Abstract
Introduction: The emergence of multidrug-resistant (MDR) E. coli has developed worldwide; therefore, the use of antibiotic combinations may be an effective strategy to target resistant bacteria and fight life-threatening infections. The current study was performed to evaluate the in vitro and in vivo efficacy of amikacin and imipenem alone and in combination against multidrug-resistant E. coli. Methods: The combination treatment was assessed in vitro using a checkerboard technique and time-killing curve and in vivo using a peritonitis mouse model. In resistant isolates, conventional PCR and quantitative real-time PCR techniques were used to detect the resistant genes of Metallo-β-lactamase gene Imipenemase (bla-IMP) and aminoglycoside 6′-N-acetyltransferase (aac (6′)-Ib). Scanning electron microscopy was used to detect the morphological changes in the resistant isolates after treatment with each drug alone and in combination. In vitro and in vivo studies showed a synergistic effect using the tested antibiotic combinations, showing fractional inhibitory concentration indices (FICIs) of ≤0.5. Regarding the in vivo study, combination therapy indicated a bactericidal effect after 24 h. E. coli isolates harboring the resistant genes Metallo-β-lactamase gene Imipenemase (bla-IMP) and aminoglycoside 6′-N-acetyltransferase (aac (6′)-Ib) represented 80% and 66.7%, respectively, which were mainly isolated from wound infections. The lowest effect on Metallo-β-lactamase gene Imipenemase (bla-IMP) and aminoglycoside 6′-N-acetyltransferase (aac (6′)-Ib) gene expression was shown in the presence of 0.25 × MIC of imipenem and 0.5 × MIC of amikacin. The scanning electron microscopy showed cell shrinkage and disruption in the outer membrane of E. coli in the presence of the antibiotic combination. Amikacin and imipenem combination can be expected to be effective in the treatment and control of serious infections caused by multidrug-resistant (MDR) E. coli and the reduction in bacterial resistance emergence.
1. Introduction
Escherichia coli is a rod-shaped Gram-negative facultative anaerobe bacterium found in the microbiota of human and animal digestive tracts [1]. E. coli pathotypes are involved in hospital-acquired pneumonia (HAP), urinary tract infections (UTI), gastrointestinal tract infections, surgical site infections (SSI), sepsis, meningitis, and hemolytic–uremic syndrome (HUS) [2]. The genetic elements of E. coli can be transferred horizontally between related bacteria or bacteria from different families [2]. E. coli is one of the important species causing infections that are characterized by high morbidity and mortality rates worldwide (about 8.5 deaths per 100,000 persons–year) [3,4]. Multidrug-resistant E. coli is defined as those strains that are resistant to one or more antibiotics in three or more antimicrobial categories [5]. The wide spread of resistance limits the therapeutic activity of last resort, efficacious antibiotics [6].
Carbapenems are considered as last resort antibiotics that can treat drug-resistant Gram-negative bacteria. The molecular structure of carbapenem together with the beta-lactam ring show high stability against most beta-lactamases [7]. Due to uncontrollable public access to antibiotics and the use of subtherapeutic doses, carbapenem resistance is reported. In addition, aminoglycoside antibiotics confer bactericidal activity in emerging systemic infections [8], as they target the bacterial ribosome causing the inhibition of protein biosynthesis [9]. Aminoglycoside-modifying enzymes and RNA methyltransferases are the main mechanisms of aminoglycoside resistance commonly encountered in carbapenem-resistant isolates [10].
Carbapenemase enzyme production is the main cause of carbapenem resistance [11], and they are classified into three groups: Ambler [12] Class A (clavulanic acid-inhibitory extended-spectrum β-lactamases) Klebsiella pneumoniae carbapenemase (KPC); Class B Metallo-β-lactamases (MBLs) that include New Delhi MBL (NDM), Verona integrin-encoded MBL (VIM), and imipenemase (IMP); and Class D oxacillinases (OXA)-type enzymes which include OXA-48-like carbapenemases. These enzymes showed variable levels of resistance among carbapenem antibiotics [13]. Limited choices are available in the treatment of carbapenem-resistant Gram-negative bacteria; therefore, there is an urgent need for new effective antimicrobials [7]. Many studies have reported the effectiveness of antibiotic combinations against multidrug-resistant (MDR) strains in comparison to the use of a single antibiotic [14,15].
Aminoglycosides are reported to be a preferred agent for combining antibiotics because of their wide spectrum and their good synergistic activity with antibiotics that affect the bacterial cell wall, such as β-lactams, glycopeptides, monobactam, and carbapenems [16,17,18,19]. Previous studies revealed that the synergistic activity among β-lactams and amikacin was not attributed to the level of the minimum inhibitory concentrations (MICs) of the antibiotics tested in combination but due to the post-antibiotic effect (PAE) of the tested amikacin and other antibiotics [20,21]. The synergistic effect of amikacin with other antimicrobials was studied by many researchers that explained the action of combinations tested based on post-antibiotic effects [20,21,22,23].
In our hospitals, imipenem and amikacin are widely prescribed antibiotics. Several studies reported the wide spread of aminoglycoside resistance mobilized with β-lactamase genes carrying integrons except for bla-SPM. Depending on the previous findings, our study aimed to evaluate the in vitro and in vivo activities of amikacin and imipenem as a monotherapy and in combination against multidrug-resistant E. coli.
2. Materials and Methods
2.1. Culture and Bacterial Identification
A total of two hundred clinical samples were collected from patients admitted to intensive care units of Minia University Hospital (Minia, Egypt) suffering from chest, wound, burn, urinary tract, and ear infections, and gastroenteritis. Their age ranged from 23 to 71 years: 67% (134/200) were male, and 33% (66/200) were female. One hundred and fifty nonrepetitive isolates were Gram-negative bacteria identified using traditional methods. E. coli was the most prevalent bacteria (60 isolates) followed by Pseudomonas. aeruginosa (45 isolates), Proteus spp. (30 isolates), Klebsiella spp. (10 isolates), and Acinetobacter baumannii (5 isolates). The need for patient consent was waived by the ethics committee. Samples were processed and grown on trypticase soy agar (Lab M, Heywood, UK) at 37 °C overnight. E. coli colonies showed a pink color on MacConkey agar and on eosin methylene blue (EMB) (Lab M, Heywood, UK), appeared as a green metallic sheen. Colonies were further identified using traditional microbiological and biochemical tests [24].
2.2. Antibiotic Susceptibility Tests
Antibiotic sensitivity tests were performed using the Kirby–Bauer disk diffusion method with cefpodoxime (30 µg), tetracycline (30 µg), amikacin (30 µg), cefadroxil (30 µg), streptomycin (10 µg), aztreonam (30 µg), ceftriaxone (30 µg), cephalothin (30 µg), gentamycin (10 µg), amoxicillin/clavulanic acid (30 µg), ceftazidime (30 µg), imipenem (10 µg), meropenem (10 µg), cefoperazone (75 µg), doxycycline (30 µg), ciprofloxacin (5 µg), nalidixic acid (30 µg), cefotaxime (30 µg), piperacillin (100 µg), cefepime(30 µg), ampicillin/sulbactam (20 µg), norfloxacin (10 µg), tobramycin (10 µg), sulphamethoxazole/trimethoprim (25 µg), nitrofurantoin (300 µg), chloramphenicol (30 µg), levofloxacin (5 µg), piperacillin/tazobactam (10 µg), ofloxacin (10 µg), and azithromycin (30 µg). Zones of inhibition were determined according to CLSI 2018 [25]. Antibiotic susceptibility was further confirmed for resistant E. coli using minimum inhibitory concentrations (MICs) for both imipenem (IMP) (MIC, R ≥ 4 µg/mL) and amikacin (AK) (MIC, R ≥ 64 µg/mL) confirmed by broth microdilution according to the Clinical and Laboratory Standards Institute 2018 [25].
2.3. PCR Detection of Metallo-β-Lactamase Gene Imipenemase (bla-IMP) and Aminoglycoside 6′-N-acetyltransferase (aac (6′)-Ib) in Selected Resistant Bacteria
The DNA template was extracted by boiling (10 min at 95 °C) [26], and PCR was performed in a thermal cycler (Bio Rad, USA) using 25-μL volumes including 12.5 μL of PCR Master Mix (500 mM of Tris-HCl pH 8.55, 1.5 mM of MgCl2, 0.2 mM of dNTPs, and 0.04 units/uL of Taq DNA polymerase). PCR primers and PCR conditions are presented in Table 1. After amplification, 10 μL of each sample was analyzed with 2% agarose gel electrophoresis for the detection of positive samples. A 1000-bp DNA ladder was used to detect the product sizes of the genes Metallo-β-lactamase gene Imipenemase (blaIMP) (488 bp) and aminoglycoside 6′-N-acetyltransferase (aac (6′)-Ib) (365 bp).

Table 1.
PCR primers.
2.4. Checkerboard Assay
The combined effect of both amikacin and imipenem were tested against clinical resistant E. coli isolates to determine the fractional inhibitory concentration (FIC) indices. FIC index is defined as the ratio between MIC alone and MIC in combination as follows:
FIC index = FIC of drug A + FIC of drug B.
FIC of drug A = MIC of drug A in combination/MIC of drug A alone.
FIC of drug B = MIC of drug B in combination/MIC of drug B alone.
Results were interpreted as follows: synergy showed as FIC index ≤ 0.5; antagonism showed as FIC index of ≥2; and additive showed as FIC index of >0.5 but ≤1 [17,30].
2.5. Time-Kill Analysis
Time-kill curves were performed at concentrations of 1/2× MIC, 1× MIC, 2× MIC, and 4× MIC for each tested antibiotic alone or in combination. Three representative isolates were chosen: one isolate was resistant to both antibiotics; one isolate was resistant to imipenem only; and the other isolate was resistant to amikacin only. They were chosen based on the checkerboard results. Bacterial counts were detected after 0, 2, 4, 8, and 24 h after incubation at 37 °C. The limit of detection was 300 CFU/mL. Synergy was defined as a 2 log10 decrease in the colony counts caused by the combination compared to the most active single drug at single time-points; additivity was a >1 log10 but <2 log10 decrease. Antagonism was defined as a >2 log10 increase in colony count caused by the combination compared to that by the most active antibiotic alone at any time-point [17]. Bacteriostatic activities were defined as the presence of ≥2 log10 but <3 log10 reductions and bactericidal activities as the presence of ≥3 log10 reductions in CFU/mL at 24 h compared to the initial inoculum [31].
2.6. In Vivo Study
Specific pathogen-free ICR (female) mice at 6 weeks old, weighing 30–38 g (National Research Center, Dokki, Giza, Egypt) were used in this study. The mice were divided into 4 groups, with 8 mice in each group. The mice were intraperitoneally infected with (0.5 MacFarland 1.5 × 108 CFU/mL; 0.2 mL) E. coli resistant to both drugs.
Animals were kept in well-ventilated cages at temperature of 25 ± 2 °C, humidity of 60 ± 10%, and normal photoperiod 12/12 h light–dark cycles under stress-free conditions with free access to food and water. Animals were observed every day for changes in body weight or any other clinical signs of bacterial infections, such as change in body posture and coat appearance, a decline in activity, and changes in food and drink intake. No specific housing requirements were provided. All the staff that handled, administered medications to, and took care of the animals were properly trained.
Animals were treated for 24 h, starting 3 h after bacterial challenge. Imipenem was administered intraperitoneally with 40 mg/kg every 4 h. Amikacin was administered 15 mg/kg every 8 h, and amikacin and imipenem in combination (doses and intervals were the same as in monotherapy). The whole experiment took 27 h. Four groups of animals were evaluated and inoculated with the E. coli resistant to both drugs. Group I: Normal control group were divided into two subgroups: the negative control group received intraperitoneal sterile saline, and the positive and tested group received intraperitoneal bacteria. (Humane endpoint was determined through overdose of thiopental.) Test groups received intraperitoneal bacteria and were treated as follows: Group II: tested group received amikacin dose (200 µL intraperitoneal) with 15 mg/kg every 8 h; Group III: tested group received imipenem dose (200 µL intraperitoneal) with 40 mg/kg every 4 h; and Group IV: tested group received intraperitoneal combination of amikacin and imipenem the same as a single dose.
Twenty µL blood samples were taken from the tail vein of 5 mice randomly selected in each group at 3, 11, and 27 h after infection. Seven two-fold serial dilutions were plated on Mueller–Hinton agar plates by adding 10 microliter aliquots and incubated overnight at 37 °C for CFU determination [32]. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, as revealed in a priori approval (8/2021) by ethical review board of Faculty of Pharmacy, Deraya University, Egypt.
2.7. Gene Expression of Metallo-β-Lactamase Gene Imipenemase (bla-IMP) and Aminoglycoside 6′-N-acetyltransferase (aac (6′)-Ib) Using Real-Time PCR
RNA was isolated from log phase cells of E. coli resistant strains to both imipenem and amikacin exposed to sub-MIC. Gene expression for bla-IMP and aac(6′)-Ib with 16S rRNA house-keeping gene as a control gene in the resistant isolate (isolate W3) was performed before and after treatment with the antibiotic.
Bacterial RNA was extracted using QuantiTect SYBR Green PCR kit (Qiagen, Hilden, Germany). The RT-PCR was performed in 25 μL reaction mixture consisting of 2× QuantiTect SYBR Green PCR Master Mix (12.5 μL), reverse transcriptase (0.25 μL), 0.5μL of each forward (20 pmol) and reverse (20 pmol) primers, RNase-free water (8.25 μL), and template RNA (3 μL). The cycling conditions are indicated in Table 1. Strata gene MX3005P software was used for the detection of amplification curves and CT values. The variation of gene expression on the RNA of the different samples was estimated. CT of each sample was compared with that of the control group according to the “ΔΔCT” method stated by Yuan, et al. [33]. Dissociation curves were compared between different samples for exclusion of false positive results.
2.8. Scanning Electron Microscopy (SEM)
E. coli-resistant colonies at the desired growth phase were suspended in a saline solution containing 0.2% Tween 80 and incubated at 37 °C with imipenem alone, amikacin alone, and in combination. The bacterial cells were centrifuged after 24 h at 8000 rpm for 15 min. The bacterial cells were then washed with 0.1 mol/L tris-acetate buffer (pH 7.1), fixed in tris-acetate buffer containing 1.5% glutaraldehyde, and then freeze-dried. Each of the bacterial cultures were observed by SEM (Hitachi, Japan) at magnifications of 7500, 10,000, and 20,000×. The bacterial suspension in saline with no antibiotics acted as a negative control [34].
2.9. Statistical Analysis
SPSS program (Statistical Package for Social Sciences) software version 25 was used to evaluate the data that had been statistically obtained. The graphic presentation was performed with the help of Microsoft Office Excel 365 software. For parametric (normally distributed) quantitative data, descriptive statistics were performed using the mean and standard deviation (SD). Shapiro–Wilk test was used to distribute the data. Analyses were carried out for parametric quantitative data between various groups or concentrations using the one-way ANOVA test, followed by the post hoc Tukey’s analysis between various groups or concentrations. The repeated measures ANOVA test was used to analyze parametric quantitative data between different periods, and it was followed by a post hoc LSD analysis between each pair of times. The significance level was set at (p value 0.05).
3. Results
3.1. E. coli Isolation and Identification
A total of 150 clinical nonrepetitive isolates of Gram-negative bacteria were identified using traditional methods. Escherichia coli was the most common Gram-negative bacteria, representing 40% of the total isolates (60/150).
3.2. Antibiotic Susceptibility Testing
High resistance to most of the tested antibiotics was shown, including amoxicillin/clavulanic acid, cefpodoxime, cefepime, imipenem, and others. On the other hand, piperacillin/tazobactam and levofloxacin were the most effective antibiotics.
E. coli isolates were found to be multidrug-resistant (MDR) where they were resistant to one or more agents in three or more antibiotic categories (Figure 1) (Table S1).
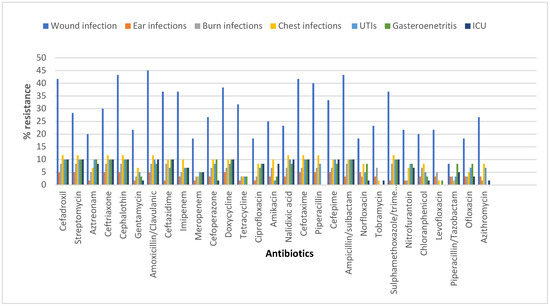
Figure 1.
Antibiotic resistance profile of E. coli according to the infection type.
3.3. Detection of MIC50 and MIC90 of Imipenem and Amikacin for E. coli Isolates
The MIC values indicated that 75% and 58.3% of isolates were resistant to amikacin (MIC ≥ 64 µg/mL) and imipenem (MIC ≥ 4 µg/mL), respectively. Imipenem MIC90 and MIC50 were 512 µg/mL and 16 µg/mL, while amikacin MIC90 and MIC50 were 512 µg/mL and 64 µg/mL.
3.4. Molecular Identification of Metallo-β-Lactamase Gene Imipenemase (bla-IMP) and Aminoglycoside 6′-N-acetyltransferase (aac (6′)-Ib)-Resistant Genes Using Conventional PCR
E. coli harboring bla-IMP had 48 isolates (80%) of the total number of E. coli. bla-IMP was commonly found among strains isolated from wound infections (47.9%), followed by patients admitted to intensive care units and from chest infections (12.5%), urinary tract infections (10.4%), gastroenteritis (8.3%), burn infections (6.3%), and ear infections (4.2%). Forty isolates (66.7%) of E. coli were positive for aac(6′)-Ib. aac(6′)-Ib was found to be the most common among wound infections (47.5%), followed by chest infections (15%), patients admitted to intensive care units (12.5%), burn infections (10%), ear infections, urinary tract infections, and gastroenteritis (5%) (Table 2). All isolates that were resistant to imipenem and amikacin were positive for both genes Metallo-β-lactamase gene Imipenemase (bla-IMP) and aminoglycoside 6′-N-acetyltransferase (aac (6′)-Ib) (Tables S2 and S3).

Table 2.
Distribution of Metallo-β-lactamase gene Imipenemase (bla-IMP) and aminoglycoside 6′-N-acetyltransferase (aac (6′)-Ib) genotype within 60 isolated E. coli.
3.5. The Combined Effect of Amikacin and Imipenem against Resistant E. coli Using the Checkerboard Dilution Technique
High synergistic activity was shown by combinations with a high reduction in MICs, which ranged from two- to eight-fold in comparison to amikacin and imipenem alone, respectively (Table 3).

Table 3.
Determination of the combined effect of amikacin and imipenem against resistant E. coli using checkerboard assay.
3.6. Time-Kill Studies
This test was conducted on three isolates: one isolate showing resistance to both imipenem and amikacin (isolate no. 3, wound source (W3)), an isolate resistant to imipenem only (W2), and an isolate resistant to amikacin only (W1). Regarding E. coli resistant to both amikacin and imipenem (W3), Figure 2 shows that at 0.5× MIC of the antibiotic combination count decreased the initial bacterial count (8.2 log10 CFU/mL) after 24 h to 7.46 log10 CFU/mL, and such a combination regimen was shown to be synergistic in comparison to each drug alone. At 1× MIC of the combination group, the colony count decreased to 4.7 log10 CFU/mL showing 3.5 log10 CFU/mL reductions which indicated a bactericidal and synergistic effect. At 2× MIC, there was a decrease in the bacterial count by 2.26 log10 CFU/mL reduction after 12 h, indicating a bacteriostatic effect and a decrease by 4.2 log10 CFU/mL reduction after 24 h, indicating bactericidal and synergistic activity in comparison to each drug alone.
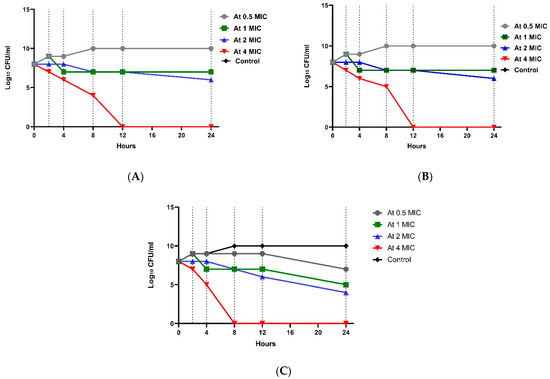
Figure 2.
Time-kill assay for isolate no. 3 resistant to both imipenem and amikacin. (A): E. coli isolate treated with amikacin at different concentrations; (B): E. coli isolate treated with imipenem at different concentrations; and (C): E. coli isolate treated with a combination at different concentrations.
At 4× MIC, the bacterial count decreased after 4 h to 4.6 log10 CFU/mL with 3.6 log10 CFU/mL reduction, indicating bactericidal activity. A significant difference between the four groups (p value ˂ 0.001) was shown at each concentration. Regarding E. coli resistant to imipenem (W2), the 0.5× MIC of combination group showed no significant effect (Figure 3). In the 1× MIC combination group, a reduction of 2.5 log10 CFU/mL was shown, indicating bacteriostatic activity after 8 h, and a reduction of 4.2 log10 CFU/mL was shown, indicating bactericidal and synergistic activity after 24 h. There was a statistically significant difference between the four groups (p-value ˂ 0.001) in each concentration. Regarding E. coli resistant to amikacin (W1), Figure 4 shows that at 0.5× MIC, the combination group showed no significant effect, while at 1× MIC combination, a decrease in count by 2.33 log10 CFU/mL reduction was observed, indicating a bacteriostatic effect after 8 h, and a reduction of 4.2 log10 CFU/mL was observed, representing a bactericidal and synergistic effect after 24 h. There was a significant difference between the four groups (p value ˂ 0.001) at each concentration (Tables S4–S6).
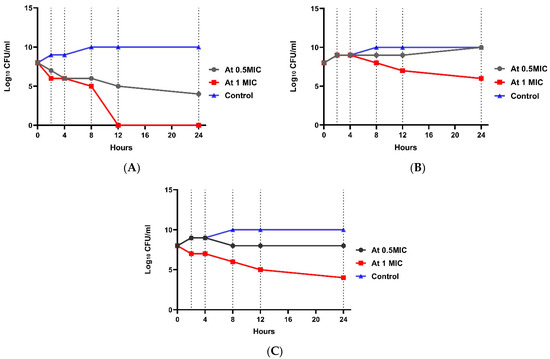
Figure 3.
Time-kill assay for isolate no. 1 resistant to imipenem. (A): E. coli isolate treated with amikacin at different concentrations; (B): E. coli isolate treated with imipenem at different concentrations; and (C): E. coli isolate treated with a combination at different concentrations.
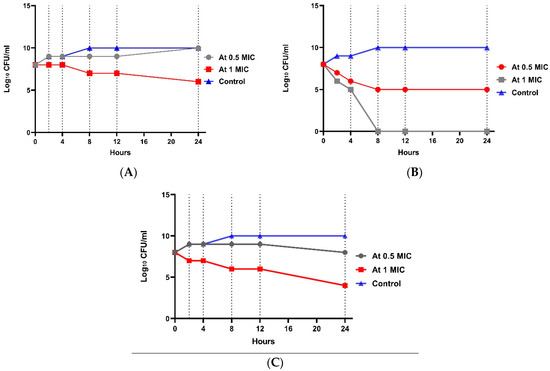
Figure 4.
Time-kill assay for isolate no. 2 resistant to amikacin. (A): E. coli isolate treated with amikacin at different concentrations; (B): E. coli isolate treated with imipenem at different concentrations; and (C): E. coli isolate treated with a combination at different concentrations.
3.7. In Vivo Studies
Figure 5 shows that the average blood bacterial counts were 9.41 log10 CFU/mL 3 h after infecting the mice (i.e., when the treatment started) with E. coli (W3). The blood microbial count constantly increased in the control group (infected, untreated) to 10.36 log10 CFU/mL after 27 h using E. coli. Meanwhile, the blood microbial count reduced in all the infected treated groups using amikacin, imipenem, and a combination to 8.71 log10 CFU/mL, 8.25 log10 CFU/mL, and 6 log10 CFU/mL, respectively. After 27 h, the combination indicated bactericidal and synergistic activity. There was a significant difference among the tested groups (p < 0.001): control group and imipenem, control and amikacin, control and combination, amikacin and imipenem, amikacin and combination, and imipenem and combination (Table S7).
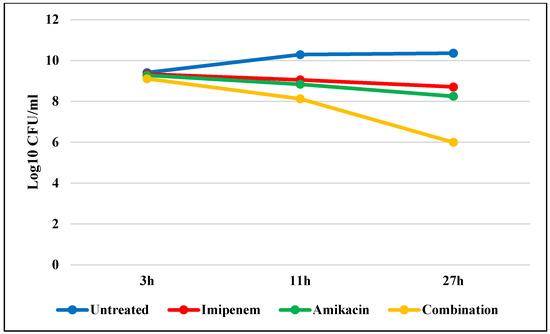
Figure 5.
Average blood bacterial count of E. coli isolated from untreated animals and animals treated with imipenem, amikacin, and a combination at different times.
3.8. Gene Expression (Real-Time PCR) Results
Figure 6 shows the gene expression for selected E. coli-resistant isolates that were treated with amikacin and imipenem separately and in combination. The control samples showed no fold change for Metallo-β-lactamase gene Imipenemase (bla-IMP) or aminoglycoside 6′-N-acetyltransferase (aac (6′)-Ib). The isolate (W3) was selected to test the effect of the combination on the expression of genes using a real-time polymerase chain. The results show a decrease in the expression for the Metallo-β-lactamase gene Imipenemase (bla-IMP) gene equivalent to 9.1896 and the aminoglycoside 6′-N-acetyltransferase (aac (6′)-Ib) gene equivalent to 6.1475 after treatment with an amikacin/imipenem combination. The isolate (W1) and isolate (W2) which showed resistance to amikacin and imipenem, respectively, were selected to determine the expression of each gene using RT-PCR. Both isolates showed over-expression of Metallo-β-lactamase gene Imipenemase (bla-IMP) and aminoglycoside 6′-N-acetyltransferase (aac (6′)-Ib) in the untreated isolates rather than the treated isolates, confirming the importance of each gene on the resistance mechanism. The results also reveal that a combination 0.25× MIC of imipenem + 0.5× MIC of amikacin presented a higher decrease in expression than that showed by 0.5× MIC of imipenem +0.25× MIC of amikacin (Table S8).
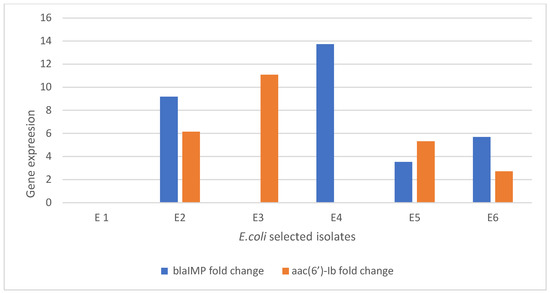
Figure 6.
Gene expression of resistant E. coli after treatment: E1: control; E2: E. coli (w3) resistant to amikacin harboring aminoglycoside 6′-N-acetyltransferase (aac (6′)-Ib) and imipenem harboring Metallo-β-lactamase gene Imipenemase (bla-IMP); E3: E. coli (W1) resistant to amikacin harboring aminoglycoside 6′-N-acetyltransferase (aac (6′)-Ib) only; E4: E. coli (W2) resistant to imipenem harboring Metallo-β-lactamase gene Imipenemase (bla-IMP) only; E5: E. coli isolate (w3) after treatment with 0.25 MIC of amikacin +0.5 MIC of imipenem; and E6: E. coli isolate (w3) after treatment with 0.25 MIC of imipenem +0.5 MIC of amikacin.
3.9. Scanning Electron Microscopy (SEM)
The effect of amikacin, imipenem, and their combination on the cell structure of E. coli (No.3) was confirmed by the SEM analysis in Figure 7A–D. In comparison to the control group, the cells treated with the tested antibiotics displayed a change in morphology in the form of cell elongation and swelling. The examined antibiotic-treated bacteria showed significant structural alterations on the outer membrane of E. coli, resulting in cell destruction, while untreated bacteria were intact (regular rod-shaped) and showed smooth surfaces. The outer membrane and cellular structure were altered by the combination of antibiotics.
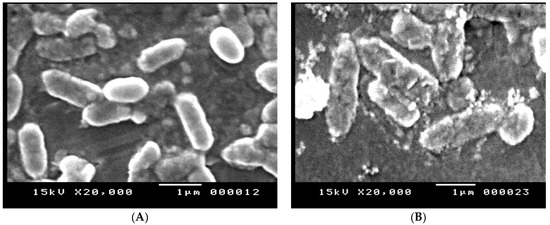
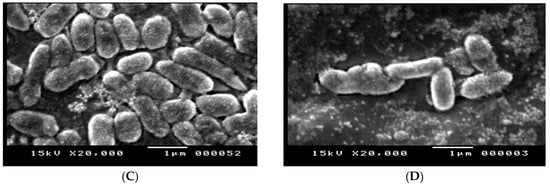
Figure 7.
(A) SEM image of E. coli control, (B) SEM image of E. coli treated with imipenem, (C) SEM image of E. coli treated with amikacin, and (D) SEM image of E. coli treated with amikacin/imipenem- treated cells.
4. Discussion
The emergence of bacterial resistance is considered a critical global issue. As infections by bacterial-resistant strains are associated with high morbidity and mortality, the World Health Organization (WHO) lists carbapenem-resistant strains, especially carbapenem-resistant Enterobacteriaceae (CRE), as one of the top tier of antibiotic-resistant “priority pathogens” that are considered as the greatest threat to human health [35,36]. Treatment with antibiotic combinations is recommended in severe infections caused by resistant strains [37,38,39,40]. In vitro and in vivo synergy tests can provide guidance for these combination therapies. This study was conducted to evaluate the effectiveness of the combination treatment of imipenem and amikacin as an example of beta-lactam and aminoglycoside antibiotics in the treatment of multidrug-resistant (MDR) E. coli using checkerboard, time-kill curve studies techniques, and in vivo studies. Our analysis showed that E. coli was the most common species (40%), which agreed with many studies [41,42]. A study by Giacobbe, et al. [43] showed that Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter spp. were the most common isolated pathogens that cause blood stream infections. On the other hand, Bhatt, et al. [44] reported a higher percentage of E. coli (50.4%). Our study indicated that multidrug-resistant (MDR) E. coli isolates were obtained from wounds, urinary tract infections, patients admitted to intensive care units, ear infections, burns, chest infections, and gastroenteritis. Antimicrobial susceptibility testing revealed that the most active antibiotics were piperacillin/tazobactam (33.3%) and gentamycin (34%). On the other hand, high resistance was observed against imipenem (61.6%), gentamycin (60%), and amikacin (58.3%). Similar results were shown by Dokla, et al. [45] and Vena, et al. [46]. Our results show that 80% and 66.7% of E. coli harbored bla-IMP and aac(6′)-Ib, respectively. Elbadawi, et al. [47] reported that 14.9% of Escherichia coli isolates harbored carbapenemase genes. Soliman, et al. [48] revealed that 33.8% Gram-negative bacteria were carbapenem-resistant GNB, including E. coli isolates (9.2%), K. pneumoniae isolates (3%), P. stuartii isolates (10.7%), and seven P. aeruginosa isolates (10.7%). Four E. coli, two K. pneumoniae, and seven P. stuartii were found to produce NDM-1, out of 1392.3% of strains; 12 were pan-aminoglycosides-resistant and had class 1 integron-carrying aac(6′)-Ib.
Our study showed synergistic activity with the combination of imipenem and amikacin using a checkerboard assay where a combination of both drugs showed MIC values below the recognized breakpoints for resistance. In addition, the results obtained by time-kill experiments approved the synergistic activity of the tested combinations. The use of different antibiotic combinations was tested by Al-Tamimi, et al. [15] who reported positive synergistic activity by cephalosporins and amoxicillin/clavulanate with cefotaxime or cefixime compared to their combinations with cefpodoxime, cefdinir, and ceftazidime. Drago, et al. [17] reported that synergistic and additive effects were detected by combinations of levofloxacin and imipenem or amikacin, and between ciprofloxacin and amikacin against ESBL-producing E. coli. Loho, et al. [16] reported the effect of doripenem and amikacin on Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae. They reported that a synergistic effect only appeared in one isolate. Additive effects were found in 24 (35.3%) isolates, and indifferent interactions appeared in 43 (63.2%) isolates. They concluded that the combination of doripenem with amikacin significantly reduced MIC in all isolated strains when compared to the MIC of each antibiotic separately. Expression changes of Metallo-β-lactamase gene Imipenemase (bla-IMP) and aminoglycoside 6′-N-acetyltransferase (aac (6′)-Ib) genes are responsible for the production of amino-glycosidase and beta-lactamase enzymes. Our study observed a reduction in gene expression using qRT-PCR in the case of treatment with 0.25× MIC imipenem + 0.5× MIC amikacin compared to the effect of a single antibiotic. These changes in expression may be due to one of the mechanisms of synergy between aminoglycosides and β-lactams by the ability of β-lactams to increase the uptake of aminoglycosides [49]. The changes in the morphology of the tested isolates were confirmed by SEM in the presence and in the absence of the tested antibiotics. The SEM observations confirmed the morphological changes and disruption to the outer membrane of the tested isolates. The scanning electron microscopy showed a great disruption to the cells treated by aminoglycosides and β-lactams. Similar results were obtained by Yadav, et al. [50] and Hayami, et al. [51].
5. Conclusions
Amikacin/imipenem combinations were found to be a therapeutic option in controlling serious infections caused by multidrug-resistant (MDR) E. coli strains according to our results. This combination can also decrease the resistance risk of monotherapy while relieving the stress of clinical treatment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/tropicalmed7100281/s1. Table S1: Distribution of resistance pattern of E. coli to different antimicrobial agents according to the infection type. Table S2: Distribution of bla-IMP genotype among 60 isolated E. coli. Table S3: Distribution of aac(6′)-Ib genotype among 60 isolated E. coli. Table S4: Time-killing curve for different groups infected with E. coli (wound, no.3) resistant to both IMP and AMK. Table S5: Time-kill studies for different groups infected with imipenem-resistant E. Coli (W2). Table S6: Time-kill studies for different groups infected with amikacin-resistant E. Coli (W1). Table S7: Average blood bacterial count of E. coli isolated from untreated animals and animals treated with imipenem, amikacin, and in combination at different times. Table S8: Gene expression of resistant E. coli after treatment.
Author Contributions
Data curation, H.R.A., D.S.M. and A.E.Z.; formal analysis, S.M.F. and A.O.E.-G.; investigation, H.R.A. and A.F.A.; methodology, R.M.A.E.-B., S.M.F., A.O.E.-G., D.S.M. and A.E.Z.; project administration, A.E.Z.; supervision, S.A., A.O.E.-G. and A.F.A.; validation, D.S.M.; visualization, R.M.A.E.-B. and S.A.; writing—original draft, R.M.A.E.-B., S.M.F., A.O.E.-G., H.R.A. and A.F.A.; and writing—review and editing, S.M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, with prior approval (8/2021) by the ethical review board of the Faculty of Pharmacy, Deraya University, Egypt.
Informed Consent Statement
Patient consent was waived by the ethics committee.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lee, C.-H.; Kang, J.-H.; Woo, H.-J.; Song, K.B. Inactivation of Listeria monocytogenes and Escherichia coli O157: H7 inoculated on fresh-cut romaine lettuce by peanut skin extract/benzethonium chloride emulsion washing. Food Control 2021, 119, 107479. [Google Scholar] [CrossRef]
- Sarowska, J.; Futoma-Koloch, B.; Jama-Kmiecik, A.; Frej-Madrzak, M.; Ksiazczyk, M.; Bugla-Ploskonska, G.; Choroszy-Krol, I. Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: Recent reports. Gut Pathog. 2019, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.D.; Bogaerts, P.; Berhin, C.; Hoebeke, M.; Bauraing, C.; Glupczynski, Y. A Multicentre Study Group. Increasing proportion of carbapenemase-producing Enterobacteriaceae and emergence of a MCR-1 producer through a multicentric study among hospital-based and private laboratories in Belgium from September to November 2015. Eurosurveillance 2017, 22, 30530. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, M.C.; McEwen, S.A.; Pearl, D.L.; Lyytikäinen, O.; Jacobsson, G.; Collignon, P.; Gregson, D.B.; Valiquette, L.; Laupland, K.B. Mortality in Escherichia coli bloodstream infections: A multinational population-based cohort study. BMC Infect. Dis. 2021, 21, 606. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.; Giske, C.; Harbarth, S.; Hindler, J.; Kahlmeter, G.; Olsson-Liljequist, B. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Sheu, C.-C.; Chang, Y.-T.; Lin, S.-Y.; Chen, Y.-H.; Hsueh, P.-R. Infections Caused by Carbapenem-Resistant Enterobacteriaceae: An Update on Therapeutic Options. Front. Microbiol. 2019, 10, 80. [Google Scholar] [CrossRef]
- Krause, K.M.; Serio, A.W.; Kane, T.R.; Connolly, L.E. Aminoglycosides: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a027029. [Google Scholar] [CrossRef]
- Lee, C.-S.; Doi, Y. Therapy of infections due to carbapenem-resistant gram-negative pathogens. Infect. Chemother. 2014, 46, 149. [Google Scholar] [CrossRef] [PubMed]
- Karah, N.; Dwibedi, C.K.; Sjostrom, K.; Edquist, P.; Johansson, A.; Wai, S.N.; Uhlin, B.E. Novel Aminoglycoside Resistance Transposons and Transposon-Derived Circular Forms Detected in Carbapenem-Resistant Acinetobacter baumannii Clinical Isolates. Antimicrob Agents Chemother 2016, 60, 1801–1818. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Price, L.S.; Poirel, L.; Bonomo, R.A.; Schwaber, M.J.; Daikos, G.L.; Cormican, M.; Cornaglia, G.; Garau, J.; Gniadkowski, M.; Hayden, M.K.; et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect. Dis. 2013, 13, 785–796. [Google Scholar] [CrossRef]
- Ambler, R.P.; Coulson, A.F.; Frère, J.-M.; Ghuysen, J.-M.; Joris, B.; Forsman, M.; Levesque, R.C.; Tiraby, G.; Waley, S.G. A standard numbering scheme for the class A beta-lactamases. Biochem. J. 1991, 276, 269. [Google Scholar] [CrossRef]
- Marsik, F.J.; Nambiar, S. Review of carbapenemases and AmpC-beta lactamases. Pediatr. Infect. Dis. J. 2011, 30, 1094–1095. [Google Scholar] [CrossRef] [PubMed]
- Jones, F.; Hu, Y.; Coates, A. The Efficacy of Using Combination Therapy against Multi-Drug and Extensively Drug-Resistant Pseudomonas aeruginosa in Clinical Settings. Antibiotics 2022, 11, 323. [Google Scholar] [CrossRef]
- Mathe, A.; Szabo, D.; Anderlik, P.; Rozgonyi, F.; Nagy, K. The effect of amikacin and imipenem alone and in combination against an extended-spectrum beta-lactamase-producing Klebsiella pneumoniae strain. Diagn. Microbiol. Infect. Dis. 2007, 58, 105–110. [Google Scholar] [CrossRef]
- Bernabeu-Wittel, M.; Pichardo, C.; Garcia-Curiel, A.; Pachon-Ibanez, M.E.; Ibanez-Martinez, J.; Jimenez-Mejias, M.E.; Pachon, J. Pharmacokinetic/pharmacodynamic assessment of the in-vivo efficacy of imipenem alone or in combination with amikacin for the treatment of experimental multiresistant Acinetobacter baumannii pneumonia. Clin. Microbiol. Infect. 2005, 11, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Al-Tamimi, M.; Abu-Raideh, J.; Albalawi, H.; Shalabi, M.; Saleh, S. Effective Oral Combination Treatment for Extended-Spectrum Beta-Lactamase-Producing Escherichia coli. Microb. Drug Resist. 2019, 25, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Loho, T.; Sukartini, N.; Astrawinata, D.A.W.; Immanuel, S.; Aulia, D.; Priatni, I. In Vitro Antibacterial Interaction of Doripenem and Amikacin against Multidrug-Resistant Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae Isolates. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 1047670. [Google Scholar] [CrossRef]
- Drago, L.; De Vecchi, E.; Nicola, L.; Legnani, D.; Lombardi, A.; Gismondo, M. In vitro synergy and selection of resistance by fluoroquinolones plus amikacin or β-lactams against extended-spectrum β-lactamase-producing Escherichia coli. J. Chemother. 2005, 17, 46–53. [Google Scholar] [CrossRef]
- Critchley, I.A.; Sahm, D.F.; Kelly, L.J.; Karlowsky, J.A. In vitro synergy studies using aztreonam and fluoroquinolone combinations against six species of Gram-negative bacilli. Chemotherapy 2003, 49, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Munckhof, W.J.; Olden, D.; Turnidge, J.D. The postantibiotic effect of imipenem: Relationship with drug concentration, duration of exposure, and MIC. Antimicrob. Agents Chemother. 1997, 41, 1735–1737. [Google Scholar] [CrossRef][Green Version]
- Sasahara, T.; Satoh, Y.; Sekiguchi, T.; Suzuki, K.; Irinoda, K.; Kitasato, H.; Okamoto, R.; Inoue, M.; Takayama, Y.; Sakamoto, A. Pretreatment of Pseudomonas aeruginosa with a sub-MIC of imipenem enhances bactericidal activity of neutrophils. J. Infect. Chemother. 2003, 9, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Knafl, D.; Thalhammer, F.; Vossen, M.G. In-vitro release pharmacokinetics of amikacin, teicoplanin and polyhexanide in a platelet rich fibrin—Layer (PRF)—A laboratory evaluation of a modern, autologous wound treatment. PLoS ONE 2017, 12, e0181090. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Kentepozidis, N.; Antonopoulou, A.; Plachouras, D.; Tsaganos, T.; Giamarellou, H. Postantibiotic effect of antimicrobial combinations on multidrug-resistant Pseudomonas aeruginosa. Diagn. Microbiol. Infect. Dis. 2005, 51, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Collee, J.G.; Mackie, T.J.; McCartney, J.E. Mackie & McCartney Practical Medical Microbiology; Harcourt Health Sciences: San Diego, CA, USA, 1996. [Google Scholar]
- Wayne, P. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; CLSI document M100-28 edition; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Abiri, R.; Mohammadi, P.; Shavani, N.; Rezaei, M. Detection and Genetic Characterization of Metallo-beta-Lactamase IMP-1 and VIM-2 in Pseudomonas aeruginosa Strains from Different Hospitals in Kermanshah, Iran. Jundishapur. J. Microbiol. 2015, 8, e22582. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Liang, Z.; Su, X.; Xiong, Y. Characterization of carbapenemase genes in Enterobacteriaceae species exhibiting decreased susceptibility to carbapenems in a university hospital in Chongqing, China. Ann. Lab. Med. 2012, 32, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Jang, J.-H.; Kim, H.; Kim, Y.-J.; Lee, K.-R.; Kim, Y.-T. Multiplex PCR for Simultaneous Detection of Aminoglycoside Resistance Genes in Escherichia coli and Klebsiella pneumoniae. Korean J. Clin. Lab. Sci. 2012, 44, 155–165. [Google Scholar]
- Tivendale, K.A.; Allen, J.L.; Ginns, C.A.; Crabb, B.S.; Browning, G.F. Association of iss and iucA, but not tsh, with plasmid-mediated virulence of avian pathogenic Escherichia coli. Infect. Immun. 2004, 72, 6554–6560. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhong, Y.; Tang, L.; Deng, Q.; Jing, L.; Zhang, J.; Zhang, Y.; Yu, F.; Ou, Y.; Guo, S.; Huang, B. Unraveling the Novel Effect of Patchouli Alcohol Against the Antibiotic Resistance of Helicobacter pylori. Front. Microbiol. 2021, 12, 1169. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.J.; Lai, C.C.; Chen, C.C.; Zhang, C.C.; Weng, T.C.; Chiu, Y.H.; Toh, H.S.; Chiang, S.R.; Yu, W.L.; Ko, W.C.; et al. Colistin-sparing regimens against Klebsiella pneumoniae carbapenemase-producing K. pneumoniae isolates: Combination of tigecycline or doxycycline and gentamicin or amikacin. J. Microbiol Immunol. Infect. 2019, 52, 273–281. [Google Scholar] [CrossRef]
- Yuan, J.S.; Reed, A.; Chen, F.; Stewart, C.N. Statistical analysis of real-time PCR data. BMC Bioinform. 2006, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Soboh, F.; Khoury, A.E.; Zamboni, A.C.; Davidson, D.; Mittelman, M.W. Effects of ciprofloxacin and protamine sulfate combinations against catheter-associated Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 1995, 39, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Willyard, C. The drug-resistant bacteria that pose the greatest health threats. Nat. News 2017, 543, 15. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Woo, J.H.; Jun, S.H.; Moon, D.C.; Lim, S.-K.; Lee, J.C. Synergy between Florfenicol and Aminoglycosides against Multidrug-Resistant Escherichia coli Isolates from Livestock. Antibiotics 2020, 9, 185. [Google Scholar] [CrossRef]
- Zusman, O.; Avni, T.; Leibovici, L.; Adler, A.; Friberg, L.; Stergiopoulou, T.; Carmeli, Y.; Paul, M. Systematic review and meta-analysis of in vitro synergy of polymyxins and carbapenems. Antimicrob. Agents Chemother. 2013, 57, 5104–5111. [Google Scholar] [CrossRef]
- Tamma, P.D.; Cosgrove, S.E.; Maragakis, L.L. Combination therapy for treatment of infections with gram-negative bacteria. Clin. Microbiol. Rev. 2012, 25, 450–470. [Google Scholar] [CrossRef]
- Lim, T.P.; Lee, W.; Tan, T.Y.; Sasikala, S.; Teo, J.; Hsu, L.Y.; Tan, T.T.; Syahidah, N.; Kwa, A.L. Effective antibiotics in combination against extreme drug-resistant Pseudomonas aeruginosa with decreased susceptibility to polymyxin B. PLoS ONE 2011, 6, e28177. [Google Scholar] [CrossRef]
- Cai, B.; Cai, Y.; Liew, Y.X.; Chua, N.G.; Teo, J.Q.; Lim, T.P.; Kurup, A.; Ee, P.L.; Tan, T.T.; Lee, W.; et al. Clinical Efficacy of Polymyxin Monotherapy versus Nonvalidated Polymyxin Combination Therapy versus Validated Polymyxin Combination Therapy in Extensively Drug-Resistant Gram-Negative Bacillus Infections. Antimicrob. Agents Chemother. 2016, 60, 4013–4022. [Google Scholar] [CrossRef]
- ECDC. Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report for 2019; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2020. [Google Scholar]
- Yousefi, A.; Torkan, S. Uropathogenic Escherichia coli in the urine samples of Iranian dogs: Antimicrobial resistance pattern and distribution of antibiotic resistance genes. BioMed Res. Int. 2017, 2017, 4180490. [Google Scholar] [CrossRef]
- Giacobbe, D.R.; Giani, T.; Bassetti, M.; Marchese, A.; Viscoli, C.; Rossolini, G.M. Rapid microbiological tests for bloodstream infections due to multidrug resistant Gram-negative bacteria: Therapeutic implications. Clin. Microbiol. Infect. 2020, 26, 713–722. [Google Scholar] [CrossRef]
- Bhatt, P.; Tandel, K.; Shete, V.; Rathi, K.R. Burden of extensively drug-resistant and pandrug-resistant Gram-negative bacteria at a tertiary-care centre. N. Microbes N. Infect. 2015, 8, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Dokla, E.M.E.; Abutaleb, N.S.; Milik, S.N.; Li, D.; El-Baz, K.; Shalaby, M.W.; Al-Karaki, R.; Nasr, M.; Klein, C.D.; Abouzid, K.A.M.; et al. Development of benzimidazole-based derivatives as antimicrobial agents and their synergistic effect with colistin against gram-negative bacteria. Eur. J. Med. Chem. 2020, 186, 111850. [Google Scholar] [CrossRef]
- Vena, A.; Giacobbe, D.R.; Castaldo, N.; Cattelan, A.; Mussini, C.; Luzzati, R.; Rosa, F.G.; Del Puente, F.; Mastroianni, C.M.; Cascio, A.; et al. Clinical Experience with Ceftazidime-Avibactam for the Treatment of Infections due to Multidrug-Resistant Gram-Negative Bacteria Other than Carbapenem-Resistant Enterobacterales. Antibiotics 2020, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Elbadawi, H.S.; Elhag, K.M.; Mahgoub, E.; Altayb, H.N.; Ntoumi, F.; Elton, L.; McHugh, T.D.; Osman, M.; Tembo, J.; Ippolito, G. Detection and Characterization of Carbapenem resistant Gram-negative bacilli isolates recovered from hospitalized patients at Soba University Hospital, Sudan. BMC Microbiol. 2021, 21, 136. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.M.; Zarad, H.O.; Nariya, H.; Shimamoto, T.; Shimamoto, T. Genetic analysis of carbapenemase-producing Gram-negative bacteria isolated from a university teaching hospital in Egypt. Infect. Genet. Evol. 2020, 77, 104065. [Google Scholar] [CrossRef] [PubMed]
- Poole, K.; Gilmour, C.; Farha, M.A.; Parkins, M.D.; Klinoski, R.; Brown, E.D. Meropenem potentiation of aminoglycoside activity against Pseudomonas aeruginosa: Involvement of the MexXY-OprM multidrug efflux system. J. Antimicrob. Chemother. 2018, 73, 1247–1255. [Google Scholar] [CrossRef]
- Yadav, R.; Bulitta, J.B.; Schneider, E.K.; Shin, B.S.; Velkov, T.; Nation, R.L.; Landersdorfer, C.B. Aminoglycoside Concentrations Required for Synergy with Carbapenems against Pseudomonas aeruginosa Determined via Mechanistic Studies and Modeling. Antimicrob. Agents Chemother. 2017, 61, e00722-17. [Google Scholar] [CrossRef]
- Hayami, H.; Goto, T.; Kawahara, M.; Ohi, Y. Activities of β-lactams, fluoroquinolones, amikacin and fosfomycin alone and in combination against Pseudomonas aeruginosa isolated from complicated urinary tract infections. J. Infect. Chemother. 1999, 5, 130–138. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).