Prevention, Risk Exposure, and Knowledge of Monkeypox in Occupational Settings: A Scoping Review
Abstract
1. Introduction
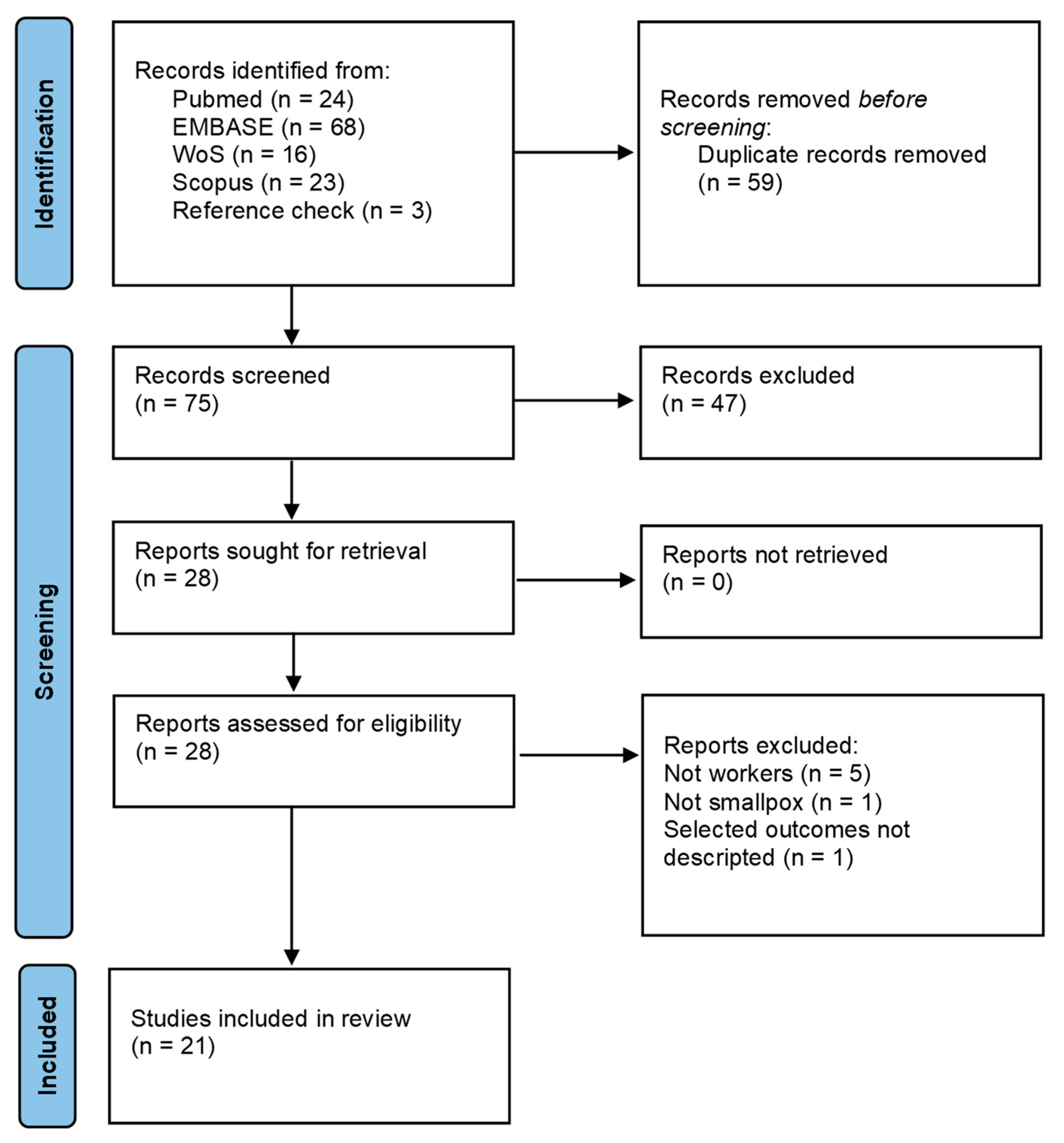
2. Materials and Methods
2.1. Objectives of the Review
- What is the occupational risk of contracting monkeypox, mainly in healthcare settings, but possibly also in other working sectors such as veterinarian environments?
- What are the preventive measures that should be implemented and were applied in previous monkeypox outbreaks to prevent contagion in working settings?
- Since the healthcare setting is the main high-risk working environment, what is the level of knowledge and attitude of healthcare workers toward the monkeypox virus?
2.2. Methods
- Population: workers
- Intervention: spread of monkeypox virus
- Comparison: not applicable
- Outcome: risk of exposure/preventive measures/knowledge and attitude
3. Results
- Risk of contagion in healthcare settings;
- Preventive measures for contagion prevention in healthcare settings, including training and risk assessment;
- Knowledge and attitudes of HCWs toward monkeypox;
- Evidence for the prevention and knowledge of monkeypox in other occupational settings.
3.1. Risk of Contagion in Healthcare Settings
Risk Assessment of Monkeypox Exposure
3.2. Preventive Measures in Healthcare Settings
3.2.1. General Hygiene Measures
3.2.2. Training and Education
3.3. Knowledge and Attitudes of HCWs toward Monkeypox and Vaccination
3.4. Other Occupational Settings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Reference | Country | Type of Article | Topic | Summary |
|---|---|---|---|---|
| Athar 2022 [26] | Iran | Editorial | Preventive measures in healthcare settings | The study is a mini-review of preventive practices for disease control in surgery rooms. |
| Atkinson 2022 [35] | UK | Case report | Risk of contagion in workplace | Environmental sampling was performed to identify MPXV contamination in an office where a worker infected with monkeypox had worked with evidence of low contamination. |
| Costello 2021 [19] | USA | Case report | Risk of contagion in healthcare settings | A total of 40 HCWs were identified as contacts. No HCW met the criteria for high-risk exposure, and no doses of the preventive smallpox vaccine were administered. Active surveillance and symptom monitoring were implemented. No disease transmission was detected. |
| Croft 2007 [33] | USA | Observational study | Risk of contagion in veterinarian settings | The study documented the occupational contagion of veterinary staff and other pet staff through contact with infected prairie dogs. |
| Dell 2020 [34] | Uganda | Cross-sectional study | Knowledge and attitudes of bushmeat traders toward monkeypox virus | Bushmeat trade workers were interviewed regarding their attitudes and practices toward zoonosis prevention. The majority believed that pathogens causing stomach ache or diarrhea (74.6%) and monkeypox (62.2%) can be transmitted by wildlife. |
| Doshi 2018 [29] | Congo | Case series | Preventive measures in healthcare settings | The study reported the response measures, including community education and surveillance strengthening, during a monkeypox outbreak in 2017 in the Republic of Congo. |
| Erez 2019 [22] | Israel | Case report | Risk of contagion in healthcare settings | A total of 11 HCWs were recognized as a monkeypox patient’s contacts; they were offered smallpox vaccination, but only 1 HCW agreed. All contacts were followed up on for 21 days; no virus transmission was detected. |
| Fleischauer 2005 [16] | USA | Cross-sectional study | Risk of contagion in healthcare settings | The study reported the exposure of HCWs to 3 cases of monkeypox, with at least 1 unprotected encounter. One asymptomatic HCW showed laboratory evidence of recent Orthopoxvirus infection, which was possibly attributable to either a recent infection or smallpox vaccination. |
| Harapan 2020a [32] | Indonesia | Cross-sectional study | Knowledge and attitudes of physicians toward monkeypox virus | Among 407 Indonesian doctors, 96% expressed acceptance of a free vaccination. Those 30 years old or younger had 2.94 times greater odds of vaccine acceptance compared to those who were older. The length of practice was also associated with the willingness of vaccination. |
| Harapan 2020b [30] | Indonesia | Cross-sectional study | Knowledge and attitudes of physicians toward monkeypox virus | In Indonesia, out of a total of 432 doctors, 10.0% and 36.5% of them had a good level of knowledge using an 80% and 70% cutoff point for the knowledge domain, respectively. |
| Hobson 2021 [21] | UK | Case report | Risk of contagion in healthcare settings | All contacts, including HCWs, identified for active surveillance completed the 21-day surveillance period from their last date of exposure and no transmissions outside the index family were identified. |
| Koenig 2022 [28] | USA | Editorial | Preventive measures in healthcare settings | A tool, 3I (identify, isolate, inform), was presented to manage patients with suspected and confirmed monkeypox. |
| Kyaw 2020 [20] | Singapore | Case report | Risk of contagion in healthcare settings and preventive measures after exposure | The level of exposure to a monkeypox patient was investigated through telephonic interviews and contact tracing. A total of 27 HCWs were identified to have had close contact, as defined by direct contact with the patient himself or the patient’s surroundings, contact with the patient’s linens, or contact with the patient’s specimens in the lab. All had protected exposure to the patient, with the appropriate and adequate use of PPE. None developed symptoms. |
| Lepellettier 2022 [25] | France | Review | Preventive measures in healthcare settings | Specific measures were provided for healthcare workers, including patient isolation and adequate PPE. |
| Nakoune 2017 [17] | Central African Republic | Case series | Risk of contagion in healthcare settings | Occupational monkeypox infections of nurses and transport clerks were reported during a familial outbreak of monkeypox in 2016 in the Central African Republic. |
| Nörz 2022 [23] | Germany | Observational study | Risk of contagion in healthcare settings | The study found a high viral load on several hospital surfaces that had been in contact with infected patients. |
| Ogoina 2019 [27] | Nigeria | Cross-sectional study | Preventive measures in healthcare settings | During the 2017 monkeypox outbreak, the hospital established a make-shift isolation ward for case management, constituted a monkeypox response team, and provided infection control resources. Training and education were major points of intervention. |
| Palmore 2022 [24] | USA | Editorial | Preventive measures in healthcare settings | The paper provided an overview of the fundamental preventive practices to be implemented in HCW settings. |
| Petersen 2019 [18] | Congo | Editorial | Risk of contagion in healthcare settings | The healthcare-associated transmission of monkeypox was observed on multiple occasions in areas where the disease is endemic, where vaccination for workers can be useful to reduce the occupational risk of disease. |
| Riccò 2022 [31] | Italy | Cross-sectional study | Knowledge and attitudes of physicians toward monkeypox virus | The knowledge status was quite unsatisfying, with substantial knowledge gaps on all aspects of MPX. An analysis of risk perception suggested a substantial overlooking of MPX as a pathogen. |
| Vaughan 2020 [15] | UK | Case report | Risk of contagion in healthcare settings | Reported the occupational contagion of a HCW from an infected patient, probably through contact with contaminated bedding. |
| Europe | European countries refer to ECDC guidelines in terms of prevention measures in workplaces, especially for healthcare facilities. National governments and the Health Ministry of the individual countries issued national prevention measures based on ECDC and WHO recommendations. |
| USA | The USA refers to CDC guidelines for workplace prevention (mainly for healthcare settings), including the use of PPE, environmental infection control, and post-exposure measures. |
| Asia | Asian countries have reported only a few cases during the current monkeypox outbreak so far [64]. Examples of monkeypox management guidelines are those issued in Taiwan and Singapore [65]. Guidelines regarding cases management and preventive measures have also been provided by the Saudi Arabia Public Authority [66]. |
| Africa | An example of monkeypox management guidelines is provided by the Nigerian Centre of Disease Prevention [67]. The WHO guidelines are a major reference point. |
References
- Monkeypox. Available online: https://www.who.int/news-room/fact-sheets/detail/monkeypox (accessed on 11 September 2022).
- Nalca, A.; Rimoin, A.W.; Bavari, S.; Whitehouse, C.A. Reemergence of Monkeypox: Prevalence, Diagnostics, and Countermeasures. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2005, 41, 1765–1771. [Google Scholar] [CrossRef]
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The Changing Epidemiology of Human Monkeypox—A Potential Threat? A Systematic Review. PLoS Negl. Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef]
- Mauldin, M.R.; McCollum, A.M.; Nakazawa, Y.J.; Mandra, A.; Whitehouse, E.R.; Davidson, W.; Zhao, H.; Gao, J.; Li, Y.; Doty, J.; et al. Exportation of Monkeypox Virus From the African Continent. J. Infect. Dis. 2022, 225, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Multi-Country Outbreak of Monkeypox, External Situation Report #5–7 September 2022. Available online: https://www.who.int/publications/m/item/multi-country-outbreak-of-monkeypox--external-situation-report--5---7-september-2022 (accessed on 9 September 2022).
- Reed, K.D.; Melski, J.W.; Graham, M.B.; Regnery, R.L.; Sotir, M.J.; Wegner, M.V.; Kazmierczak, J.J.; Stratman, E.J.; Li, Y.; Fairley, J.A.; et al. The Detection of Monkeypox in Humans in the Western Hemisphere. N. Engl. J. Med. 2004, 350, 342–350. [Google Scholar] [CrossRef] [PubMed]
- CDC Monkeypox in the U.S. Available online: https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html (accessed on 11 September 2022).
- Monkeypox Situation Update, as of 6 September 2022. Available online: https://www.ecdc.europa.eu/en/news-events/monkeypox-situation-update (accessed on 11 September 2022).
- McCollum, A.M.; Damon, I.K. Human Monkeypox. Clin. Infect. Dis. 2014, 58, 260–267. [Google Scholar] [CrossRef]
- Risk Assessment: Monkeypox Multi-Country Outbreak. Available online: https://www.ecdc.europa.eu/en/publications-data/risk-assessment-monkeypox-multi-country-outbreak (accessed on 11 September 2022).
- Weinstein, R.A.; Weber, D.J.; Rutala, W.A. Risks and Prevention of Nosocomial Transmission of Rare Zoonotic Diseases. Clin. Infect. Dis. 2001, 32, 446–456. [Google Scholar] [CrossRef]
- PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation—Annals of Internal Medicine. Available online: https://www.acpjournals.org/doi/10.7326/M18-0850 (accessed on 10 September 2022).
- PRISMA-S: An Extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews—Systematic Reviews—Full Text. Available online: https://systematicreviewsjournal.biomedcentral.com/articles/10.1186/s13643-020-01542-z (accessed on 10 September 2022).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, A.; Aarons, E.; Astbury, J.; Brooks, T.; Chand, M.; Flegg, P.; Hardman, A.; Harper, N.; Jarvis, R.; Mawdsley, S.; et al. Human-to-Human Transmission of Monkeypox Virus, United Kingdom, October 2018. Emerg. Infect. Dis. 2020, 26, 782. [Google Scholar] [CrossRef]
- Fleischauer, A.T.; Kile, J.C.; Davidson, M.; Fischer, M.; Karem, K.L.; Teclaw, R.; Messersmith, H.; Pontones, P.; Beard, B.A.; Braden, Z.H.; et al. Evaluation of Human-to-Human Transmission of Monkeypox from Infected Patients to Health Care Workers. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2005, 40, 689–694. [Google Scholar] [CrossRef]
- Nakoune, E.; Lampaert, E.; Ndjapou, S.G.; Janssens, C.; Zuniga, I.; Van Herp, M.; Fongbia, J.P.; Koyazegbe, T.D.; Selekon, B.; Komoyo, G.F.; et al. A Nosocomial Outbreak of Human Monkeypox in the Central African Republic. Open Forum Infect. Dis. 2017, 4, ofx168. [Google Scholar] [CrossRef] [PubMed]
- Petersen, B.W.; Kabamba, J.; McCollum, A.M.; Lushima, R.S.; Wemakoy, E.O.; Tamfum, J.-J.M.; Nguete, B.; Hughes, C.M.; Monroe, B.P.; Reynolds, M.G. Vaccinating against Monkeypox in the Democratic Republic of the Congo. Antivir. Res. 2019, 162, 171–177. [Google Scholar] [CrossRef]
- Costello, V.; Sowash, M.; Gaur, A.; Cardis, M.; Pasieka, H.; Wortmann, G.; Ramdeen, S. Imported Monkeypox from International Traveler, Maryland, USA, 2021. Emerg. Infect. Dis. 2022, 28, 1002–1005. [Google Scholar] [CrossRef] [PubMed]
- Kyaw, W.M.; Vasoo, S.; Ho, H.J.A.; Chan, M.; Yeo, T.W.; Manauis, C.M.; Ang, H.; de Pratim, P.; Ang, B.S.P.; Chow, A.L.P. Monitoring Healthcare Professionals after Monkeypox Exposure: Experience from the First Case Imported to Asia. Infect. Control Hosp. Epidemiol. 2020, 41, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Hobson, G.; Adamson, J.; Adler, H.; Firth, R.; Gould, S.; Houlihan, C.; Johnson, C.; Porter, D.; Rampling, T.; Ratcliffe, L.; et al. Family Cluster of Three Cases of Monkeypox Imported from Nigeria to the United Kingdom, May 2021. Eurosurveillance 2021, 26, 2100745. [Google Scholar] [CrossRef]
- Erez, N.; Achdout, H.; Milrot, E.; Schwartz, Y.; Wiener-Well, Y.; Paran, N.; Politi, B.; Tamir, H.; Israely, T.; Weiss, S.; et al. Diagnosis of Imported Monkeypox, Israel, 2018. Emerg. Infect. Dis. 2019, 25, 980–983. [Google Scholar] [CrossRef]
- Nörz, D.; Pfefferle, S.; Brehm, T.T.; Franke, G.; Grewe, I.; Knobling, B.; Aepfelbacher, M.; Huber, S.; Klupp, E.M.; Jordan, S.; et al. Evidence of Surface Contamination in Hospital Rooms Occupied by Patients Infected with Monkeypox, Germany, June 2022. Eurosurveillance 2022, 27, 2200477. [Google Scholar] [CrossRef]
- Palmore, T.N.; Henderson, D.K. Adding New Fuel to the Fire: Monkeypox in the Time of COVID-19-Implications for Health Care Personnel. Ann. Intern. Med. 2022, 175, 1183–1184. [Google Scholar] [CrossRef]
- Lepelletier, D.; Pozzetto, B.; Chauvin, F.; Chidiac, C.; High Council for Public Health (HCSP) National Working Group; staff Members of the General Secretary. Management of Patients with Monkeypox Virus (MPXV) Infection and Contacts in the Community and in Healthcare Settings: A French Position Paper. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2022, in press. [Google Scholar] [CrossRef]
- Athar, M.M.T.; Sarvipour, N.; Shafiee, A. Advancing Surgical Setting: A Paradigm for Healthcare Workers during the Monkeypox Outbreak. Ann. Med. Surg. 2022, 81, 104343. [Google Scholar] [CrossRef]
- Ogoina, D.; Izibewule, J.H.; Ogunleye, A.; Ederiane, E.; Anebonam, U.; Neni, A.; Oyeyemi, A.; Etebu, E.N.; Ihekweazu, C. The 2017 Human Monkeypox Outbreak in Nigeria-Report of Outbreak Experience and Response in the Niger Delta University Teaching Hospital, Bayelsa State, Nigeria. PLoS ONE 2019, 14, e0214229. [Google Scholar] [CrossRef]
- Koenig, K.L.; Beÿ, C.K.; Marty, A.M. Monkeypox 2022: A Primer and Identify-Isolate-Inform (3I) Tool for Emergency Medical Services Professionals. Prehospital Disaster Med. 2022, 37, 1–6. [Google Scholar] [CrossRef]
- Doshi, R.H.; Guagliardo, S.A.J.; Dzabatou-Babeaux, A.; Likouayoulou, C.; Ndakala, N.; Moses, C.; Olson, V.; McCollum, A.M.; Petersen, B.W. Strengthening of Surveillance during Monkeypox Outbreak, Republic of the Congo, 2017. Emerg. Infect. Dis. 2018, 24, 1158–1160. [Google Scholar] [CrossRef]
- Harapan, H.; Setiawan, A.M.; Yufika, A.; Anwar, S.; Wahyuni, S.; Asrizal, F.W.; Sufri, M.R.; Putra, R.P.; Wijayanti, N.P.; Salwiyadi, S.; et al. Knowledge of Human Monkeypox Viral Infection among General Practitioners: A Cross-Sectional Study in Indonesia. Pathog. Glob. Health 2020, 114, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Ferraro, P.; Camisa, V.; Satta, E.; Zaniboni, A.; Ranzieri, S.; Baldassarre, A.; Zaffina, S.; Marchesi, F. When a Neglected Tropical Disease Goes Global: Knowledge, Attitudes and Practices of Italian Physicians towards Monkeypox, Preliminary Results. Trop. Med. Infect. Dis. 2022, 7, 135. [Google Scholar] [CrossRef]
- Harapan, H.; Setiawan, A.M.; Yufika, A.; Anwar, S.; Wahyuni, S.; Asrizal, F.W.; Sufri, M.R.; Putra, R.P.; Wijayanti, N.P.; Salwiyadi, S.; et al. Physicians’ Willingness to Be Vaccinated with a Smallpox Vaccine to Prevent Monkeypox Viral Infection: A Cross-Sectional Study in Indonesia. Clin. Epidemiol. Glob. Health 2020, 8, 1259–1263. [Google Scholar] [CrossRef]
- Croft, D.R.; Sotir, M.J.; Williams, C.J.; Kazmierczak, J.J.; Wegner, M.V.; Rausch, D.; Graham, M.B.; Foldy, S.L.; Wolters, M.; Damon, I.K.; et al. Occupational Risks during a Monkeypox Outbreak, Wisconsin, 2003. Emerg. Infect. Dis. 2007, 13, 1150–1157. [Google Scholar] [CrossRef]
- Dell, B.M.; Souza, M.J.; Willcox, A.S. Attitudes, Practices, and Zoonoses Awareness of Community Members Involved in the Bushmeat Trade near Murchison Falls National Park, Northern Uganda. PLoS ONE 2020, 15, e0239599. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, B.; Gould, S.; Spencer, A.; Onianwa, O.; Furneaux, J.; Grieves, J.; Summers, S.; Crocker-Buqué, T.; Fletcher, T.; Bennett, A.M.; et al. Monkeypox Virus Contamination in an Office-Based Workplace Environment. J. Hosp. Infect. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Filho, W.L.; Ternova, L.; Parasnis, S.A.; Kovaleva, M.; Nagy, G.J. Climate Change and Zoonoses: A Review of Concepts, Definitions, and Bibliometrics. Int. J. Environ. Res. Public. Health 2022, 19, 893. [Google Scholar] [CrossRef] [PubMed]
- Worobey, M.; Levy, J.I.; Serrano, L.M.; Crits-Christoph, A.; Pekar, J.E.; Goldstein, S.A.; Rasmussen, A.L.; Kraemer, M.U.G.; Newman, C.; Koopmans, M.P.G.; et al. The Huanan Seafood Wholesale Market in Wuhan Was the Early Epicenter of the COVID-19 Pandemic. Science 2022, 377, 951–959. [Google Scholar] [CrossRef]
- León-Figueroa, D.A.; Bonilla-Aldana, D.K.; Pachar, M.; Romaní, L.; Saldaña-Cumpa, H.M.; Anchay-Zuloeta, C.; Diaz-Torres, M.; Franco-Paredes, C.; Suárez, J.A.; Ramirez, J.D.; et al. The Never-Ending Global Emergence of Viral Zoonoses after COVID-19? The Rising Concern of Monkeypox in Europe, North America and Beyond. Travel Med. Infect. Dis. 2022, 49, 102362. [Google Scholar] [CrossRef]
- Sah, R.; Mohanty, A.; Reda, A.; Siddiq, A.; Mohapatra, R.K.; Dhama, K. Marburg Virus Re-Emerged in 2022: Recently Detected in Ghana, Another Zoonotic Pathogen Coming up amid Rising Cases of Monkeypox and Ongoing COVID-19 Pandemic- Global Health Concerns and Counteracting Measures. Vet. Q. 2022, 42, 167–171. [Google Scholar] [CrossRef]
- Chauhan, R.P.; Dessie, Z.G.; Noreddin, A.; El Zowalaty, M.E. Systematic Review of Important Viral Diseases in Africa in Light of the ‘One Health’ Concept. Pathogens 2020, 9, 301. [Google Scholar] [CrossRef] [PubMed]
- Alakunle, E.; Moens, U.; Nchinda, G.; Okeke, M.I. Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution. Viruses 2020, 12, E1257. [Google Scholar] [CrossRef] [PubMed]
- Farahat, R.A.; Ali, I.; Al-Ahdal, T.; Benmelouka, A.Y.; Albakri, K.; El-Sakka, A.A.; Abdelaal, A.; Abdelazeem, B.; Anwar, M.M.; Mehta, R.; et al. Monkeypox and Human Transmission: Are We on the Verge of Another Pandemic? Travel Med. Infect. Dis. 2022, 49, 102387. [Google Scholar] [CrossRef] [PubMed]
- Hemati, S.; Farhadkhani, M.; Sanami, S.; Mohammadi-Moghadam, F. A Review on Insights and Lessons from COVID-19 to the Prevent of Monkeypox Pandemic. Travel Med. Infect. Dis. 2022, 50, 102441. [Google Scholar] [CrossRef]
- Nakoune, E.; Olliaro, P. Waking up to Monkeypox. BMJ 2022, 377, o1321. [Google Scholar] [CrossRef]
- Petersen, E.; Kantele, A.; Koopmans, M.; Asogun, D.; Yinka-Ogunleye, A.; Ihekweazu, C.; Zumla, A. Human Monkeypox: Epidemiologic and Clinical Characteristics, Diagnosis, and Prevention. Infect. Dis. Clin. N. Am. 2019, 33, 1027–1043. [Google Scholar] [CrossRef]
- Seang, S.; Burrel, S.; Todesco, E.; Leducq, V.; Monsel, G.; Pluart, D.L.; Cordevant, C.; Pourcher, V.; Palich, R. Evidence of Human-to-Dog Transmission of Monkeypox Virus. Lancet 2022, 400, 658–659. [Google Scholar] [CrossRef]
- Geng, Y.; Wang, Y. Stability and Transmissibility of SARS-CoV-2 in the Environment. J. Med. Virol. 2022, 1, 11. [Google Scholar] [CrossRef]
- Khatib, M.N.; Sinha, A.; Mishra, G.; Quazi, S.Z.; Gaidhane, S.; Saxena, D.; Gaidhane, A.M.; Bhardwaj, P.; Sawleshwarkar, S.; Zahiruddin, Q.S. WASH to Control COVID-19: A Rapid Review. Front. Public Health 2022, 10, 976423. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Yuan, Z.; Huang, Y. Disinfectants against SARS-CoV-2: A Review. Viruses 2022, 14, 1721. [Google Scholar] [CrossRef] [PubMed]
- Mailhe, M.; Beaumont, A.-L.; Thy, M.; Le Pluart, D.; Perrineau, S.; Houhou-Fidouh, N.; Deconinck, L.; Bertin, C.; Ferré, V.M.; Cortier, M.; et al. Clinical Characteristics of Ambulatory and Hospitalised Patients with Monkeypox Virus Infection: An Observational Cohort Study. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2022, in press. [Google Scholar] [CrossRef]
- Juneau, C.-E.; Pueyo, T.; Bell, M.; Gee, G.; Collazzo, P.; Potvin, L. Lessons from Past Pandemics: A Systematic Review of Evidence-Based, Cost-Effective Interventions to Suppress COVID-19. Syst. Rev. 2022, 11, 90. [Google Scholar] [CrossRef]
- Mei, Y.; Guo, X.; Chen, Z.; Chen, Y. An Effective Mechanism for the Early Detection and Containment of Healthcare Worker Infections in the Setting of the COVID-19 Pandemic: A Systematic Review and Meta-Synthesis. Int. J. Environ. Res. Public. Health 2022, 19, 5943. [Google Scholar] [CrossRef]
- Spagnolo, L.; Vimercati, L.; Caputi, A.; Benevento, M.; De Maria, L.; Ferorelli, D.; Solarino, B. Role and Tasks of the Occupational Physician during the COVID-19 Pandemic. Med. Kaunas Lith. 2021, 57, 479. [Google Scholar] [CrossRef]
- Surveillance, Case Investigation and Contact Tracing for Monkeypox: Interim Guidance. Available online: https://www.who.int/publications-detail-redirect/WHO-MPX-Surveillance-2022.3 (accessed on 12 September 2022).
- Simpson, K.; Heymann, D.; Brown, C.S.; Edmunds, W.J.; Elsgaard, J.; Fine, P.; Hochrein, H.; Hoff, N.A.; Green, A.; Ihekweazu, C.; et al. Human Monkeypox—After 40 Years, an Unintended Consequence of Smallpox Eradication. Vaccine 2020, 38, 5077–5081. [Google Scholar] [CrossRef]
- Rao, A.K. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for Preexposure Vaccination of Persons at Risk for Occupational Exposure to Orthopoxviruses: Recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 734–742. [Google Scholar] [CrossRef]
- Vaccines and Immunization for Monkeypox: Interim Guidance, 24 August 2022. Available online: https://www.who.int/publications/i/item/WHO-MPX-Immunization-2022.2-eng (accessed on 12 September 2022).
- Peterson, C.J.; Lee, B.; Nugent, K. COVID-19 Vaccination Hesitancy among Healthcare Workers-A Review. Vaccines 2022, 10, 948. [Google Scholar] [CrossRef]
- Maltezou, H.C.; Gargalianos, P.; Nikolaidis, P.; Katerelos, P.; Tedoma, N.; Maltezos, E.; Lazanas, M. Attitudes towards Mandatory Vaccination and Vaccination Coverage against Vaccine-Preventable Diseases among Health-Care Workers in Tertiary-Care Hospitals. J. Infect. 2012, 64, 319–324. [Google Scholar] [CrossRef]
- Zachary, K.C.; Shenoy, E.S. Monkeypox Transmission Following Exposure in Healthcare Facilities in Nonendemic Settings: Low Risk but Limited Literature. Infect. Control Hosp. Epidemiol. 2022, 43, 920–924. [Google Scholar] [CrossRef]
- Lulli, L.G.; Giorgi, G.; Pandolfi, C.; Foti, G.; Finstad, G.L.; Arcangeli, G.; Mucci, N. Identifying Psychosocial Risks and Protective Measures for Workers’ Mental Wellbeing at the Time of COVID-19: A Narrative Review. Sustainability 2021, 13, 13869. [Google Scholar] [CrossRef]
- Baldassarre, A.; Giorgi, G.; Alessio, F.; Lulli, L.G.; Arcangeli, G.; Mucci, N. Stigma and Discrimination (SAD) at the Time of the SARS-CoV-2 Pandemic. Int. J. Environ. Res. Public. Health 2020, 17, E6341. [Google Scholar] [CrossRef] [PubMed]
- Grazzini, M.; Lulli, L.G.; Mucci, N.; Paolini, D.; Baldassarre, A.; Gallinoro, V.; Chiarelli, A.; Niccolini, F.; Arcangeli, G. Return to Work of Healthcare Workers after SARS-CoV-2 Infection: Determinants of Physical and Mental Health. Int. J. Environ. Res. Public. Health 2022, 19, 6811. [Google Scholar] [CrossRef] [PubMed]
- Multi-Country Outbreak of Monkeypox, External Situation Report #2–25 July 2022. Available online: https://www.who.int/publications/m/item/multi-country-outbreak-of-monkeypox--external-situation-report--2---25-july-2022 (accessed on 26 September 2022).
- Webb, E.; Rigby, I.; Michelen, M.; Dagens, A.; Cheng, V.; Rojek, A.M.; Dahmash, D.; Khader, S.; Gedela, K.; Norton, A.; et al. Availability, Scope and Quality of Monkeypox Clinical Management Guidelines Globally: A Systematic Review. BMJ Glob. Health 2022, 7, e009838. [Google Scholar] [CrossRef] [PubMed]
- Keblawi, B. Interim Guidelines for Monkeypox. 2022. Available online: https://covid19.cdc.gov.sa/wp-content/uploads/2022/08/Monkeypox-Guidelines-Aug15-V1.3_eng.pdf (accessed on 26 September 2022).
- Nigeria Centre for Disease Control. Available online: https://ncdc.gov.ng/ (accessed on 26 September 2022).
| Database | Search String |
|---|---|
| Pubmed | “MONKEYPOX VIRUS” AND (WORKER* OR OCCUPATION*) |
| Scopus | (TITLE-ABS-KEY (“MONKEYPOX VIRUS”) AND TITLE-ABS-KEY (WORKER*)) |
| Embase | (‘MONKEYPOX’/exp OR MONKEYPOX) AND (WORKER* OR ‘OCCUPATION’/exp OR OCCUPATION) |
| Web of Science | “MONKEYPOX VIRUS” (All Fields) AND “WORKER*” (All Fields) |
| Topic | Key Findings | Practical Implications |
|---|---|---|
| Occupational exposure | - Occupational exposure and infections have been reported in HCWs and in workers in contact with animals in previous outbreaks; - A limited number of studies are available, despite the endemic diffusion of monkeypox in central Africa since 1970; - The monkeypox virus can remain on surfaces for a long time, but further studies are needed to detect its infection potential. | - HCWs can be considered at a high risk; - Surveillance of exposed workers for 21 days is necessary; - Exposed workers should not work with immunocompromised patients; - Evidence on the possible transmission of monkeypox to animals (domestic and farm) are missing, but in the future, other job categories may be at risk. |
| Preventive measures | - PPE (disposable gown and gloves, eye protection, and FFP2 masks) are needed; - Education and training are preventive measures; - Risk assessment, contact tracing, and vaccination are valid post-exposure prevention measures; - Editorials, reviews, and WHO, CDC, and ECDC guidelines provide indications about appropriate practices. | - The widespread availability of PPE is mandatory, including in outpatient clinics; - Knowledge of the disease and confidence in the diagnosis are fundamental parts of the overall prevention strategy; - As learned from the COVID-19 pandemic, training and education are measures that also protect workers’ mental health; - Vaccination with VARV vaccines, predominantly as a secondary prevention act, is effective up to 85%. |
| Knowledge and attitudes of healthcare workers | - Outside endemic regions, healthcare knowledge about monkeypox is limited; - The attitude towards vaccination in the selected studies on the topic is moderate; - Only a few cross-sectional studies are available on this topic. | - Specific programs of education and training for the early recognition of the disease are necessary, especially in non-endemic countries; - Awareness of the biological risk is necessary in healthcare settings to implement prevention procedures; - Programs of vaccine sensibilization may be useful. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lulli, L.G.; Baldassarre, A.; Mucci, N.; Arcangeli, G. Prevention, Risk Exposure, and Knowledge of Monkeypox in Occupational Settings: A Scoping Review. Trop. Med. Infect. Dis. 2022, 7, 276. https://doi.org/10.3390/tropicalmed7100276
Lulli LG, Baldassarre A, Mucci N, Arcangeli G. Prevention, Risk Exposure, and Knowledge of Monkeypox in Occupational Settings: A Scoping Review. Tropical Medicine and Infectious Disease. 2022; 7(10):276. https://doi.org/10.3390/tropicalmed7100276
Chicago/Turabian StyleLulli, Lucrezia Ginevra, Antonio Baldassarre, Nicola Mucci, and Giulio Arcangeli. 2022. "Prevention, Risk Exposure, and Knowledge of Monkeypox in Occupational Settings: A Scoping Review" Tropical Medicine and Infectious Disease 7, no. 10: 276. https://doi.org/10.3390/tropicalmed7100276
APA StyleLulli, L. G., Baldassarre, A., Mucci, N., & Arcangeli, G. (2022). Prevention, Risk Exposure, and Knowledge of Monkeypox in Occupational Settings: A Scoping Review. Tropical Medicine and Infectious Disease, 7(10), 276. https://doi.org/10.3390/tropicalmed7100276









