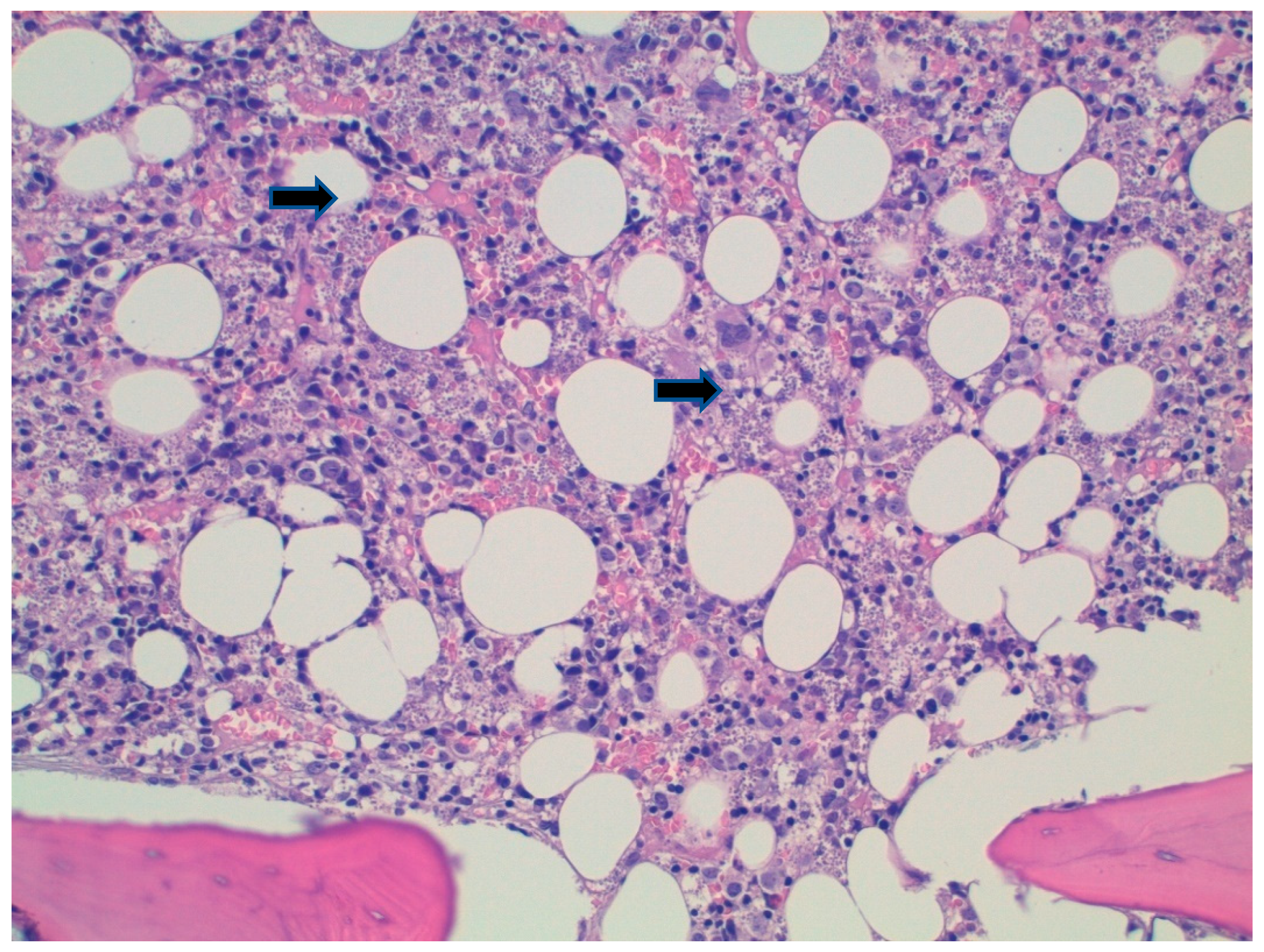
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blauvelt, A.; Chiricozzi, A. The Immunologic Role of IL-17 in Psoriasis and Psoriatic Arthritis Pathogenesis. Clin. Rev. Allergy Immunol. 2018, 55, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Keijsers, R.R.M.C.; Joosten, I.; van Erp, P.E.J.; Koenen, H.J.P.M.; van de Kerkhof, P.C.M. Cellular sources of IL-17 in psoriasis: A paradigm shift? Exp. Dermatol. 2014, 23, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Lande, R.; Botti, E.; Jandus, C.; Dojcinovic, D.; Fanelli, G.; Conrad, C.; Chamilos, G.; Feldmeyer, L.; Marinari, B.; Chon, S.; et al. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat. Commun. 2014, 5, 5621. [Google Scholar] [CrossRef] [PubMed]
- Pitta, M.G.; Romano, A.; Cabantous, S.; Henri, S.; Hammad, A.; Kouriba, B.; Argiro, L.; El Kheir, M.; Bucheton, B.; Mary, C.; et al. IL-17 and IL-22 are associated with protection against human kala azar caused by Leishmania donovani. J. Clin. Investig. 2009, 119, 2379–2387. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Bhattacharya, P.; Joshi, A.B.; Ismail, N.; Dey, R.; Nakhasi, H.L. Role of pro-inflammatory cytokine IL-17 in Leishmania pathogenesis and in protective immunity by Leishmania vaccines. Cell Immunol. 2016, 309, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.S.; Carregaro, V.; Lima-Júnior, D.S.; Costa, D.L.; Ryffel, B.; Duthie, M.S.; de Jesus, A.; de Almeida, R.P.; da Silva, J.S. Interleukin 17A acts synergistically with interferon gamma to promote protection against Leishmania infantum infection. J. Infect. Dis. 2015, 211, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Reserch 2017, 26, 750. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, G.; Rovaris, M.; Veraldi, S. Leishmaniasis: A Disease With Many Names. JAMA Dermatol. 2014, 150, 1204. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Regional Office for the Americas (WHO PAHO). Leishmaniasis. Washington: WHO PAHO. Available online: https://www.paho.org/hq/dmdocuments/2017/2017-cha-leishmaniasis-factsheet-work.pdf (accessed on 24 September 2022).
- Ferroglio, E.; Battisti, E.; Zanet, S.; Bolla, C.; Concialdi, E.; Trisciuoglio, A.; Khalili, S.; Biglino, A. Epidemiological evaluation of Leishmania infantum zoonotic transmission risk in the recently established endemic area of Northwestern Italy. Zoonoses Public Health 2018, 65, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Colomba, C.; Saporito, L.; Bonura, S.; Campisi, G.; Di Carlo, P.; Panzarella, V.; Caputo, V.; Cascio, A. Leishmania infection in psoriasis. J. Infect. 2020, 80, 578–606. [Google Scholar] [CrossRef] [PubMed]
- Pagliano, P.; Esposito, S. Visceral leishmaniosis in immunocompromised host: An update and literature review. J. Chemother. 2017, 29, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Lupia, T.; Corcione, S.; Boglione, L.; Cariti, G.; DE Rosa, F.G. Visceral leishmaniasis in a patient with active HBV/HDV co-infection. J. Infect. Public. Health 2020, 13, 306–308. [Google Scholar] [CrossRef] [PubMed]
- Ghoreschi, K.; Balato, A.; Enerbäck, C.; Sabat, R. Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet 2021, 397, 754–766. [Google Scholar] [CrossRef]
- O’Daly, J.A.; Lezama, R.; Gleason, J. Isolation of Leishmania amastigote protein fractions which induced lymphocyte stimulation and remission of psoriasis. Arch. Dermatol. Res. 2009, 301, 411–427. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-de-Albuquerque, S.D.C.; Pessoa-E-Silva, R.; Trajano-Silva, L.A.M.; de Goes, T.C.; de Morais, R.C.S.; da COliveira, C.N.; De Lorena, V.M.B.; De Paiva-Cavalcanti, M. The Equivocal role of Th17 cells and neutrophils on immunopathogenesis of leishmaniasis. Front. Immunol. 2017, 8, 1437. [Google Scholar] [CrossRef] [PubMed]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 Family of Cytokines in Health and Disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Chang, C.; Lu, Q. The inflammatory response in psoriasis: A comprehensive review. Clin. Rev. Allergy Immunol. 2016, 50, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Kurizky, P.S.; Gomes, C.M.; Cesetti, M.V.; Martins, G.A.; Regattieri, N.A.T.; Marianelli, F.F.; Sevilha Santos, L.; Medeiros Silva, V.; de Paula, N.A.; Frade, M.A.C.; et al. Cross-sectional screening study for Leishmania DNA and antibodies in biologic-treated patients with psoriasis living in an area endemic for leishmaniasis. Br. J. Dermatol. 2019, 81, 1337–1339. [Google Scholar] [CrossRef] [PubMed]
- Surveillance of Leishmaniasis in the WHO European Region, 2016 and Global Leishmaniasis Surveillance Update, 1998–2016. Available online: https://www.who.int/publications/i/item/who-wer9340 (accessed on 19 August 2022).
- Maritati, M.; Trentini, A.; Michel, G.; Bellini, T.; Almugadam, S.; Hanau, S.; Govoni, M.; Marty, P.; Contini, C. Subclinical Leishmania infection in patients with rheumatic diseases under biological drugs. Infection 2018, 46, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Akhoundi, M.; Downing, T.; Votýpka, J.; Kuhls, K.; Lukeš, J.; Cannet, A.; Ravel, C.; Marty, P.; Delaunay, P.; Kasbari, M.; et al. Leishmania infections: Molecular targets and diagnosis. Mol. Asp. Med. 2017, 57, 1–29. [Google Scholar] [CrossRef] [PubMed]

| Day 0 | Day 5 | Day 10 | Day 38 | 1 Month PT | 3 Months PT | |
|---|---|---|---|---|---|---|
| Total WBC count (103/uL) | 1.44 | 2.35 | 3.0 | 3.27 | 6.07 | 5.40 |
| Lymphocytes (absolute) | 530 | 770 | 770 | 940 | 1370 | 1550 |
| Neutrophils (absolute) | 620 | 1.220 | 1710 | 1880 | 3990 | 3220 |
| Hb (g/dL) | 8.2 | 8.4 | 8.5 | 9.6 | 11.0 | 11.8 |
| PLTS (103/uL) | 53 | 98 | 134 | 197 | 191 | 161 |
| LDH (U/L) | 351 | NA | NA | NA | NA | NA |
| IgG (g/L) | 24 | NA | NA | 21 | 22.6 | NA |
| Albumin (g/L) | 26 | NA | NA | NA | NA | NA |
| Ferritin (ng/mL) | 1075 | NA | NA | NA | NA | NA |
| GOT | 19 | 24 | 14 | 16 | NA | 12 |
| GPT | 20 | 29 | 9 | 13 | NA | 8 |
| Bilirubin Total (mg/dL) | 0.9 | NA | 0.6 | NA | NA | NA |
| Creatinine (mg/dL) | 1.45 | 2.5 | 1.65 | 1.42 | NA | 1.4 |
| Protein C-reactive (mg/dL) | 93.3 | 15.7 | 29.7 | 43.5 | 33.5 | 10.7 |
| Procalcitonin (ng/mL) | 0.8 | 0.18 | 0.08 | NA | 0.10 | 0.04 |
| Protrombin Time | 66% | NA | NA | NA | NA | NA |
| Microbiological Screening on Admission | |
|---|---|
| HIV ½ Ab and p24 | Negative |
| HBsAg, antiHbs, anti-HBc | Negative |
| HCV Ab | Negative |
| Treponema total Ab | Negative |
| CMV | IgM negative, IgG positive |
| EBV | VCA IgG, Positive, EBNA IgG positive |
| HSV 1 & 2 | IgM negative, IgG positive |
| HHV-6 | IgM negative, IgG positive |
| Adenovirus | IgM negative, IgG negative |
| Parvovirus B19 | IgM negative, IgG negative |
| Mycobacterium tuberculosis Quantiferon | Negative |
| Galactomannan Ag | Negative |
| Beta D-glucan Ag | Negative |
| Leishmania spp. | WB p14 positive, p16 positive. PCR: negative |
| COVID-19 | Negative |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupia, T.; Corcione, S.; Fornari, V.; Rizzello, B.; Bosio, R.; Brusa, M.T.; De Rosa, F.G. Visceral Leishmaniasis after Anti-Interleukin 17A (IL-17A) Therapy in a Patient Affected by Psoriatic Arthritis. Trop. Med. Infect. Dis. 2022, 7, 319. https://doi.org/10.3390/tropicalmed7100319
Lupia T, Corcione S, Fornari V, Rizzello B, Bosio R, Brusa MT, De Rosa FG. Visceral Leishmaniasis after Anti-Interleukin 17A (IL-17A) Therapy in a Patient Affected by Psoriatic Arthritis. Tropical Medicine and Infectious Disease. 2022; 7(10):319. https://doi.org/10.3390/tropicalmed7100319
Chicago/Turabian StyleLupia, Tommaso, Silvia Corcione, Valentina Fornari, Barbara Rizzello, Roberta Bosio, Maria Teresa Brusa, and Francesco Giuseppe De Rosa. 2022. "Visceral Leishmaniasis after Anti-Interleukin 17A (IL-17A) Therapy in a Patient Affected by Psoriatic Arthritis" Tropical Medicine and Infectious Disease 7, no. 10: 319. https://doi.org/10.3390/tropicalmed7100319
APA StyleLupia, T., Corcione, S., Fornari, V., Rizzello, B., Bosio, R., Brusa, M. T., & De Rosa, F. G. (2022). Visceral Leishmaniasis after Anti-Interleukin 17A (IL-17A) Therapy in a Patient Affected by Psoriatic Arthritis. Tropical Medicine and Infectious Disease, 7(10), 319. https://doi.org/10.3390/tropicalmed7100319







