Seropositivity of Anti-Toxoplasma gondii Antibodies in Owners and Their Dogs Living on Island and Mainland Seashore Areas of Southern Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
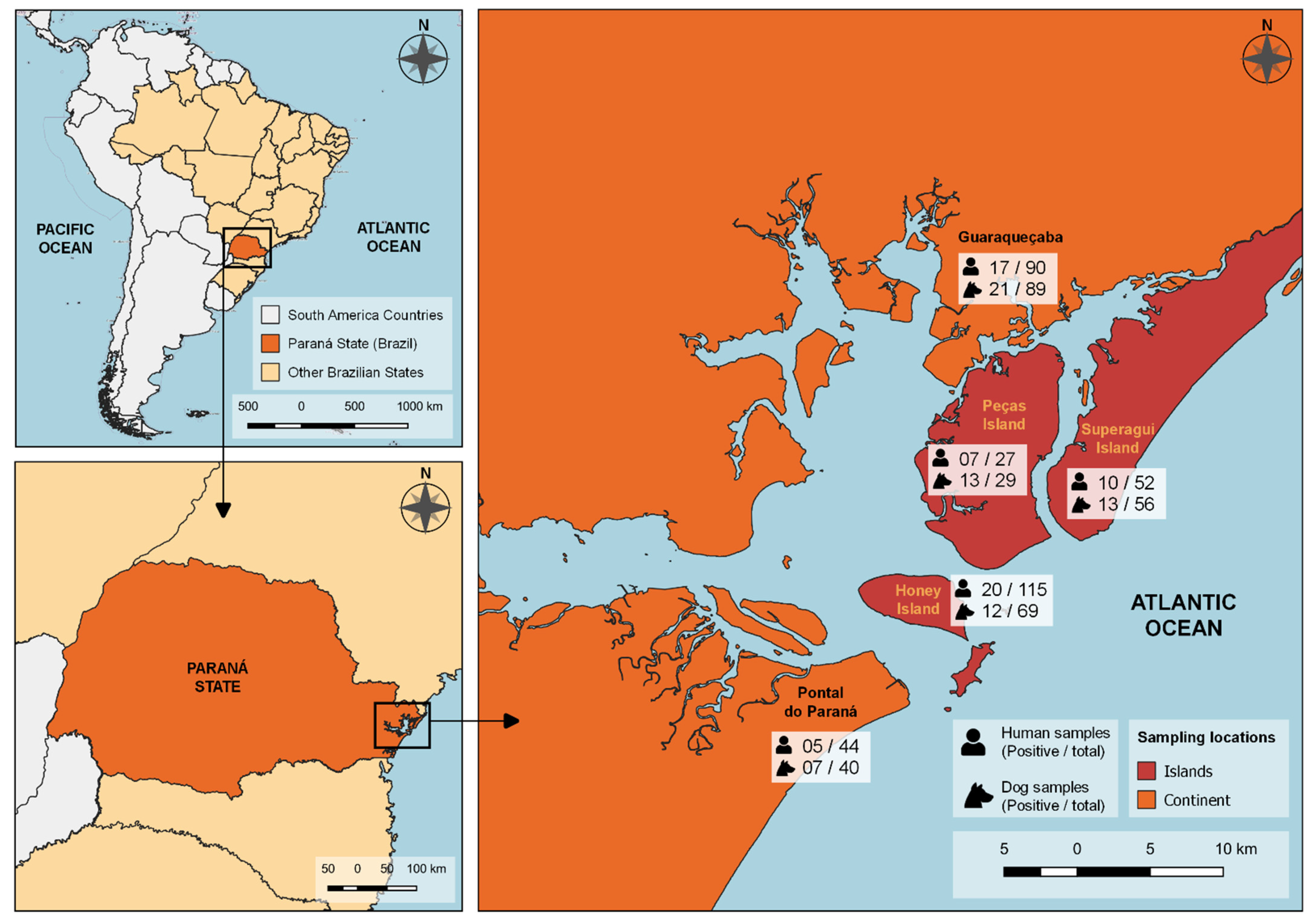
2.2. Local of Study
2.3. Blood Sample Collection
2.4. Serological Testing
2.5. Epidemiological Data Collection
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Povos e Comunidades Tradicionais—Ministério da Cidadania Secretaria Especial do Desenvolvimento Social, (n.d.). Available online: http://mds.gov.br/assuntos/seguranca-alimentar/direito-a-alimentacao/povos-e-comunidades-tradicionais (accessed on 8 November 2021).
- Plano Nacional de Operacionalização da Vacinação contra COVID-19—Português (Brasil), (n.d.). Available online: https://www.gov.br/saude/pt-br/coronavirus/publicacoes-tecnicas/guias-e-planos/plano-nacional-de-vacinacao-covid-19/view (accessed on 5 November 2021).
- Carvalho, M.D.C.; Ribeiro-Andrade, M.; de Melo, R.P.B.; Guedes, D.M.; Junior, J.W.P.; Cavalcanti, E.F.T.S.F.; Magalhães, F.J.R.; Mota, R.A. Cross-sectional survey for Toxoplasma gondii infection in humans in Fernando de Noronha island, Brazil. Rev. Bras. Parasitol. Vet. 2021, 30, e005121. [Google Scholar] [CrossRef]
- Magalhães, F.J.; Ribeiro-Andrade, M.; Souza, F.M.; Filho, C.D.L.; Biondo, A.W.; Vidotto, O.; Navarro, I.T.; Mota, R.A. Seroprevalence and spatial distribution of Toxoplasma gondii infection in cats, dogs, pigs and equines of the Fernando de Noronha Island, Brazil. Parasitol. Int. 2017, 66, 43–46. [Google Scholar] [CrossRef]
- Delai, R.R.; Freitas, A.R.; Kmetiuk, L.B.; Merigueti, Y.F.F.B.; Ferreira, I.B.; Lescano, S.A.Z.; Gonzáles, W.H.R.; Brandão, A.P.D.; de Barros-Filho, I.R.; Pettan-Brewer, C.; et al. One Health approach on human seroprevalence of anti-Toxocara antibodies, Toxocara spp. eggs in dogs and sand samples between seashore mainland and island areas of southern Brazil. One Health 2021, 13, 100353. [Google Scholar] [CrossRef]
- Acha, P.N.; Szyfres, B. Zoonosis y Enfermedades Transmisibles Comunes al Hombre y a los Animales; Volumen I: Bacteriosis y micosis; Pan American Health Organization: Washington, DC, USA, 2001; p. 398.
- Navarrete, M.G.; Cordeiro, M.D.; Batista, Y.; Alonso, J.C.; Márquez, M.; Roque, E.; Fonseca, A. Serological detection of Toxoplasma gondii in domestic dogs in the western region of Cuba. Veter. Parasitol. Reg. Stud. Rep. 2017, 9, 9–12. [Google Scholar] [CrossRef]
- Silva, R.; De Souza, L.C.; Langoni, H.; Tanaka, E.M.; De Lima, V.Y.; Silva, A. Risk factors and presence of antibodies to Toxoplasma gondii in dogs from the coast of São Paulo State, Brazil. Pesqui. Veterinária. Bras. 2010, 30, 161–166. [Google Scholar] [CrossRef]
- Da Cunha, G.R.; Pellizzaro, M.; Martins, C.M.; Rocha, S.M.; Yamakawa, A.C.; Da Silva, E.C.; Dos Santos, A.P.; Morikawa, V.M.; Langoni, H.; Biondo, A.W. Spatial serosurvey of anti-Toxoplasma gondii antibodies in individuals with animal hoarding disorder and their dogs in Southern Brazil. PLoS ONE 2020, 15, e0233305. [Google Scholar] [CrossRef]
- Meireles, L.R.; Galisteo, A.J.; Pompeu, E.; Andrade, H.F. Toxoplasma gondii spreading in an urban area evaluated by seroprevalence in free-living cats and dogs. Trop. Med. Int. Health 2004, 9, 876–881. [Google Scholar] [CrossRef]
- CDC-Parasites-Neglected Parasitic Infections (NPIs) in the United States, (n.d.). Available online: https://www.cdc.gov/parasites/npi/index.html (accessed on 24 March 2022).
- Parque Estadual da Ilha do Mel (PEIM) | Instituto Água e Terra, (n.d.). Available online: http://www.iat.pr.gov.br/Pagina/Parque-Estadual-da-Ilha-do-Mel-PEIM (accessed on 24 November 2021).
- IBGE | Cidades@ | Paraná | Pontal do Paraná | Panorama, (n.d.). Available online: https://cidades.ibge.gov.br/brasil/pr/pontal-do-parana/panorama (accessed on 24 November 2021).
- IBGE | Cidades@ | Paraná | Guaraqueçaba | Panorama, (n.d.). Available online: https://cidades.ibge.gov.br/brasil/pr/guaraquecaba/panorama (accessed on 24 November 2021).
- Cola, G.A.; Garcia, J.L.; Da Costa, L.; Ruffolo, B.; Navarro, I.T.; Freire, R.L. Freire, Comparison of the indirect fluorescent antibody test and modified agglutination test for detection of anti-Toxoplasma gondii antibodies in rats. Semin. Ciências Agrárias 2010, 31, 717–722. [Google Scholar] [CrossRef][Green Version]
- Comparação do ensaio imunoenzimático indireto (ELISA-teste) com a reação da imunofluorescência indireta na detecção de anticorpos IgG anti-Toxoplasma gondii em ovinos naturalmente infectados. Ci. Vet. Tróp. 2014, 17, 16–20. Available online: https://pesquisa.bvsalud.org/portal/resource/pt/vti-688292 (accessed on 11 June 2022).
- Uchôa, C.M.A.; Duarte, R.; Laurentino-Silva, V.; Alexandre, G.M.C.; Ferreira, H.G.; Amendoeira, M.R.R. Padronização de ensaio imunoenzimático para pesquisa de anticorpos das classes IgM e IgG anti-Toxoplasma gondii e comparação com a técnica de imunofluorescência indireta. Rev. Soc. Bras. Med. Trop. 1999, 32, 661–669. [Google Scholar] [CrossRef][Green Version]
- Franco, W.A.C.; Bergamaschi, D.P.; Richtzenhain, L.J.; Nogueira, Y.; Camargo, L.M.A.; de Souza, S.L.P.; Gennari, S.M. Evaluation of the performance of the modified direct agglutination test (MAT) for detection of Toxoplasma gondii antibodies in dogs. Braz. J. Veter. Res. Anim. Sci. 2003, 40, 452–456. [Google Scholar] [CrossRef]
- Dubey, J.P.; Hattel, A.L.; Lindsay, D.; Topper, M.J. Neonatal Neospora caninum infection in dogs: Isolation of the causative agent and experimental transmission. J. Am. Veter. Med Assoc. 1988, 193, 1259–1263. [Google Scholar]
- Dubey, J.P. Toxoplasmosis of Animals and Humans; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Mantovan, K.B.; Luiz, C.G.S.; Menozzi, B.D.; Nilsson, M.G.; Latosinski, G.S.; Langoni, H. Inquérito soroepidemiológico para toxoplasmose, leptospirose e leishmaniose visceral canina. Veterinária Zootec. 2021, 28, 1–10. [Google Scholar] [CrossRef]
- Callefe, J.L.R.; Langoni, H.; Mantovan, K.B. Detecção de imunoglobulinas anti- Toxoplasma gondii e anti- neospora caninum em bovinos no rio grande do sul, brasil. Veterinária Zootec. 2021, 28, 1–7. [Google Scholar] [CrossRef]
- R: The R Project for Statistical Computing, (n.d.). Available online: https://www.r-project.org/ (accessed on 4 November 2021).
- Subirana, I.; Sanz, H.; Vila, J. Building Bivariate Tables: The compare Groups Package for R. J. Stat. Softw. 2014, 57, 1–16. [Google Scholar] [CrossRef]
- Prevalence of Human Toxoplasmosis in san Carlos Island, Venezuela, (n.d.). Available online: http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0378-18442003000800005 (accessed on 4 November 2021).
- Ramos, R.C.F.; Palmer, J.P.S.; Dib, L.V.; Lobão, L.F.; Pinheiro, J.L.; Dos Santos, C.R.; Uchôa, C.M.A.; Bastos, O.M.P.; Da Silva, H.P.; Fonseca, A.B.M.; et al. Soropositividade e fatores de risco associados à infecção por Toxoplasma gondii em pacientes atendidos no Laboratório Municipal de Oriximiná, estado do Pará, Brasil. Rev. Pan Amaz. Saúde 2021, 12, 1–11. [Google Scholar] [CrossRef]
- Benitez, A.D.N.; Martins, F.D.C.; Mareze, M.; Santos, N.; Ferreira, F.P.; Martins, C.M.; Garcia, J.L.; Mitsuka-Breganó, R.; Freire, R.L.; Biondo, A.W.; et al. Spatial and simultaneous representative seroprevalence of anti-Toxoplasma gondii antibodies in owners and their domiciled dogs in a major city of southern Brazil. PLoS ONE 2017, 12, e0180906. [Google Scholar] [CrossRef]
- Machado, F.P.; Kmetiuk, L.B.; Teider-Junior, P.I.; Pellizzaro, M.; Yamakawa, A.C.; Martins, C.M.; Bach, R.V.W.; Morikawa, V.M.; De Barros-Filho, I.R.; Langoni, H.; et al. Seroprevalence of anti-Toxoplasma gondii antibodies in wild boars (Sus scrofa), hunting dogs, and hunters of Brazil. PLoS ONE 2019, 14, e0223474. [Google Scholar] [CrossRef]
- Etheredge, G.D.; Michael, G.; Muehlenbein, M.P.; Frenkel, J.K. The roles of cats and dogs in the transmission of Toxoplasma infection in Kuna and Embera children in eastern Panama. Rev. Panam. Salud Pública 2004, 16, 176–186. [Google Scholar] [CrossRef][Green Version]
- Xin, S.; Su, R.; Jiang, N.; Zhang, L.; Yang, Y. Low Prevalence of Antibodies Against Toxoplasma gondii in Chinese Populations. Front. Cell. Infect. Microbiol. 2020, 10, 302. [Google Scholar] [CrossRef]
- Jones, J.L.; Kruszon-Moran, D.; Elder, S.; Rivera, H.N.; Press, C.; Montoya, J.G.; McQuillan, G.M. Toxoplasma gondii Infection in the United States, 2011–2014. Am. J. Trop. Med. Hyg. 2018, 98, 551–557. [Google Scholar] [CrossRef]
- Dias, R.A.; Abrahão, C.R.; Micheletti, T.; Mangini, P.R.; Gasparotto, V.P.D.O.; Pena, H.F.D.J.; Ferreira, F.; Russell, J.C.; Silva, J.C.R. Prospects for domestic and feral cat management on an inhabited tropical island. Biol. Invasions 2017, 19, 2339–2353. [Google Scholar] [CrossRef]
- Fernando de Noronha (PE) | Cidades e Estados | IBGE. Available online: https://www.ibge.gov.br/cidades-e-estados/pe/fernando-de-noronha.html (accessed on 7 June 2022).
- Dubey, J.P. Toxoplasma gondii oocyst survival under defined temperatures. J. Parasitol. 1998, 84, 862. [Google Scholar] [CrossRef]
- Almeria, S.; Dubey, J. Foodborne transmission of Toxoplasma gondii infection in the last decade. An overview. Res. Veter. Sci. 2021, 135, 371–385. [Google Scholar] [CrossRef]
- Smith, N.C.; Goulart, C.; Hayward, J.A.; Kupz, A.; Miller, C.M.; van Dooren, G.G. Control of human toxoplasmosis. Int. J. Parasitol. 2021, 51, 95–121. [Google Scholar] [CrossRef]
- Pinto-Ferreira, F.; Caldart, E.T.; Freire, R.L.; Mitsuka-Breganó, R.; De Freitas, F.M.; Miura, A.C.; Mareze, M.; Martins, F.D.C.; Urbano, M.; Seifert, A.L.; et al. The effect of water source and soil supplementation on parasite contamination in organic vegetable gardens. Rev. Bras. Parasitol. Veterinária 2018, 27, 327–337. [Google Scholar] [CrossRef]
- Bahia-Oliveira, L.; Gomez-Marin, J.; Shapiro, K. Toxoplasma gondii. In Water and Sanitation for the 21st Century: Health and Microbiological Aspects of Excreta and Wastewater Management (Global Water Pathogen Project); Michigan State University: East Lansing, MI, USA, 2019. [Google Scholar] [CrossRef]
- De Moura, L.; Bahia-Oliveira, L.M.G.; Wada, M.Y.; Jones, J.L.; Tuboi, S.H.; Carmo, E.H.; Ramalho, W.M.; Camargo, N.J.; Trevisan, R.; Graça, R.M.T.; et al. Waterborne Toxoplasmosis, Brazil, from Field to Gene. Emerg. Infect. Dis. 2006, 12, 326–329. [Google Scholar] [CrossRef]
- e Silva, G.N.D.R.; Branco, M.D.R.F.C.; Rodrigues, Z.M.R.; dos Santos, A.M.; Pereira, P.R.M.; da Silva, M.D.S.; Nunes, A.T.D.S.; Júnior, A.R.G.; Medeiros, M.N.L.; Azevedo, C.D.M.P.E.S.D.; et al. Toxoplasmosis outbreak in Brazil, 2006-Revisited. Parasite Epidemiol. Control 2019, 7, e00117. [Google Scholar] [CrossRef]
- Minuzzi, C.E.; Fernandes, F.D.; Portella, L.P.; Bräunig, P.; Sturza, D.A.F.; Giacomini, L.; Salvagni, E.; Ribeiro, J.D.S.; Silva, C.R.; Difante, C.M.; et al. Contaminated water confirmed as source of infection by bioassay in an outbreak of toxoplasmosis in South Brazil. Transbound. Emerg. Dis. 2020, 68, 767–772. [Google Scholar] [CrossRef]
- Eng, S.B.; Werker, D.H.; King, A.S.; Marion, S.A.; Bell, A.; Issac-Renton, J.L.; Irwin, G.S.; Bowie, W.R. Computer-Generated Dot Maps as an Epidemiologic Tool: Investigating an Outbreak of Toxoplasmosis. Emerg. Infect. Dis. 1999, 5, 815–819. [Google Scholar] [CrossRef]
- Dumètre, A.; Dubey, J.P.; Ferguson, D.J. Effect of household bleach on the structure of the sporocyst wall of Toxoplasma gondii. Parasite 2021, 28, 68. [Google Scholar] [CrossRef]
- Pinto-Ferreira, F.; Paschoal, A.T.P.; Pasquali, A.K.S.; Bernardes, J.C.; Caldart, E.T.; Freire, R.L.; Mitsuka-Breganó, R.; Navarro, I.T. Techniques for inactivating Toxoplasma gondii oocysts: A systematic review. Orgao Off. Do Col. Bras. Parasitol. Vet. 2021, 30, e026420. [Google Scholar] [CrossRef]
- Felipetto, L.G.; Teider-Junior, P.I.; Da Silva, F.F.V.; Yamakawa, A.C.; Kmetiuk, L.B.; Couto, A.C.D.; Martins, C.M.; Vaz, E.S.; Ullmann, L.S.; Langoni, H.; et al. Serosurvey of Anti-Toxoplasma gondii Antibodies in Homeless Persons of São Paulo City, Southeastern Brazil. Front. Public Health 2020, 8, 580637. [Google Scholar] [CrossRef]
- Pena, H.F.J.; Alves, B.F.; Soares, H.S.; Oliveira, S.; Ferreira, M.N.; Bricarello, P.A.; Machado, T.M.P.; Castro, B.B.P.; Gennari, S.M. Free-range chickens from Santa Catarina state, southern Brazil, as asymptomatic intermediate hosts for Toxoplasma gondii clonal type I and typical Brazilian genotypes. Veter. Parasitol. Reg. Stud. Rep. 2018, 13, 55–59. [Google Scholar] [CrossRef]
- Gomes, D.F.C.; Krawczak, F.D.S.; De Oliveira, C.H.S.; Júnior, F.; Fernandes, E.; Lopes, W.D.Z.; Sevá, A.D.P.; Gennari, S.M. Toxoplasma gondii in cattle in Brazil: A review. Rev. Bras. Parasitol. Vet. 2020, 29, e015719. [Google Scholar] [CrossRef]
- Deiró, A.G.D.J.; Montargil, S.M.A.; Carvalho, F.S.; Munhoz, A.D.; Albuquerque, G.R. Antibody occurrence of Anti-Toxoplasma gondii, Leishmania sp. and Ehrlichia canis in dogs in Bahia State. Semin. Ciências Agrárias 2018, 39, 199–210. [Google Scholar] [CrossRef]
- Ocurrence of anti-Toxoplasma gondii antibodies and the risk factors associated with canine infection at ilhéus-itabuna region in the state of bahia. Braz. J. Vet. Med. 2010, 32, 115–121. Available online: https://rbmv.org/BJVM/article/view/840 (accessed on 5 November 2021).
- Arruda, I.F.; Millar, P.R.; Barbosa, A.D.S.; Abboud, L.C.D.S.; dos Reis, I.C.; Moreira, A.S.d.C.; Guimarães, M.P.d.P.; Amendoeira, M.R.R. Toxoplasma gondii in domiciled dogs and cats in urban areas of Brazil: Risk factors and spatial distribution. Parasite 2021, 28, 56. [Google Scholar] [CrossRef]
- Sheng, Z.; Jin, Y.; Yao, Y.; El-Ashram, S.; Shen, J.; Wang, X.-L.; Ji, Y. Seroprevalence of Toxoplasma gondii Infection in Pet Dogs in Anhui Province, China. Iran. J. Parasitol. 2020, 15, 446–451. [Google Scholar] [CrossRef]
- Duan, G.; Tian, Y.-M.; Li, B.-F.; Yang, J.-F.; Liu, Z.-L.; Yuan, F.-Z.; Zhu, X.-Q.; Zou, F.-C. Seroprevalence of Toxoplasma gondii infection in pet dogs in Kunming, Southwest China. Parasites Vectors 2012, 5, 118. [Google Scholar] [CrossRef]
- Frenkel, J.K.; Parker, B.B. An Apparent Role of Dogs in the Transmission of Toxoplasma gondii: The Probable Importance of Xenosmophilia. Ann. N. Y. Acad. Sci. 1996, 791, 402–407. [Google Scholar] [CrossRef]
- Sepúlveda-Arias, J.C.; Gómez-Marin, J.E.; Bobić, B.; Naranjo-Galvis, C.A.; Djurković-Djaković, O. Toxoplasmosis as a travel risk. Travel Med. Infect. Dis. 2014, 12, 592–601. [Google Scholar] [CrossRef]
| Variable | Bivariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Anti-T. gondii Antibodies | OR (95% IC) | p Value | OR (95% IC) | p Value | ||
| Seropositive No. (%) | Seronegative No. (%) | |||||
| 59/328 (18.0) | 269/328 (82.0) | |||||
| Household Location | 0.562 | |||||
| Seashore mainland | 22 (37.3) | 112 (41.6) | Ref | |||
| Island | 37 (62.7) | 157 (58.4) | 0.8 (0.47–1.49) | |||
| Gender | 0.098 | |||||
| Female | 31 (52.5) | 175 (65.1) | Ref | Ref | ||
| Male | 28 (47.5) | 94 (34.9) | 1.7 (0.94–2.98) | 1.6 (0.87–2.85) | 0.149 | |
| Age (Years Old) | 0.322 | |||||
| >18 | 55 (93.2) | 258 (95.9) | Ref | |||
| <18 | 4 (6.8) | 11 (4.1) | 0.6 (0.19–0.20) | |||
| Education Level | 0.682 | |||||
| >Elementary school | 37 (62.7) | 179 (66.5) | Ref | |||
| ≤Elementary school | 22 (37.3) | 90 (33.5) | 1.2 (0.65–2.12) | |||
| Income | 0.627 | |||||
| >1 minimum wage | 40 (70.2) | 165 (65.7) | Ref | |||
| ≤1 minimum wage | 17 (29.8) | 86 (34.3) | 0.8 (0.43–1.51) | |||
| Dog Owner | 0.856 | |||||
| No | 7 (11.9) | 27 (10.0) | Ref | |||
| Yes | 52 (88.1) | 242 (90.0) | 0.8 (0.35–2.15) | |||
| Cat Owner | 0.579 | |||||
| No | 39 (66.1) | 164 (61.2) | Ref | |||
| Yes | 20 (33.9) | 104 (38.8) | 0.8 (0.44–1.46) | |||
| Water Consumption | ||||||
| Treated water | 0.047 * | |||||
| No | 27 (45.8) | 84 (31.2) | Ref | |||
| Yes | 32 (54.2) | 185 (68.8) | 0.5 (0.30–0.96) | |||
| Well Water | 1 | |||||
| No | 52 (88.1) | 236 (87.7) | Ref | |||
| Yes | 7 (11.9) | 33 (12.3) | 1.0 (0.38–2.23) | |||
| Spring Water | 0.016 | |||||
| No | 33 (55.9) | 196 (72.9) | Ref | Ref | ||
| Yes | 26 (44.1) | 73 (27.1) | 2.1 (1.17–3.78) | 2.0 (1.08–3.57) | 0.027 | |
| Wash Fruits/Vegetables | ||||||
| Before consumption | 0.118 | |||||
| No | 4 (6.8) | 7 (2.6) | Ref | Ref | ||
| Yes | 55 (93.2) | 260 (97.4) | 0.4 (0.10–1.49) | 0.3 (0.06–1.11) | 0.054 | |
| Only Water | 0.177 | |||||
| No | 14 (23.7) | 90 (33.8) | Ref | Ref | ||
| Yes | 45 (76.3) | 176 (66.2) | 1.6 (0.87–3.24) | 1.8 (0.87–4.0) | 0.132 | |
| Water and Vinegar | 0.546 | |||||
| No | 48 (81.4) | 204 (76.7) | Ref | |||
| Yes | 11 (18.6) | 62 (23.3) | 0.8 (0.35–1.51) | |||
| Water with Sodium Hypochlorite | 0.297 | |||||
| No | 56 (94.9) | 238 (89.5) | Ref | |||
| Yes | 3 (5.08) | 28 (10.5) | 0.5 (0.11–1.42) | |||
| Consumption of Raw or Undercooked Meat | 0.3 | |||||
| No | 36 (61.0) | 185 (69.0) | Ref | |||
| Yes | 23 (39.0) | 83 (31.0) | 1.4 (0.78–2.55) | |||
| Cattle | 0.276 | |||||
| No | 38 (64.4) | 193 (72.6) | Ref | |||
| Yes | 21 (35.6) | 73 (27.4) | 1.5 (0.79–2.65) | |||
| Pig | 1 | |||||
| No | 56 (94.9) | 249 (93.6) | Ref | |||
| Yes | 3 (5.1) | 17 (6.4) | 0.8 (0.18–2.57) | |||
| Chicken | 0.746 | |||||
| No | 57 (96.6) | 252 (94.7) | Ref | |||
| Yes | 2 (3.4) | 14 (5.3) | 0.7 (0.09–2.53) | |||
| Fish | 0.924 | |||||
| No | 49 (83.1) | 225 (84.6) | Ref | |||
| Yes | 10 (16.9) | 41 (15.4) | 1.1 (0.50–2.34) | |||
| Variable | Bivariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Anti-T. gondii Antibodies | OR (95% CI) | p Value | OR (95% CI) | p Value | ||
| Seropositive (%) | Seronegative (%) | |||||
| 66/283 (23.3) | 217/283 (76.7) | |||||
| Household Location | 0.655 | |||||
| Seashore mainland | 38 (57.6) | 116 (53.5) | Ref | |||
| Island | 28 (42.4) | 101 (46.5) | 0.9 (0.48–1.48) | |||
| Sex | 1 | |||||
| Female | 32 (48.5) | 106 (48.8) | Ref | |||
| Male | 34 (51.5) | 111 (51.2) | 1.0 (0.58–1.77) | |||
| Cohabitate with Another Dog | 0.485 | |||||
| No | 58 (87.9) | 199 (91.7) | Ref | |||
| Yes | 8 (12.1) | 18 (8.29) | 1.5 (0.60–3.64) | |||
| Dog and Cat Cohabitate | 1 | |||||
| No | 64 (97.0) | 211 (97.2%) | Ref | |||
| Yes | 2 (3.03) | 6 (2.76) | 1.2 (0.15–5.33) | |||
| Seropositive Owner | 0.016 | |||||
| No | 52 (78.8) | 197 (90.8) | Ref | Ref. | ||
| Yes | 14 (21.2) | 20 (9.22) | 2.7 (1.23–5.60) | 2.8 (1.30–6.0) | 0.008 | |
| Dog Diet | ||||||
| Dry Food | 0.532 | |||||
| No | 2 (3.03) | 12 (5.56) | Ref. | |||
| Yes | 64 (97.0) | 204 (94.4) | 1.8 (0.46–12.7) | |||
| Home Cooked | 0.054 | |||||
| No | 19 (28.8) | 93 (43.1) | Ref | Ref | ||
| Yes | 47 (71.2) | 123 (56.9) | 1.9 (1.03–3.45) | 1.7 (0.93–3.20) | 0.089 | |
| Meat Consumption | ||||||
| Cattle | 0.042 * | |||||
| No | 50 (75.8) | 189 (87.1) | Ref | |||
| Yes | 16 (24.2) | 28 (12.9) | 2.2(1.06–4.28) | |||
| Pig | 0.22 | |||||
| No | 62 (93.9) | 212 (97.7) | Ref | |||
| Yes | 4 (6.06) | 5 (2.30) | 2.7(0.63–11.1) | |||
| Chicken | 0.026 | |||||
| No | 57 (86.4) | 206 (94.9) | Ref | Ref | ||
| Yes | 9 (13.6) | 11 (5.07) | 3.0 (1.12–7.56) | 2.9 (1.10–7.51) | 0.028 | |
| Fish | 0.101 * | |||||
| No | 60 (90.9) | 209 (96.3) | Ref | |||
| Yes | 6 (9.09) | 8 (3.69) | 2.6 (0.81–7.97) | |||
| Water Consumption | ||||||
| Tap Water | 0.243 | |||||
| No | 29 (43.9) | 76 (35.0) | Ref | |||
| Yes | 37 (56.1) | 141 (65.0) | 0.7 (0.39–1.21) | |||
| Well Water | 0.923 | |||||
| No | 59 (89.4) | 197 (90.8) | Ref | |||
| Yes | 7 (10.6) | 20 (9.22) | 1.2 (0.44–2.84) | |||
| Beach Access | 0.673 | |||||
| No | 23 (34.8) | 84 (38.7) | Ref | |||
| Yes | 43 (65.2) | 133 (61.3) | 1.2 (0.67–2.12) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, A.R.; Delai, R.R.; Kmetiuk, L.B.; da Silva, E.C.; Martini, R.; Brandão, A.P.D.; Giuffrida, R.; de Barros-Filho, I.R.; Costa da Silva, R.; Langoni, H.; et al. Seropositivity of Anti-Toxoplasma gondii Antibodies in Owners and Their Dogs Living on Island and Mainland Seashore Areas of Southern Brazil. Trop. Med. Infect. Dis. 2022, 7, 252. https://doi.org/10.3390/tropicalmed7100252
Freitas AR, Delai RR, Kmetiuk LB, da Silva EC, Martini R, Brandão APD, Giuffrida R, de Barros-Filho IR, Costa da Silva R, Langoni H, et al. Seropositivity of Anti-Toxoplasma gondii Antibodies in Owners and Their Dogs Living on Island and Mainland Seashore Areas of Southern Brazil. Tropical Medicine and Infectious Disease. 2022; 7(10):252. https://doi.org/10.3390/tropicalmed7100252
Chicago/Turabian StyleFreitas, Aaronson Ramathan, Ruana Renostro Delai, Louise Bach Kmetiuk, Evelyn Cristine da Silva, Rafaella Martini, Ana Pérola Drulla Brandão, Rogério Giuffrida, Ivan Roque de Barros-Filho, Rodrigo Costa da Silva, Hélio Langoni, and et al. 2022. "Seropositivity of Anti-Toxoplasma gondii Antibodies in Owners and Their Dogs Living on Island and Mainland Seashore Areas of Southern Brazil" Tropical Medicine and Infectious Disease 7, no. 10: 252. https://doi.org/10.3390/tropicalmed7100252
APA StyleFreitas, A. R., Delai, R. R., Kmetiuk, L. B., da Silva, E. C., Martini, R., Brandão, A. P. D., Giuffrida, R., de Barros-Filho, I. R., Costa da Silva, R., Langoni, H., Figueiredo, F. B., Pimpão, C. T., Dos Santos, A. P., Santarém, V. A., & Biondo, A. W. (2022). Seropositivity of Anti-Toxoplasma gondii Antibodies in Owners and Their Dogs Living on Island and Mainland Seashore Areas of Southern Brazil. Tropical Medicine and Infectious Disease, 7(10), 252. https://doi.org/10.3390/tropicalmed7100252









