Acalculous Cholecystitis in a Young Adult with Scrub Typhus: A Case Report and Epidemiology of Scrub Typhus in the Maldives
Abstract
1. Introduction
2. Materials and Methods
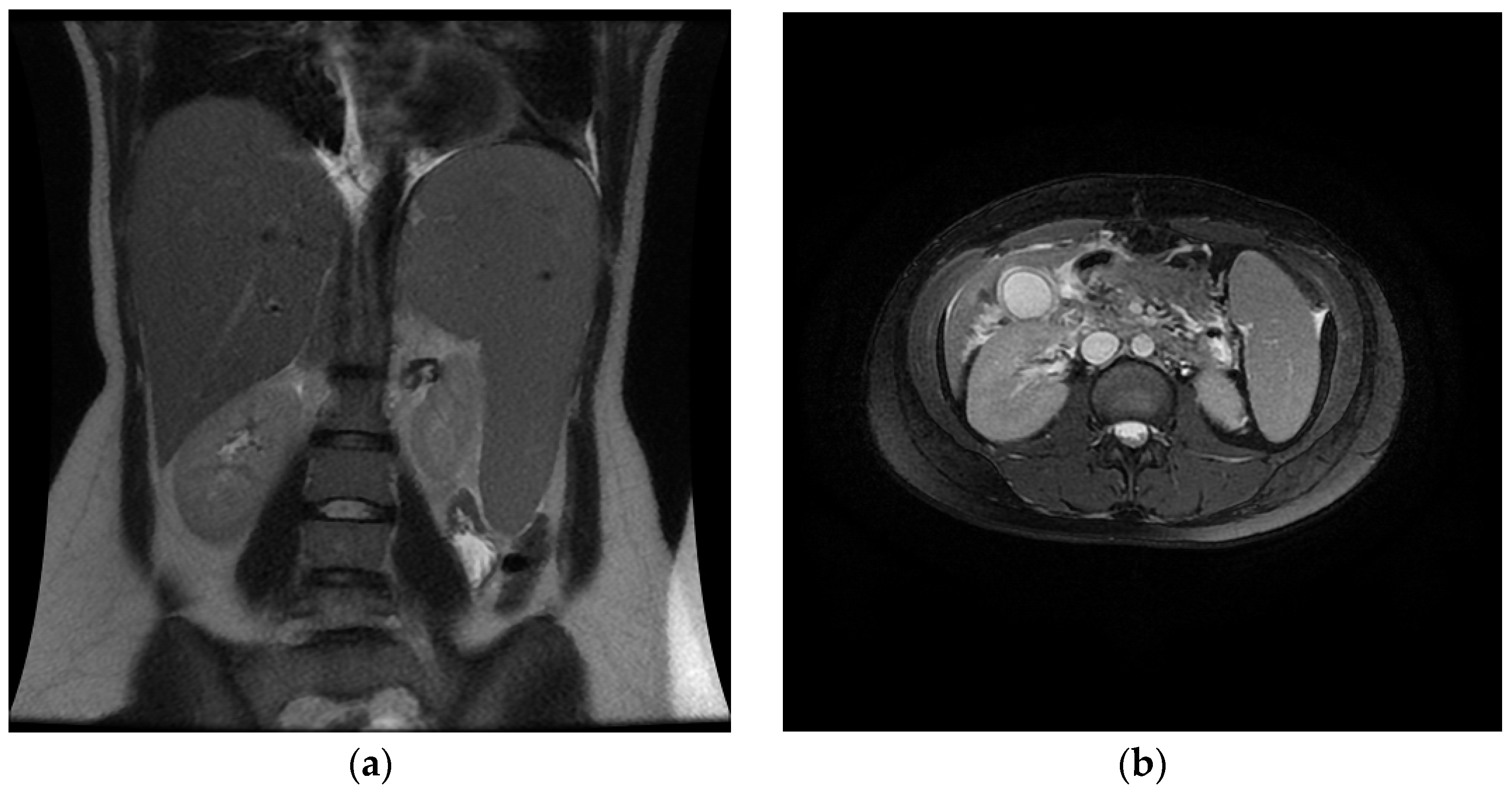
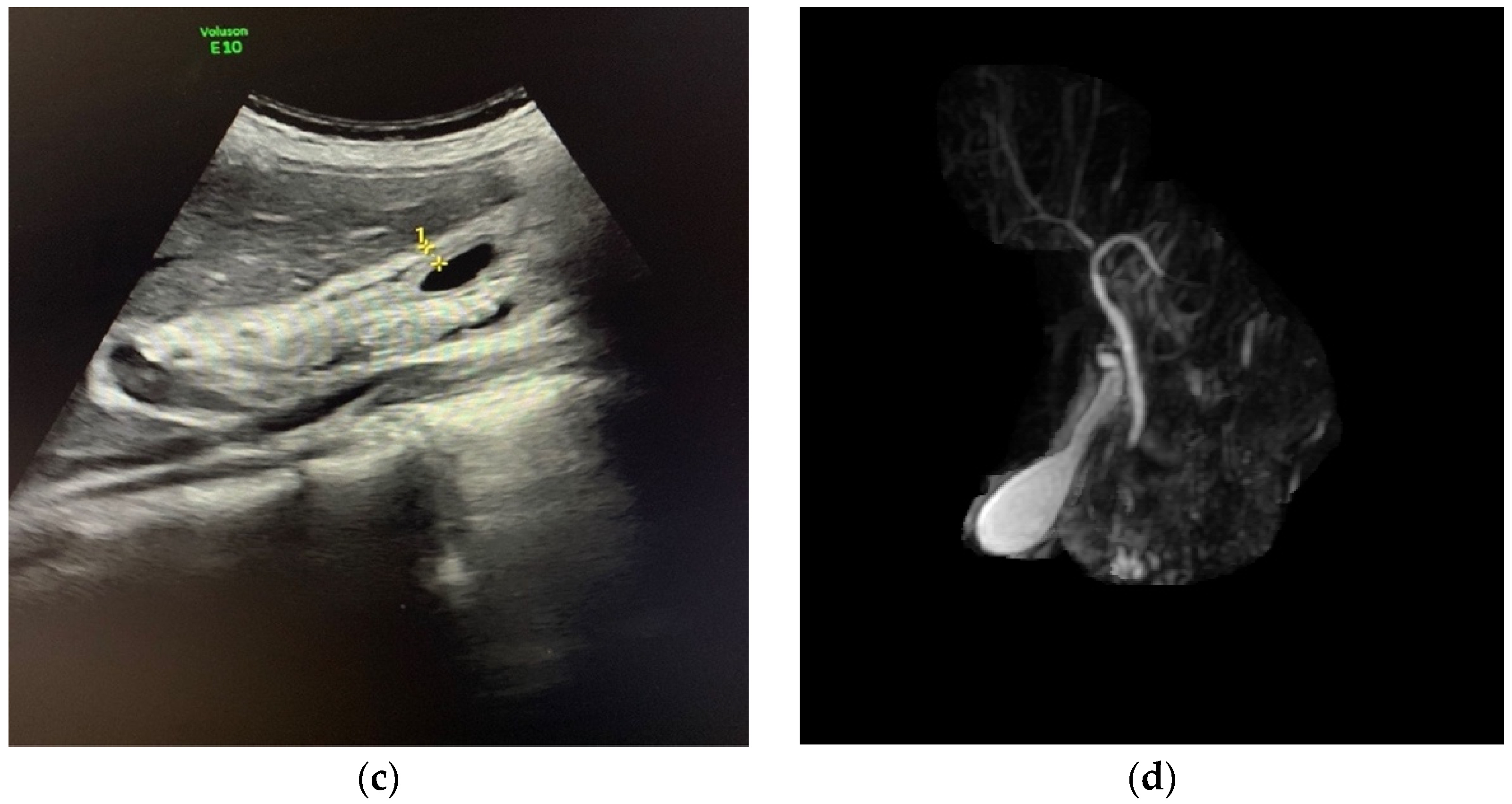
3. Case Report
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paris, D.H. Special Issue “The Past and Present Threat of Rickettsial Diseases”. Trop. Med. Infect. Dis. 2020, 5, 187. [Google Scholar] [CrossRef]
- Kelly, D.J.; Fuerst, P.A.; Richards, A.L. Origins, Importance and Genetic Stability of the Prototype Strains Gilliam, Karp and Kato of Orientia tsutsugamushi. Trop. Med. Infect. Dis. 2019, 4, 75. [Google Scholar] [CrossRef]
- Richards, A.L.; Jiang, J. Scrub Typhus: Historic Perspective and Current Status of the Worldwide Presence of Orientia Species. Trop. Med. Infect. Dis. 2020, 5, 49. [Google Scholar] [CrossRef]
- Nagayo, M.; Miyagawa, Y.; Mitamura, T.; Imamura, A. On the Nymph and Prosopon of the Tsutsugamushi, Leptotrombidium Akamushi, N. Sp. (Trombidium Akamushi Brumpt), Carrier of the Tsutsugamushi Disease. J. Exp. Med. 1917, 25, 255–272. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Imad, H.A.; Tanyaratsrisakul, S.; Piyaphanee, W.; Wattanagoon, Y. Skin lesion from Maldives: Classic but forgotten. Travel Med. Infect. Dis. 2017, 17, 74–75. [Google Scholar] [CrossRef] [PubMed]
- Hase, T.; Roberts, L.W.; Hildebrandt, P.K.; Cavanaugh, D.C. Stylostome Formation by Leptotrombidium Mites (Acari: Trombiculidae). J. Parasitol. 1978, 64, 712. [Google Scholar] [CrossRef] [PubMed]
- Megaw, J.W.D. Scrub Typhus as a War Disease. BMJ 1945, 2, 109–112. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Philip, C.B. Tsutsugamushi disease in World War II. J. Parasitol. 1948, 34, 169–191. [Google Scholar] [CrossRef]
- Luce-Fedrow, A.; Lehman, M.L.; Kelly, D.J.; Mullins, K.; Maina, A.N.; Stewart, R.L.; Ge, H.; John, H.S.; Jiang, J.; Richards, A.L. A Review of Scrub Typhus (Orientia tsutsugamushi and Related Organisms): Then, Now, and Tomorrow. Trop. Med. Infect. Dis. 2018, 3, 8. [Google Scholar] [CrossRef]
- Giles, H.M.; Symington, T. Chloromycetin in scrub-typhus. Lancet 1950, 255, 16–19. [Google Scholar] [CrossRef]
- Weitzel, T.; la Fuente, M.C.S.-D.; Martínez-Valdebenito, C.; Stekolnikov, A.A.; Pérez, C.; Pérez, R.; Vial, C.; Abarca, K.; Acosta-Jamett, G. Novel Vector of Scrub Typhus in Sub-Antarctic Chile: Evidence from Human Exposure. Clin. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Izzard, L.; Fuller, A.; Blacksell, S.D.; Paris, D.H.; Richards, A.L.; Aukkanit, N.; Nguyen, C.; Jiang, J.; Fenwick, S.; Day, N.P.J.; et al. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J. Clin. Microbiol. 2010, 48, 4404–4409. [Google Scholar] [CrossRef]
- Ghorbani, R.P.; Ghorbani, A.J.; Jain, M.K.; Walker, D.H. A Case of Scrub Typhus Probably Acquired in Africa. Clin. Infect. Dis. 1997, 25, 1473–1474. [Google Scholar] [CrossRef]
- Osuga, K.; Kimura, M.; Goto, H.; Shimada, K.; Suto, T. A case of tsutsugamushi disease probably contracted in Africa. Eur. J. Clin. Microbiol. Infect. Dis. 1991, 10, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Horton, K.C.; Maina, A.; Dueger, E.; Pimentel, G.; Jiang, J.; Richards, A.L.; Zayed, A.; Ahmed, A.A. Evidence of Rickettsia and Orientia Infections among Abattoir Workers in Djibouti. Am. J. Trop. Med. Hyg. 2016, 95, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Thiga, J.W.; Mutai, B.K.; Eyako, W.K.; Ng’Ang’A, Z.; Jiang, J.; Richards, A.L.; Waitumbi, J.N. High Seroprevalence of Antibodies against Spotted Fever and Scrub Typhus Bacteria in Patients with Febrile Illness, Kenya. Emerg. Infect. Dis. 2015, 21, 688–691. [Google Scholar] [CrossRef]
- Masakhwe, C.; Linsuwanon, P.; Kimita, G.; Mutai, B.; Leepitakrat, S.; Yalwala, S.; Abuom, D.; Auysawasi, N.; Gilbreath, T.; Wanja, E.; et al. Identification and Characterization of Orientia chuto in Trombiculid Chigger Mites Collected from Wild Rodents in Kenya. J. Clin. Microbiol. 2018, 56, e01124-18. [Google Scholar] [CrossRef]
- Yen, T.-Y.; Zhang, Z.; Chao, C.-C.; Ching, W.-M.; Shu, P.-Y.; Tseng, L.-F.; Carvalho, A.V.D.A.; Tsai, K.-H. Serologic Evidence for Orientia Exposure in the Democratic Republic of Sao Tome and Principe. Vector-Borne Zoonotic Dis. 2019, 19, 821–827. [Google Scholar] [CrossRef]
- Lewis, M.D.; Yousuf, A.A.; Lerdthusnee, K.; Razee, A.; Chandranoi, K.; Jones, J.W. Scrub typhus reemergence in the Maldives. Emerg. Infect. Dis. 2003, 9, 1638–1641. [Google Scholar] [CrossRef]
- Scrub Typhus Surveillance Data; Health Protection Agency, Ministry of Health: Malé, Maldives, 2021.
- Rajapakse, S.; Weeratunga, P.; Sivayoganathan, S.; Fernando, S.D. Clinical manifestations of scrub typhus. Trans. R. Soc. Trop. Med. Hyg. 2017, 111, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.; Goutos, I.; Dheansa, B. Acute Acalculous Cholecystitis in Burns: A Review. J. Burn Care Res. 2018, 39, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Dong, Z.; Wang, C.; Yang, W. Chinese Obesity and Metabolic Surgery Collaborative. Acute Gangrenous Acalculous Cholecystitis After Laparoscopic Roux-en-Y Gastric Bypass: A Case Report. Obes. Surg. 2021, 1–2. [Google Scholar] [CrossRef]
- Shapiro, M.J.; Luchtefeld, W.B.; Kurzweil, S.; Kaminski, D.L.; Durham, R.M.; Mazuski, J.E. Acute acalculous cholecystitis in the critically ill. Am. Surg. 1994, 60, 335–339. [Google Scholar] [PubMed]
- McChesney, J.A.; Northup, P.G.; Bickston, S.J. Acute Acalculous Cholecystitis Associated with Systemic Sepsis and Visceral Arterial Hypoperfusion: A Case Series and Review of Pathophysiology. Dig. Dis. Sci. 2003, 48, 1960–1967. [Google Scholar] [CrossRef]
- Markaki, I.; Konsoula, A.; Markaki, L.; Spernovasilis, N.; Papadakis, M. Acute acalculous cholecystitis due to infectious causes. World J. Clin. Cases 2021, 9, 6674–6685. [Google Scholar] [CrossRef]
- Reed, A.C. Scrub Typhus. Cal. West Med. 1944, 61, 62–63. [Google Scholar]
- Wang, N.-C.; Ni, Y.-H.; Peng, M.-Y.; Chang, F.-Y. Acute acalculous cholecystitis and pancreatitis in a patient with concomitant leptospirosis and scrub typhus. J. Microbiol. Immunol. Infect. 2003, 36, 285–287. [Google Scholar]
- Hayakawa, K.; Oki, M.; Moriya, Y.; Mizuma, A.; Ohnuki, Y.; Yanagi, H.; Fukuda, R.; Ozawa, H.; Takizawa, S.; Takagi, A. A case of scrub typhus with acalculous cholecystitis, aseptic meningitis and mononeuritis multiplex. J. Med. Microbiol. 2012, 61, 291–294. [Google Scholar] [CrossRef]
- Lee, H.; Ji, M.; Hwang, J.-H.; Lee, J.-Y.; Lee, J.-H.; Chung, K.M.; Lee, C.-S. Acute Cholecystitis in Patients with Scrub Typhus. J. Korean Med. Sci. 2015, 30, 1698–1700. [Google Scholar] [CrossRef]
- Lee, S.J.; Cho, Y.H.; Lee, S.Y.; Jeong, D.W.; Choi, E.J.; Kim, Y.J.; Lee, J.G.; Lee, Y.H. A Case of Scrub Typhus Complicated by Acute Calculous Cholecystitis. Korean J. Fam. Med. 2012, 33, 243–246. [Google Scholar] [CrossRef]
- Charoenphak, S.; Rattanawong, P.; Sungkanuparph, S. Acute Cholecystitis as an Unusual Presentation of Scrub Typhus: A Report of Two Cases and Review of The Literature. S. Asian J. Trop. Med. Public Health 2017, 48, 143–149. [Google Scholar]
- Acharya, S.; Yadav, J.K.; Khanal, N.; Bhandari, R.; Ghimire, B. Acute Severe Calculous Cholecystitis with Multiorgan Failure Complicated by Scrub Typhus. Case Rep. Surg. 2019, 2019, 1–4. [Google Scholar] [CrossRef]
- Gagneux-Brunon, A.; Suy, F.; Pouvaret, A.; Pillet, S.; Tarantino, E.; Bouchet, D.; Fresard, A.; Cazorla, C.; Guglielminotti, C.; Lucht, F.; et al. Acute acalculous cholecystitis, a rare complication of Epstein-Barr virus primary infection: Report of two cases and review. J. Clin. Virol. 2014, 61, 173–175. [Google Scholar] [CrossRef]
- Aydin Teke, T.; Tanir, G.; Ozel, A.; Timur, O.M.; Eksioglu, A.S. A case of acute acalculous cholecystitis during the course of reactive Epstein-Barr virus infection. Turk. J. Gastroenterol. 2013, 24, 571–572. [Google Scholar] [CrossRef]
- Vermaak, J.S. Epstein–Barr virus acute acalculous cholecystitis. Can. Med. Assoc. J. 2021, 193, E1143. [Google Scholar] [CrossRef] [PubMed]
- Harvey, K.G.; Tice, J.G.; Sigal, A. Epstein-Barr Virus Causing Clinical Jaundice and Acute Acalculous Cholecystitis in a Previously Healthy 17-Year-Old Girl. Am. J. Case Rep. 2021, 22, e932285. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Miyata, Y. Epstein–Barr virus infection associated with acute acalculous cholecystitis in a 20-year-old woman. Can. Med. Assoc. J. 2021, 193, E696. [Google Scholar] [CrossRef]
- Wolstenholme, R.J. The disease spectrum in a Maldivian (Adduan) population. Trans. R. Soc. Trop. Med. Hyg. 1984, 78, 505–507. [Google Scholar] [CrossRef]
- Kang, N.; Lan, N.; Ye, H. Clinical analysis of tsutsugamushi disease misdiagnosed as tonsillitis. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi J. Clin. Otorhinolaryngol. Head Neck Surg. 2014, 28, 425–426. [Google Scholar]
- Lee, J.-H.; Lee, J.; Chung, K.M.; Kim, E.S.; Kwak, Y.G.; Moon, C.; Lee, C.-S. Dynamics of Clinical Symptoms in Patients with Scrub Typhus. Jpn. J. Infect. Dis. 2013, 66, 155–157. [Google Scholar] [CrossRef]
- Aung, T.; Supanaranond, W.; Phumiratanaprapin, W.; Phonrat, B.; Chinprasatsak, S.; Ratanajaratroj, N. Gastrointestinal manifestations of septic patients with scrub typhus in Maharat Nakhon Ratchasima Hospital. S. Asian J. Trop. Med. Public Health 2004, 35, 845–851. [Google Scholar]
- Lee, J.; Kim, D.-M.; Yun, N.R.; Kim, Y.D.; Park, C.G.; Kim, M.W. The Correlation of Endoscopic Findings and Clinical Features in Korean Patients with Scrub Typhus: A Cohort Study. PLoS ONE 2016, 11, e0155810. [Google Scholar] [CrossRef] [PubMed]
- Moron, C.G.; Popov, V.L.; Feng, H.-M.; Wear, D.; Walker, D.H. Identification of the Target Cells of Orientia tsutsugamushi in Human Cases of Scrub Typhus. Mod. Pathol. 2001, 14, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, S.I.; Yi, Y.-S.; Lee, H.; Hwang, J.-H.; Park, E.C.; Jun, S.; Lee, C.-S. Transmission Electron Microscopy Confirmation of Orientia tsutsugamushi in Human Bile. Emerg. Infect. Dis. 2020, 26, 3101–3103. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Kim, S.; Wook, Y.D.; Lee, J.W.; Kim, K.-I.; Lee, S.H. Scrub Typhus: Clinical, Pathologic, and Imaging Findings. RadioGraphics 2007, 27, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.A.; Hauptmann, M.; Kolbaum, J.; Gharaibeh, M.H.; Neumann, M.; Glatzel, M.; Fleischer, B. Dissemination of Orientia tsutsugamushi and Inflammatory Responses in a Murine Model of Scrub Typhus. PLoS Negl. Trop. Dis. 2014, 8, e3064. [Google Scholar] [CrossRef]
- Lee, K.H.; Heo, S.T.; Jeong, S.U.; Kim, M.-Y.; Jeong, W.S.; Hyun, C.L.; Kim, Y.-K.; Yoo, J.R. Acute Cholangitis Caused by Boryong Strain of Orientia tsutsugamushi. Infect. Chemother. 2020, 52, 621–625. [Google Scholar] [CrossRef]
- Yang, C.H.; Young, T.G.; Peng, M.Y.; Hsu, G.J. Unusual presentation of acute abdomen in scrub typhus: A report of two cases. Zhonghua Yi Xue Za Zhi (Taipei) 1995, 55, 401–404. [Google Scholar]
- El Sayed, I.; Liu, Q.; Wee, I.; Hine, P. Antibiotics for treating scrub typhus. Cochrane Database Syst. Rev. 2018, 9, CD002150. [Google Scholar] [CrossRef]
- Hilmy, A.I.; Dey, R.K.; Imad, H.A.; Yoosuf, A.A.; Nazeem, A.; Latheef, A.A. Coronavirus disease 2019 and dengue: Two case reports. J. Med. Case Rep. 2021, 15, 1–5. [Google Scholar] [CrossRef]
- Miqdhaadh, A.; Imad, H.A.; Fazeena, A.; Ngamprasertchai, T.; Nguitragool, W.; Nakayama, E.E.; Shioda, T. Multisystem Inflammatory Syndrome Associated with SARS-CoV-2 Infection in an Adult: A Case Report from the Maldives. Trop. Med. Infect. Dis. 2021, 6, 187. [Google Scholar] [CrossRef] [PubMed]
- Brummaier, T.; Kittitrakul, C.; Choovichian, V.; Lawpoolsri, S.; Namaik-Larp, C.; Wattanagoon, Y. Clinical manifestations and treatment outcomes of scrub typhus in a rural health care facility on the Thailand-Myanmar border. J. Infect. Dev. Ctries. 2017, 11, 407–413. [Google Scholar] [CrossRef]
- Jhuria, L.; Muthu, V.; Gupta, S.; Singh, M.P.; Biswal, M.; Goyal, K.; Pannu, A.K.; Kumari, S.; Bhalla, A.; Mohindra, R.; et al. Coinfection of H1N1 Influenza and Scrub Typhus-A Review. QJM: Int. J. Med. 2020, 113, 465–468. [Google Scholar] [CrossRef]
- Wilairatana, P.; Kuraeiad, S.; Rattaprasert, P.; Kotepui, M. Prevalence of malaria and scrub typhus co-infection in febrile patients: A systematic review and meta-analysis. Parasites Vectors 2021, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Dhar, M.; Mittal, G.; Bhat, N.K.; Shirazi, N.; Kalra, V.; Sati, H.C.; Gupta, V. A comparative hospital-based observational study of mono- and co-infections of malaria, dengue virus and scrub typhus causing acute undifferentiated fever. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 705–711. [Google Scholar] [CrossRef]
- Mahajan, S.K.; Nm, S.B.; Singh, D.; Kanga, A.; Kaushal, S.S. Scrub typhus and leptospirosis co-infection in Himalayan region. Trop. Dr. 2012, 42, 176–177. [Google Scholar] [CrossRef]
- Panda, P.K.; Mehta, V.; Bhasi, A.; Gupta, P. A coinfection of severe leptospirosis and scrub typhus in Indian Himalayas. J. Fam. Med. Prim. Care 2019, 8, 3416–3418. [Google Scholar] [CrossRef]
- Borkakoty, B.; Jakharia, A.; Biswas, D.; Mahanta, J. Co-infection of scrub typhus and leptospirosis in patients with pyrexia of unknown origin in Longding district of Arunachal Pradesh in 2013. Indian J. Med. Microbiol. 2016, 34, 88–91. [Google Scholar] [CrossRef]
- Das, B.K.; Mohanty, S.; Sahoo, P.K. Association of leptospirosis and scrub typhus in acute encephalitis syndrome in a tertiary care hospital in Odisha, India. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-S.; Ou, T.-Y.; Chen, F.-L.; Hsu, C.-W.; Jean, S.-S. Co-infection with Orientia tsutsugamushi and Mycoplasma pneumoniae in a traveler. J. Microbiol. Immunol. Infect. 2015, 48, 121–122. [Google Scholar] [CrossRef] [PubMed]
- Seow, C.W.-X.; Logarajah, V.; Tan, N.W.H. Typhoid and Scrub Typhus Coinfection in a Returned Traveler. Glob. Pediatric Health 2017, 4, 2333794X17726941. [Google Scholar] [CrossRef] [PubMed]
- Princess, I.; Ebenezer, R.; Ramakrishnan, N.; Nandini, S. Pulmonary nocardiosis and scrub typhus in an immunocompromised host. J. Glob. Infect. Dis. 2018, 10, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, S.; Bhandari, S.; Sapkota, S.; Hamal, R. Dengue and Scrub Typhus Coinfection in a Patient Presenting with Febrile Illness. Case Rep. Infect. Dis. 2017, 2017, 6214083. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Sharma, M.; Biswal, M.; Taneja, S.; Batra, N.; Kumar, A.; Dhiman, R.K. Hepatitis E Virus Induced Acute Liver Failure with Scrub Typhus Coinfection in a Pregnant Woman. J. Clin. Exp. Hepatol. 2017, 7, 158–160. [Google Scholar] [CrossRef]
- Pathak, S.; Chaudhary, N.; Dhakal, P.; Yadav, S.R.; Gupta, B.K.; Kurmi, O.P. Comparative Study of Chikungunya Only and Chikungunya-Scrub Typhus Coinfection in Children: Findings from a Hospital-Based Observational Study from Central Nepal. Int. J. Pediatr. 2021, 2021, 1–6. [Google Scholar] [CrossRef]
- Chandramohan, A.; Venkatesh, S.; Dhandapany, G.; Stephen, S. Scrub Typhus Co-infection in an Adolescent Girl with Varicella. Indian Pediatr. 2015, 52, 891–892. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Viswanathan, S.; Remalayam, B.; Muthu, V.; George, T. Pancreatitis and MODS Due to Scrub Typhus and Dengue Co-Infection. Trop. Med. Health 2012, 40, 19–21. [Google Scholar] [CrossRef]
- Akiyama, Y.; Ishikane, M.; Ohmagari, N. Epstein-Barr virus induced skin rash in infectious mononucleosis. IDCases 2021, 26, e01298. [Google Scholar] [CrossRef]
- Fedyanina, O.S.; Filippova, A.E.; Demina, O.I.; Zhuliabina, O.A.; Tikhomirov, D.S.; Filatov, A.V.; Chebotareva, T.A.; Kuznetsova, S.A. The Nature and Clinical Significance of Atypical Mononuclear Cells in Infectious Mononucleosis Caused by the Epstein-Barr Virus in Children. J. Infect. Dis. 2020, 223, 1699–1706. [Google Scholar] [CrossRef]
- Jones, J.F.; Straus, S.E. Chronic Epstein-Barr virus infection. Annu. Rev. Med. 1987, 38, 195–209. [Google Scholar] [CrossRef]
- Hirsiger, J.R.; Fuchs, P.S.; Hausermann, P.; Muller-Durovic, B.; Daikeler, T.; Recher, M.; Hirsch, H.H.; Erracciano, L.; Berger, C.T. Syphilis Reactivates Latent Epstein-Barr Virus Reservoir via Toll-Like Receptor 2 and B-Cell Receptor Activation. Open Forum Infect. Dis. 2019, 6, ofz317. [Google Scholar] [CrossRef] [PubMed]
- Karrasch, M.; Herfurth, K.; Kläver, M.; Miethke, J.; Mayer-Scholl, A.; Luge, E.; Straube, E.; Busch, M. Severe leptospirosis complicated by Epstein–Barr Virus reactivation. Infection 2015, 43, 763–769. [Google Scholar] [CrossRef]
- Watanabe, Y.; Mashimo, S.; Ichige, H.; Nagata, H.; Kojima, M. Scrub typhus mimicking the clinical course of infectious mononucleosis: A case report. J. Rural. Med. 2021, 16, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Hjalgrim, H.; Friborg, J.; Melbye, M. The epidemiology of EBV and its association with malignant disease. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Arvin, A., Campadelli-Fiume, G., Mocarski, E., Moore, P.S., Roizman, B., Whitley, R., Yamanishi, K., Eds.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Imad, H.A.; Phadungsombat, J.; Nakayama, E.E.; Suzuki, K.; Ibrahim, A.M.; Afaa, A.; Azeema, A.; Nazfa, A.; Ahmed, A.; Saeed, A.; et al. Clinical Features of Acute Chikungunya Virus Infection in Children and Adults during an Outbreak in the Maldives. Am. J. Trop. Med. Hyg. 2021, 105, 946–954. [Google Scholar] [CrossRef] [PubMed]
| Day of Illness (Days) | 9 | 14 |
|---|---|---|
| Leukocyte/µL | 6650 | 8030 |
| Neutrophils/µL | 4788 | 4496 |
| Lymphocytes/µL | 1349 | 2328 |
| Monocytes/µL | 435 | 1003 |
| Eosinophils/µL | 0 | 24 |
| Basophils/µL | 0 | 0 |
| Platelets/µL | 269,000 | 545,000 |
| Hemoglobin (g/dL) | 10.6 | 7.8 |
| Hematocrit (%) | 32.9 | 24.6 |
| Total Bilirubin (mg/dL) | 4.7 | 3.5 |
| Direct Bilirubin (mg/dL) | 3.5 | 2.6 |
| Total protein (g/dL) | 6.9 | 6.8 |
| Albumin (g/dL) | 3.4 | 2.8 |
| Alkaline phosphatase (IU/L) | 288 | 250 |
| Aspartate aminotransferase (IU/L) | 140 | 76 |
| Alanine aminotransferase (IU/L) | 157 | 93 |
| Creatinine (mg/dL) | 0.8 | 0.6 |
| Urea (mg/dL) | 10 | 6.4 |
| CRP (mg/dL) | 7.7 | 2.8 |
| Sodium (mmol/L) | 131 | 134 |
| Potassium (mmol/L) | 4.5 | 4.3 |
| Ferritin (ng/mL) | 1299.3 | |
| LDH (IU/L) | 726 | |
| EBV-VCA IgM (IU/mL) | 63.6 | |
| EBV-VCA IgG (IU/mL) | 89.9 | |
| EBV-EA IgG (IU/mL) | 16.1 | |
| EBV-NA IgG (IU/mL) | 574.0 | |
| Blood culture | no growth | |
| Orientia tsutsugamushi IgM | positive | |
| Orientia tsutsugamushi IgG | positive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imad, H.A.; Ali, A.A.; Nahuza, M.; Gurung, R.; Ubaid, A.; Maeesha, A.; Didi, S.A.; Dey, R.K.; Hilmy, A.I.; Hareera, A.; et al. Acalculous Cholecystitis in a Young Adult with Scrub Typhus: A Case Report and Epidemiology of Scrub Typhus in the Maldives. Trop. Med. Infect. Dis. 2021, 6, 208. https://doi.org/10.3390/tropicalmed6040208
Imad HA, Ali AA, Nahuza M, Gurung R, Ubaid A, Maeesha A, Didi SA, Dey RK, Hilmy AI, Hareera A, et al. Acalculous Cholecystitis in a Young Adult with Scrub Typhus: A Case Report and Epidemiology of Scrub Typhus in the Maldives. Tropical Medicine and Infectious Disease. 2021; 6(4):208. https://doi.org/10.3390/tropicalmed6040208
Chicago/Turabian StyleImad, Hisham Ahmed, Aishath Azna Ali, Mariyam Nahuza, Rajan Gurung, Abdulla Ubaid, Aishath Maeesha, Sariu Ali Didi, Rajib Kumar Dey, Abdullah Isneen Hilmy, Aishath Hareera, and et al. 2021. "Acalculous Cholecystitis in a Young Adult with Scrub Typhus: A Case Report and Epidemiology of Scrub Typhus in the Maldives" Tropical Medicine and Infectious Disease 6, no. 4: 208. https://doi.org/10.3390/tropicalmed6040208
APA StyleImad, H. A., Ali, A. A., Nahuza, M., Gurung, R., Ubaid, A., Maeesha, A., Didi, S. A., Dey, R. K., Hilmy, A. I., Hareera, A., Afzal, I., Matsee, W., Nguitragool, W., Nakayama, E. E., & Shioda, T. (2021). Acalculous Cholecystitis in a Young Adult with Scrub Typhus: A Case Report and Epidemiology of Scrub Typhus in the Maldives. Tropical Medicine and Infectious Disease, 6(4), 208. https://doi.org/10.3390/tropicalmed6040208







