Current Trends and Limitations in Dengue Antiviral Research
Abstract
1. Introduction
1.1. Disease Burden, Clinical Importance, and Manifestations
1.2. Viral Serotypes and Antibody-Dependent Enhancement (ADE)
1.3. Dengue Vaccines and Antiviral Agents: Current State
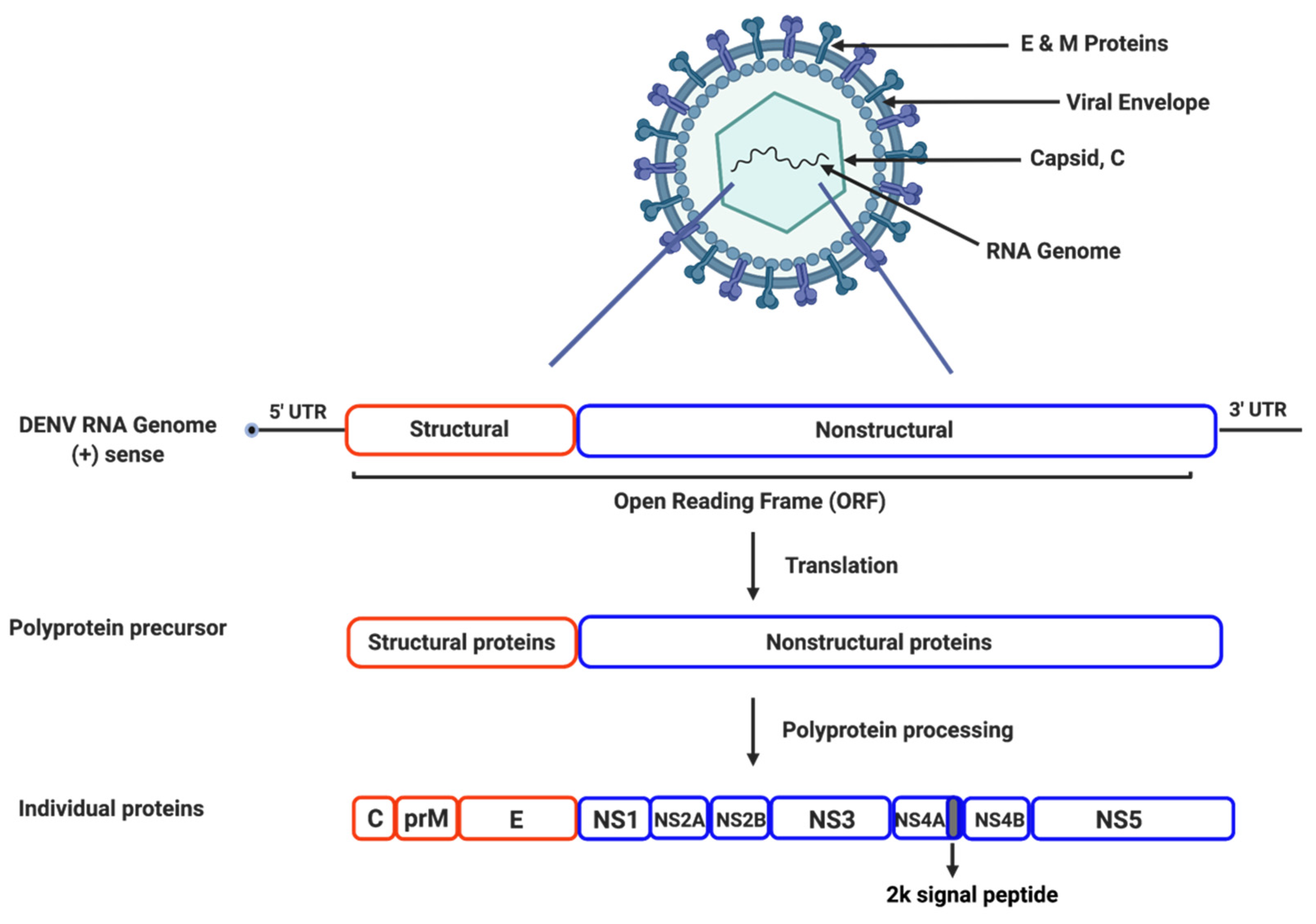
2. Dengue Viral Life Cycle and Proteins
2.1. Genome Structure and Organization
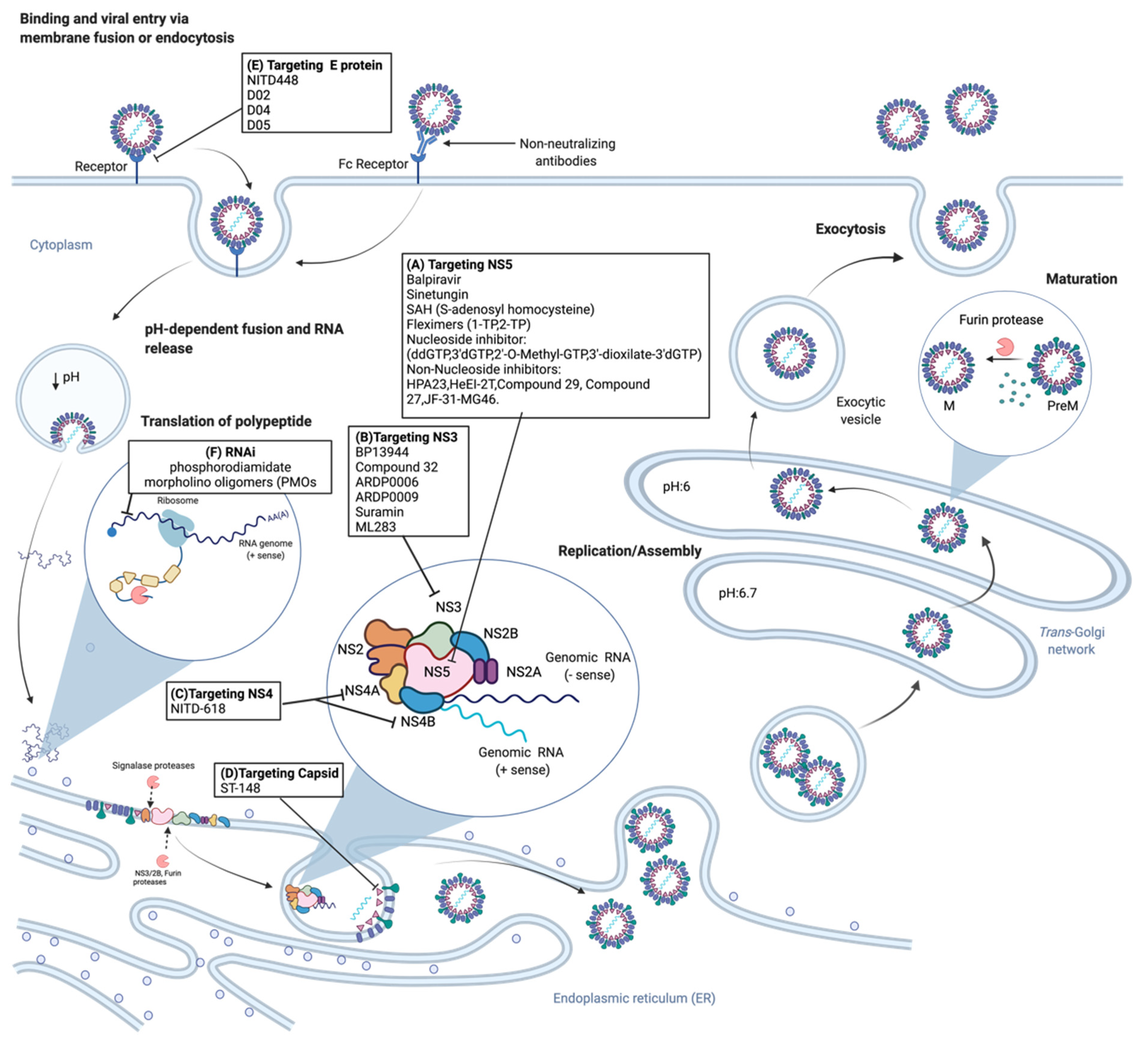
2.2. Viral Life Cycle
3. Dengue Antiviral Research and Development
3.1. Classical Antiviral Targets
3.1.1. NS3 Protease
3.1.2. NS3 Helicase
3.1.3. NS4B
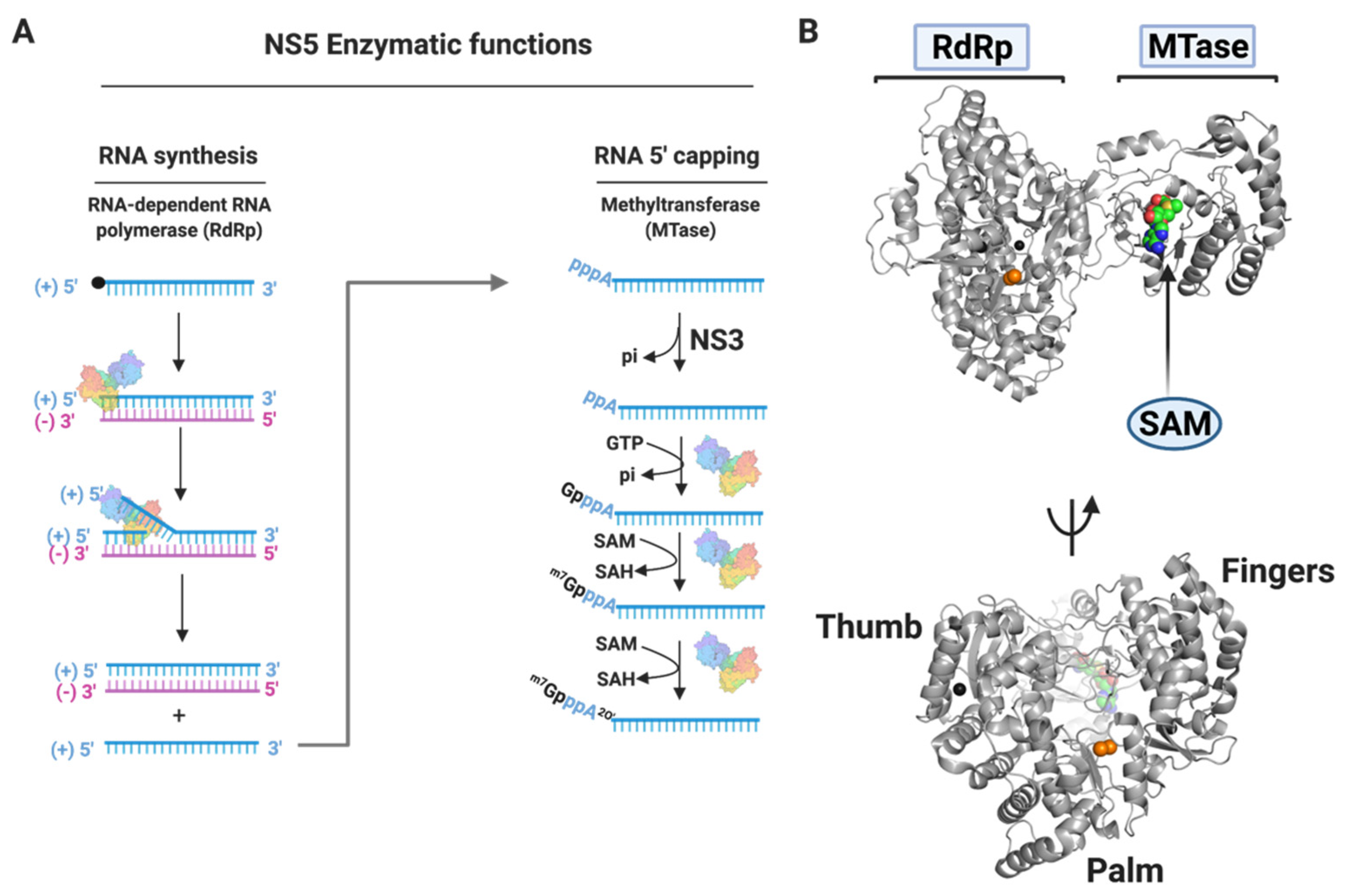
3.1.4. NS5
Nucleoside Inhibitors
Non-Nucleoside Inhibitors
3.2. Other Dengue Antiviral Targets
3.2.1. NS1
3.2.2. Envelope Protein
3.2.3. Viral Capsid
3.3. DENV Small Molecule Inhibitors in Clinical Trials
3.4. Alternative Therapeutic Approaches
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitra, A.K.; Mawson, A.R. Neglected Tropical Diseases: Epidemiology and Global Burden. Trop. Med. Infect. Dis. 2017, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife 2015, 4, e08347. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Harris, E. Dengue. Lancet 2015, 385, 453–465. [Google Scholar] [CrossRef]
- Back, A.T.; Lundkvist, A. Dengue viruses—An overview. Infect. Ecol. Epidemiol. 2013, 3, 19839. [Google Scholar] [CrossRef]
- Wilder-Smith, A.; Ooi, E.E.; Horstick, O.; Wills, B. Dengue. Lancet 2019, 393, 350–363. [Google Scholar] [CrossRef]
- Rocklöv, J.; Quam, M.B.; Sudre, B.; German, M.; Kraemer, M.U.G.; Brady, O.; Bogoch, I.I.; Liu-Helmersson, J.; Wilder-Smith, A.; Semenza, J.C.; et al. Assessing Seasonal Risks for the Introduction and Mosquito-borne Spread of Zika Virus in Europe. EBioMedicine 2016, 9, 250–256. [Google Scholar] [CrossRef]
- Guzman, M.G.; Gubler, D.J.; Izquierdo, A.; Martinez, E.; Halstead, S.B. Dengue infection. Nat. Rev. Dis. Primers 2016, 2, 16055. [Google Scholar] [CrossRef]
- Messina, J.P.; Brady, O.J.; Scott, T.W.; Zou, C.; Pigott, D.M.; Duda, K.A.; Bhatt, S.; Katzelnick, L.; Howes, R.E.; Battle, K.E.; et al. Global spread of dengue virus types: Mapping the 70 year history. Trends Microbiol. 2014, 22, 138–146. [Google Scholar] [CrossRef]
- WHO. Dengue and Severe Dengue. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 5 July 2021).
- Hadinegoro, S.R.; Arredondo-García, J.L.; Capeding, M.R.; Deseda, C.; Chotpitayasunondh, T.; Dietze, R.; Muhammad Ismail, H.I.; Reynales, H.; Limkittikul, K.; Rivera-Medina, D.M.; et al. Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. N. Engl. J. Med. 2015, 373, 1195–1206. [Google Scholar] [CrossRef]
- Gubler, D.J. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 1998, 11, 480–496. [Google Scholar] [CrossRef]
- Endy, T.P.; Anderson, K.B.; Nisalak, A.; Yoon, I.K.; Green, S.; Rothman, A.L.; Thomas, S.J.; Jarman, R.G.; Libraty, D.H.; Gibbons, R.V. Determinants of inapparent and symptomatic dengue infection in a prospective study of primary school children in Kamphaeng Phet, Thailand. PLoS Negl. Trop. Dis. 2011, 5, e975. [Google Scholar] [CrossRef]
- Amarasinghe, A.; Kuritsk, J.N.; Letson, G.W.; Margolis, H.S. Dengue virus infection in Africa. Emerg. Infect. Dis. 2011, 17, 1349–1354. [Google Scholar] [CrossRef]
- Gubler, D.J. Dengue, Urbanization and Globalization: The Unholy Trinity of the 21(st) Century. Trop. Med. Health 2011, 39, 3–11. [Google Scholar] [CrossRef]
- Murray, C.J.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef]
- Shepard, D.S.; Undurraga, E.A.; Halasa, Y.A.; Stanaway, J.D. The global economic burden of dengue: A systematic analysis. Lancet Infect. Dis. 2016, 16, 935–941. [Google Scholar] [CrossRef]
- Shepard, D.S.; Undurraga, E.A.; Betancourt-Cravioto, M.; Guzmán, M.G.; Halstead, S.B.; Harris, E.; Mudin, R.N.; Murray, K.O.; Tapia-Conyer, R.; Gubler, D.J. Approaches to refining estimates of global burden and economics of dengue. PLoS Negl. Trop. Dis. 2014, 8, e3306. [Google Scholar] [CrossRef] [PubMed]
- Messina, J.P.; Brady, O.J.; Golding, N.; Kraemer, M.U.G.; Wint, G.R.W.; Ray, S.E.; Pigott, D.M.; Shearer, F.M.; Johnson, K.; Earl, L.; et al. The current and future global distribution and population at risk of dengue. Nat. Microbiol. 2019, 4, 1508–1515. [Google Scholar] [CrossRef]
- Martina, B.E.; Koraka, P.; Osterhaus, A.D. Dengue virus pathogenesis: An integrated view. Clin. Microbiol. Rev. 2009, 22, 564–581. [Google Scholar] [CrossRef]
- Joob, B.; Wiwanitkit, V. Fifth serotype of dengue virus: What we should prepare for? Med. J. Armed Forces India 2016, 72, 194–195. [Google Scholar] [CrossRef][Green Version]
- Mustafa, M.S.; Rasotgi, V.; Jain, S.; Gupta, V. Discovery of fifth serotype of dengue virus (DENV-5): A new public health dilemma in dengue control. Med. J. Armed Forces India 2015, 71, 67–70. [Google Scholar] [CrossRef]
- Montoya, M.; Gresh, L.; Mercado, J.C.; Williams, K.L.; Vargas, M.J.; Gutierrez, G.; Kuan, G.; Gordon, A.; Balmaseda, A.; Harris, E. Symptomatic versus inapparent outcome in repeat dengue virus infections is influenced by the time interval between infections and study year. PLoS Negl. Trop. Dis. 2013, 7, e2357. [Google Scholar] [CrossRef]
- Snow, G.E.; Haaland, B.; Ooi, E.E.; Gubler, D.J. Review article: Research on dengue during World War II revisited. Am. J. Trop. Med. Hyg. 2014, 91, 1203–1217. [Google Scholar] [CrossRef]
- Guzman, M.G.; Halstead, S.B.; Artsob, H.; Buchy, P.; Farrar, J.; Gubler, D.J.; Hunsperger, E.; Kroeger, A.; Margolis, H.S.; Martínez, E.; et al. Dengue: A continuing global threat. Nat. Rev. Microbiol. 2010, 8, S7–S16. [Google Scholar] [CrossRef]
- Halstead, S.B.; Nimmannitya, S.; Cohen, S.N. Observations related to pathogenesis of dengue hemorrhagic fever. IV. Relation of disease severity to antibody response and virus recovered. Yale J. Biol. Med. 1970, 42, 311–328. [Google Scholar] [PubMed]
- Halstead, S.B.; O’Rourke, E.J. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J. Exp. Med. 1977, 146, 201–217. [Google Scholar] [CrossRef]
- Chan, K.R.; Ong, E.Z.; Tan, H.C.; Zhang, S.L.; Zhang, Q.; Tang, K.F.; Kaliaperumal, N.; Lim, A.P.; Hibberd, M.L.; Chan, S.H.; et al. Leukocyte immunoglobulin-like receptor B1 is critical for antibody-dependent dengue. Proc. Natl. Acad. Sci. USA 2014, 111, 2722–2727. [Google Scholar] [CrossRef]
- Ong, E.Z.; Zhang, S.L.; Tan, H.C.; Gan, E.S.; Chan, K.R.; Ooi, E.E. Dengue virus compartmentalization during antibody-enhanced infection. Sci. Rep. 2017, 7, 40923. [Google Scholar] [CrossRef] [PubMed]
- Rothman, A.L. Immunity to dengue virus: A tale of original antigenic sin and tropical cytokine storms. Nat. Rev. Immunol. 2011, 11, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, S.; Wertheim, H.; Simmons, C.P.; Screaton, G.; Wills, B. Microvascular and endothelial function for risk prediction in dengue: An observational study. Lancet 2015, 385 (Suppl. 1), S102. [Google Scholar] [CrossRef]
- Messer, W.B.; Yount, B.; Hacker, K.E.; Donaldson, E.F.; Huynh, J.P.; de Silva, A.M.; Baric, R.S. Development and characterization of a reverse genetic system for studying dengue virus serotype 3 strain variation and neutralization. PLoS Negl. Trop. Dis. 2012, 6, e1486. [Google Scholar] [CrossRef] [PubMed]
- Bara, J.J.; Clark, T.M.; Remold, S.K. Susceptibility of larval Aedes aegypti and Aedes albopictus (Diptera: Culicidae) to dengue virus. J. Med. Entomol. 2013, 50, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Rico-Hesse, R. Microevolution and virulence of dengue viruses. Adv. Virus Res. 2003, 59, 315–341. [Google Scholar] [CrossRef]
- Halstead, S.B. Dengue virus-mosquito interactions. Annu. Rev. Entomol. 2008, 53, 273–291. [Google Scholar] [CrossRef]
- Gubler, D.J.; Reed, D.; Rosen, L.; Hitchcock, J.R. Epidemiologic, clinical, and virologic observations on dengue in the Kingdom of Tonga. Am. J. Trop. Med. Hyg. 1978, 27, 581–589. [Google Scholar] [CrossRef]
- Rosen, L. The Emperor’s New Clothes revisited, or reflections on the pathogenesis of dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 1977, 26, 337–343. [Google Scholar] [CrossRef]
- Godói, I.P.; Lemos, L.L.; de Araújo, V.E.; Bonoto, B.C.; Godman, B.; Guerra Júnior, A.A. CYD-TDV dengue vaccine: Systematic review and meta-analysis of efficacy, immunogenicity and safety. J. Comp. Eff. Res. 2017, 6, 165–180. [Google Scholar] [CrossRef]
- Osorio, J.E.; Wallace, D.; Stinchcomb, D.T. A recombinant, chimeric tetravalent dengue vaccine candidate based on a dengue virus serotype 2 backbone. Expert Rev. Vaccines 2016, 15, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, S.S. Development of TV003/TV005, a single dose, highly immunogenic live attenuated dengue vaccine; what makes this vaccine different from the Sanofi-Pasteur CYD™ vaccine? Expert Rev. Vaccines 2016, 15, 509–517. [Google Scholar] [CrossRef]
- Torresi, J.; Ebert, G.; Pellegrini, M. Vaccines licensed and in clinical trials for the prevention of dengue. Hum. Vaccin. Immunother. 2017, 13, 1059–1072. [Google Scholar] [CrossRef]
- Low, J.G.; Sung, C.; Wijaya, L.; Wei, Y.; Rathore, A.P.S.; Watanabe, S.; Tan, B.H.; Toh, L.; Chua, L.T.; Hou, Y.; et al. Efficacy and safety of celgosivir in patients with dengue fever (CELADEN): A phase 1b, randomised, double-blind, placebo-controlled, proof-of-concept trial. Lancet Infect. Dis. 2014, 14, 706–715. [Google Scholar] [CrossRef]
- Nguyen, N.M.; Tran, C.N.; Phung, L.K.; Duong, K.T.; Huynh, H.e.A.; Farrar, J.; Nguyen, Q.T.; Tran, H.T.; Nguyen, C.V.; Merson, L.; et al. A randomized, double-blind placebo controlled trial of balapiravir, a polymerase inhibitor, in adult dengue patients. J. Infect. Dis. 2013, 207, 1442–1450. [Google Scholar] [CrossRef]
- Natali, E.N.; Babrak, L.M.; Miho, E. Prospective Artificial Intelligence to Dissect the Dengue Immune Response and Discover Therapeutics. Front. Immunol. 2021, 12, 574411. [Google Scholar] [CrossRef]
- Low, J.G.; Ooi, E.E.; Vasudevan, S.G. Current Status of Dengue Therapeutics Research and Development. J. Infect. Dis. 2017, 215, S96–S102. [Google Scholar] [CrossRef]
- Modis, Y.; Ogata, S.; Clements, D.; Harrison, S.C. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc. Natl. Acad. Sci. USA 2003, 100, 6986–6991. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Zhang, M.; Dayton, A.I. Development of dengue virus replicons expressing HIV-1 gp120 and other heterologous genes: A potential future tool for dual vaccination against dengue virus and HIV. BMC Microbiol. 2001, 1, 28. [Google Scholar] [CrossRef]
- Pang, X.; Zhang, M.; Dayton, A.I. Development of Dengue virus type 2 replicons capable of prolonged expression in host cells. BMC Microbiol. 2001, 1, 18. [Google Scholar] [CrossRef]
- Alvarez, D.E.; Lodeiro, M.F.; Filomatori, C.V.; Fucito, S.; Mondotte, J.A.; Gamarnik, A.V. Structural and functional analysis of dengue virus RNA. Novartis. Found. Symp. 2006, 277, 120–132. [Google Scholar]
- Gamarnik, A. Role of the dengue virus 5′ and 3′ untranslated regions in viral replication. In Frontiers in Dengue Virus Research; Hanley, K.A., Weaver, S.C., Eds.; Caister Academic Press: Norfolk, UK, 2010; pp. 55–78. [Google Scholar]
- Padmanabhan, R.; Strongin, A.Y. Translation and processing of the dengue virus polyprotein. In Frontiers in Dengue Virus Research; Hanley, K.A., Weaver, S.C., Eds.; Caister Academic Press: Norfolk, UK, 2010. [Google Scholar]
- Yu, L.; Nomaguchi, M.; Padmanabhan, R.; Markoff, L. Specific requirements for elements of the 5′ and 3′ terminal regions in flavivirus RNA synthesis and viral replication. Virology 2008, 374, 170–185. [Google Scholar] [CrossRef]
- Heinz, F.X.; Allison, S.L. Flavivirus structure and membrane fusion. Adv. Virus Res. 2003, 59, 63–97. [Google Scholar] [CrossRef]
- Chen, Y.; Maguire, T.; Hileman, R.E.; Fromm, J.R.; Esko, J.D.; Linhardt, R.J.; Marks, R.M. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat. Med. 1997, 3, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Tassaneetrithep, B.; Burgess, T.H.; Granelli-Piperno, A.; Trumpfheller, C.; Finke, J.; Sun, W.; Eller, M.A.; Pattanapanyasat, K.; Sarasombath, S.; Birx, D.L.; et al. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J. Exp. Med. 2003, 197, 823–829. [Google Scholar] [CrossRef]
- Miller, J.L.; de Wet, B.J.; de Wet, B.J.; Martinez-Pomares, L.; Radcliffe, C.M.; Dwek, R.A.; Rudd, P.M.; Gordon, S. The mannose receptor mediates dengue virus infection of macrophages. PLoS Pathog. 2008, 4, e17. [Google Scholar] [CrossRef]
- Chen, S.T.; Lin, Y.L.; Huang, M.T.; Wu, M.F.; Cheng, S.C.; Lei, H.Y.; Lee, C.K.; Chiou, T.W.; Wong, C.H.; Hsieh, S.L. CLEC5A is critical for dengue-virus-induced lethal disease. Nature 2008, 453, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.A.; Lebedev, A.A.; Hall, B.A.; Fenton-May, A.E.; Vagin, A.A.; Dejnirattisai, W.; Felce, J.; Mongkolsapaya, J.; Palma, A.S.; Liu, Y.; et al. Structural flexibility of the macrophage dengue virus receptor CLEC5A: Implications for ligand binding and signaling. J. Biol. Chem. 2011, 286, 24208–24218. [Google Scholar] [CrossRef]
- Clyde, K.; Kyle, J.L.; Harris, E. Recent advances in deciphering viral and host determinants of dengue virus replication and pathogenesis. J. Virol. 2006, 80, 11418–11431. [Google Scholar] [CrossRef] [PubMed]
- Stadler, K.; Allison, S.L.; Schalich, J.; Heinz, F.X. Proteolytic activation of tick-borne encephalitis virus by furin. J. Virol. 1997, 71, 8475–8481. [Google Scholar] [CrossRef]
- Guirakhoo, F.; Heinz, F.X.; Mandl, C.W.; Holzmann, H.; Kunz, C. Fusion activity of flaviviruses: Comparison of mature and immature (prM-containing) tick-borne encephalitis virions. J. Gen. Virol. 1991, 72 Pt 6, 1323–1329. [Google Scholar] [CrossRef]
- Guirakhoo, F.; Bolin, R.A.; Roehrig, J.T. The Murray Valley encephalitis virus prM protein confers acid resistance to virus particles and alters the expression of epitopes within the R2 domain of E glycoprotein. Virology 1992, 191, 921–931. [Google Scholar] [CrossRef]
- Zhang, Y.; Corver, J.; Chipman, P.R.; Zhang, W.; Pletnev, S.V.; Sedlak, D.; Baker, T.S.; Strauss, J.H.; Kuhn, R.J.; Rossmann, M.G. Structures of immature flavivirus particles. EMBO J. 2003, 22, 2604–2613. [Google Scholar] [CrossRef]
- Garcia, L.L.; Padilla, L.; Castano, J.C. Inhibitors compounds of the flavivirus replication process. Virol. J. 2017, 14, 95. [Google Scholar] [CrossRef] [PubMed]
- Apte-Sengupta, S.; Sirohi, D.; Kuhn, R.J. Coupling of replication and assembly in flaviviruses. Curr. Opin. Virol. 2014, 9, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Vasudevan, S.G.; Lescar, J. The flavivirus NS2B-NS3 protease-helicase as a target for antiviral drug development. Antiviral Res. 2015, 118, 148–158. [Google Scholar] [CrossRef]
- Zou, G.; Chen, Y.L.; Dong, H.; Lim, C.C.; Yap, L.J.; Yau, Y.H.; Shochat, S.G.; Lescar, J.; Shi, P.Y. Functional analysis of two cavities in flavivirus NS5 polymerase. J. Biol. Chem. 2011, 286, 14362–14372. [Google Scholar] [CrossRef]
- Yang, C.C.; Hu, H.S.; Wu, R.H.; Wu, S.H.; Lee, S.J.; Jiaang, W.T.; Chern, J.H.; Huang, Z.S.; Wu, H.N.; Chang, C.M.; et al. A novel dengue virus inhibitor, BP13944, discovered by high-throughput screening with dengue virus replicon cells selects for resistance in the viral NS2B/NS3 protease. Antimicrob. Agents Chemother. 2014, 58, 110–119. [Google Scholar] [CrossRef]
- Steuer, C.; Gege, C.; Fischl, W.; Heinonen, K.H.; Bartenschlager, R.; Klein, C.D. Synthesis and biological evaluation of α-ketoamides as inhibitors of the Dengue virus protease with antiviral activity in cell-culture. Bioorg. Med. Chem. 2011, 19, 4067–4074. [Google Scholar] [CrossRef]
- Tomlinson, S.M.; Watowich, S.J. Anthracene-based inhibitors of dengue virus NS2B-NS3 protease. Antiviral Res. 2011, 89, 127–135. [Google Scholar] [CrossRef][Green Version]
- Bollati, M.; Alvarez, K.; Assenberg, R.; Baronti, C.; Canard, B.; Cook, S.; Coutard, B.; Decroly, E.; de Lamballerie, X.; Gould, E.A.; et al. Structure and functionality in flavivirus NS-proteins: Perspectives for drug design. Antiviral Res. 2010, 87, 125–148. [Google Scholar] [CrossRef]
- Noble, C.G.; Chen, Y.L.; Dong, H.; Gu, F.; Lim, S.P.; Schul, W.; Wang, Q.Y.; Shi, P.Y. Strategies for development of Dengue virus inhibitors. Antiviral Res. 2010, 85, 450–462. [Google Scholar] [CrossRef]
- Lim, S.P.; Sonntag, L.S.; Noble, C.; Nilar, S.H.; Ng, R.H.; Zou, G.; Monaghan, P.; Chung, K.Y.; Dong, H.; Liu, B.; et al. Small molecule inhibitors that selectively block dengue virus methyltransferase. J. Biol. Chem. 2011, 286, 6233–6240. [Google Scholar] [CrossRef]
- Benarroch, D.; Egloff, M.P.; Mulard, L.; Guerreiro, C.; Romette, J.L.; Canard, B. A structural basis for the inhibition of the NS5 dengue virus mRNA 2’-O-methyltransferase domain by ribavirin 5’-triphosphate. J. Biol. Chem. 2004, 279, 35638–35643. [Google Scholar] [CrossRef]
- Benmansour, F.; Trist, I.; Coutard, B.; Decroly, E.; Querat, G.; Brancale, A.; Barral, K. Discovery of novel dengue virus NS5 methyltransferase non-nucleoside inhibitors by fragment-based drug design. Eur. J. Med. Chem. 2017, 125, 865–880. [Google Scholar] [CrossRef]
- Noble, C.G.; Li, S.H.; Dong, H.; Chew, S.H.; Shi, P.Y. Crystal structure of dengue virus methyltransferase without S-adenosyl-L-methionine. Antiviral Res. 2014, 111, 78–81. [Google Scholar] [CrossRef]
- Brecher, M.B.; Li, Z.; Zhang, J.; Chen, H.; Lin, Q.; Liu, B.; Li, H. Refolding of a fully functional flavivirus methyltransferase revealed that S-adenosyl methionine but not S-adenosyl homocysteine is copurified with flavivirus methyltransferase. Protein Sci. 2015, 24, 117–128. [Google Scholar] [CrossRef]
- Chung, K.Y.; Dong, H.; Chao, A.T.; Shi, P.Y.; Lescar, J.; Lim, S.P. Higher catalytic efficiency of N-7-methylation is responsible for processive N-7 and 2′-O methyltransferase activity in dengue virus. Virology 2010, 402, 52–60. [Google Scholar] [CrossRef]
- Thames, J.E.; Waters, C.D.; Valle, C.; Bassetto, M.; Aouadi, W.; Martin, B.; Selisko, B.; Falat, A.; Coutard, B.; Brancale, A.; et al. Synthesis and biological evaluation of novel flexible nucleoside analogues that inhibit flavivirus replication in vitro. Bioorg. Med. Chem. 2020, 28, 115713. [Google Scholar] [CrossRef]
- Lim, S.P.; Noble, C.G.; Seh, C.C.; Soh, T.S.; El Sahili, A.; Chan, G.K.; Lescar, J.; Arora, R.; Benson, T.; Nilar, S.; et al. Potent Allosteric Dengue Virus NS5 Polymerase Inhibitors: Mechanism of Action and Resistance Profiling. PLoS Pathog. 2016, 12, e1005737. [Google Scholar] [CrossRef]
- Tarantino, D.; Cannalire, R.; Mastrangelo, E.; Croci, R.; Querat, G.; Barreca, M.L.; Bolognesi, M.; Manfroni, G.; Cecchetti, V.; Milani, M. Targeting flavivirus RNA dependent RNA polymerase through a pyridobenzothiazole inhibitor. Antiviral Res. 2016, 134, 226–235. [Google Scholar] [CrossRef]
- Yokokawa, F.; Nilar, S.; Noble, C.G.; Lim, S.P.; Rao, R.; Tania, S.; Wang, G.; Lee, G.; Hunziker, J.; Karuna, R.; et al. Discovery of Potent Non-Nucleoside Inhibitors of Dengue Viral RNA-Dependent RNA Polymerase from a Fragment Hit Using Structure-Based Drug Design. J. Med. Chem. 2016, 59, 3935–3952. [Google Scholar] [CrossRef]
- Shimizu, H.; Saito, A.; Mikuni, J.; Nakayama, E.E.; Koyama, H.; Honma, T.; Shirouzu, M.; Sekine, S.I.; Shioda, T. Discovery of a small molecule inhibitor targeting dengue virus NS5 RNA-dependent RNA polymerase. PLoS Negl. Trop. Dis. 2019, 13, e0007894. [Google Scholar] [CrossRef]
- Noble, C.G.; Lim, S.P.; Chen, Y.L.; Liew, C.W.; Yap, L.; Lescar, J.; Shi, P.Y. Conformational flexibility of the Dengue virus RNA-dependent RNA polymerase revealed by a complex with an inhibitor. J. Virol. 2013, 87, 5291–5295. [Google Scholar] [CrossRef]
- Noble, C.G.; Lim, S.P.; Arora, R.; Yokokawa, F.; Nilar, S.; Seh, C.C.; Wright, S.K.; Benson, T.E.; Smith, P.W.; Shi, P.Y. A Conserved Pocket in the Dengue Virus Polymerase Identified through Fragment-based Screening. J. Biol. Chem. 2016, 291, 8541–8548. [Google Scholar] [CrossRef]
- Yildiz, M.; Ghosh, S.; Bell, J.A.; Sherman, W.; Hardy, J.A. Allosteric inhibition of the NS2B-NS3 protease from dengue virus. ACS Chem. Biol. 2013, 8, 2744–2752. [Google Scholar] [CrossRef]
- Noble, C.G.; Seh, C.C.; Chao, A.T.; Shi, P.Y. Ligand-bound structures of the dengue virus protease reveal the active conformation. J. Virol. 2012, 86, 438–446. [Google Scholar] [CrossRef]
- Mueller, N.H.; Yon, C.; Ganesh, V.K.; Padmanabhan, R. Characterization of the West Nile virus protease substrate specificity and inhibitors. Int. J. Biochem. Cell Biol. 2007, 39, 606–614. [Google Scholar] [CrossRef]
- Yao, Y.; Huo, T.; Lin, Y.L.; Nie, S.; Wu, F.; Hua, Y.; Wu, J.; Kneubehl, A.R.; Vogt, M.B.; Rico-Hesse, R.; et al. Discovery, X-ray Crystallography and Antiviral Activity of Allosteric Inhibitors of Flavivirus NS2B-NS3 Protease. J. Am. Chem. Soc. 2019, 141, 6832–6836. [Google Scholar] [CrossRef]
- Luo, D.; Xu, T.; Watson, R.P.; Scherer-Becker, D.; Sampath, A.; Jahnke, W.; Yeong, S.S.; Wang, C.H.; Lim, S.P.; Strongin, A.; et al. Insights into RNA unwinding and ATP hydrolysis by the flavivirus NS3 protein. EMBO J. 2008, 27, 3209–3219. [Google Scholar] [CrossRef]
- Sweeney, N.L.; Hanson, A.M.; Mukherjee, S.; Ndjomou, J.; Geiss, B.J.; Steel, J.J.; Frankowski, K.J.; Li, K.; Schoenen, F.J.; Frick, D.N. Benzothiazole and Pyrrolone Flavivirus Inhibitors Targeting the Viral Helicase. ACS Infect. Dis. 2015, 1, 140–148. [Google Scholar] [CrossRef]
- Basavannacharya, C.; Vasudevan, S.G. Suramin inhibits helicase activity of NS3 protein of dengue virus in a fluorescence-based high throughput assay format. Biochem. Biophys. Res. Commun. 2014, 453, 539–544. [Google Scholar] [CrossRef]
- Xie, X.; Zou, J.; Wang, Q.Y.; Shi, P.Y. Targeting dengue virus NS4B protein for drug discovery. Antiviral Res. 2015, 118, 39–45. [Google Scholar] [CrossRef]
- Lim, S.P.; Wang, Q.Y.; Noble, C.G.; Chen, Y.L.; Dong, H.; Zou, B.; Yokokawa, F.; Nilar, S.; Smith, P.; Beer, D.; et al. Ten years of dengue drug discovery: Progress and prospects. Antiviral Res. 2013, 100, 500–519. [Google Scholar] [CrossRef]
- van Cleef, K.W.; Overheul, G.J.; Thomassen, M.C.; Kaptein, S.J.; Davidson, A.D.; Jacobs, M.; Neyts, J.; van Kuppeveld, F.J.; van Rij, R.P. Identification of a new dengue virus inhibitor that targets the viral NS4B protein and restricts genomic RNA replication. Antiviral Res. 2013, 99, 165–171. [Google Scholar] [CrossRef]
- Sampath, A.; Padmanabhan, R. Molecular targets for flavivirus drug discovery. Antiviral Res. 2009, 81, 6–15. [Google Scholar] [CrossRef]
- Yap, T.L.; Xu, T.; Chen, Y.L.; Malet, H.; Egloff, M.P.; Canard, B.; Vasudevan, S.G.; Lescar, J. Crystal structure of the dengue virus RNA-dependent RNA polymerase catalytic domain at 1.85-angstrom resolution. J. Virol. 2007, 81, 4753–4765. [Google Scholar] [CrossRef]
- Malet, H.; Massé, N.; Selisko, B.; Romette, J.L.; Alvarez, K.; Guillemot, J.C.; Tolou, H.; Yap, T.L.; Vasudevan, S.; Lescar, J.; et al. The flavivirus polymerase as a target for drug discovery. Antiviral Res. 2008, 80, 23–35. [Google Scholar] [CrossRef]
- Najera, I. Resistance to HCV nucleoside analogue inhibitors of hepatitis C virus RNA-dependent RNA polymerase. Curr. Opin. Virol. 2013, 3, 508–513. [Google Scholar] [CrossRef]
- Ferrer-Orta, C.; Arias, A.; Escarmís, C.; Verdaguer, N. A comparison of viral RNA-dependent RNA polymerases. Curr. Opin. Struct. Biol. 2006, 16, 27–34. [Google Scholar] [CrossRef]
- Zhao, Y.; Soh, T.S.; Zheng, J.; Chan, K.W.; Phoo, W.W.; Lee, C.C.; Tay, M.Y.; Swaminathan, K.; Cornvik, T.C.; Lim, S.P.; et al. A crystal structure of the Dengue virus NS5 protein reveals a novel inter-domain interface essential for protein flexibility and virus replication. PLoS Pathog. 2015, 11, e1004682. [Google Scholar] [CrossRef]
- Bujalowski, P.J.; Bujalowski, W.; Choi, K.H. Interactions between the Dengue Virus Polymerase NS5 and Stem-Loop A. J. Virol. 2017, 91, 11. [Google Scholar] [CrossRef]
- Wang, B.; Thurmond, S.; Zhou, K.; Sánchez-Aparicio, M.T.; Fang, J.; Lu, J.; Gao, L.; Ren, W.; Cui, Y.; Veit, E.C.; et al. Structural basis for STAT2 suppression by flavivirus NS5. Nat. Struct. Mol. Biol. 2020, 27, 875–885. [Google Scholar] [CrossRef]
- Lim, S.P.; Noble, C.G.; Shi, P.Y. The dengue virus NS5 protein as a target for drug discovery. Antiviral Res. 2015, 119, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Schul, W.; Butters, T.D.; Yip, A.; Liu, B.; Goh, A.; Lakshminarayana, S.B.; Alonzi, D.; Reinkensmeier, G.; Pan, X.; et al. Combination of α-glucosidase inhibitor and ribavirin for the treatment of dengue virus infection in vitro and in vivo. Antiviral Res. 2011, 89, 26–34. [Google Scholar] [CrossRef]
- Malinoski, F.J.; Hasty, S.E.; Ussery, M.A.; Dalrymple, J.M. Prophylactic ribavirin treatment of dengue type 1 infection in rhesus monkeys. Antiviral Res. 1990, 13, 139–149. [Google Scholar] [CrossRef]
- Yates, M.K.; Raje, M.R.; Chatterjee, P.; Spiropoulou, C.F.; Bavari, S.; Flint, M.; Soloveva, V.; Seley-Radtke, K.L. Flex-nucleoside analogues—Novel therapeutics against filoviruses. Bioorg. Med. Chem. Lett. 2017, 27, 2800–2802. [Google Scholar] [CrossRef] [PubMed]
- Peters, H.L.; Jochmans, D.; de Wilde, A.H.; Posthuma, C.C.; Snijder, E.J.; Neyts, J.; Seley-Radtke, K.L. Design, synthesis and evaluation of a series of acyclic fleximer nucleoside analogues with anti-coronavirus activity. Bioorg. Med. Chem. Lett. 2015, 25, 2923–2926. [Google Scholar] [CrossRef] [PubMed]
- Seley, K.L.; Zhang, L.; Hagos, A.; Quirk, S. "Fleximers". Design and synthesis of a new class of novel shape-modified nucleosides(1). J. Org. Chem. 2002, 67, 3365–3373. [Google Scholar] [CrossRef]
- Seley, K.L.; Quirk, S.; Salim, S.; Zhang, L.; Hagos, A. Unexpected inhibition of S-adenosyl-L-homocysteine hydrolase by a guanosine nucleoside. Bioorg. Med. Chem. Lett. 2003, 13, 1985–1988. [Google Scholar] [CrossRef]
- Quirk, S.; Seley, K.L. Substrate discrimination by the human GTP fucose pyrophosphorylase. Biochemistry 2005, 44, 10854–10863. [Google Scholar] [CrossRef]
- Eyer, L.; Nencka, R.; de Clercq, E.; Seley-Radtke, K.; Růžek, D. Nucleoside analogs as a rich source of antiviral agents active against arthropod-borne flaviviruses. Antivir. Chem. Chemother. 2018, 26, 2040206618761299. [Google Scholar] [CrossRef]
- Ackermann, M.; Padmanabhan, R. De novo synthesis of RNA by the dengue virus RNA-dependent RNA polymerase exhibits temperature dependence at the initiation but not elongation phase. J. Biol. Chem. 2001, 276, 39926–39937. [Google Scholar] [CrossRef]
- Nomaguchi, M.; Teramoto, T.; Yu, L.; Markoff, L.; Padmanabhan, R. Requirements for West Nile virus (−)- and (+)-strand subgenomic RNA synthesis in vitro by the viral RNA-dependent RNA polymerase expressed in Escherichia coli. J. Biol. Chem. 2004, 279, 12141–12151. [Google Scholar] [CrossRef]
- Jordheim, L.P.; Durantel, D.; Zoulim, F.; Dumontet, C. Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases. Nat. Rev. Drug Discov. 2013, 12, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Benhamou, Y.; Tubiana, R.; Thibault, V. Tenofovir disoproxil fumarate in patients with HIV and lamivudine-resistant hepatitis B virus. N. Engl. J. Med. 2003, 348, 177–178. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.S.; Fordyce, M.W.; Hitchcock, M.J. Tenofovir alafenamide: A novel prodrug of tenofovir for the treatment of Human Immunodeficiency Virus. Antiviral Res. 2016, 125, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Stedman, C. Sofosbuvir, a NS5B polymerase inhibitor in the treatment of hepatitis C: A review of its clinical potential. Therap. Adv. Gastroenterol. 2014, 7, 131–140. [Google Scholar] [CrossRef]
- Huang, Y.S.; Chang, S.Y.; Sheng, W.H.; Sun, H.Y.; Lee, K.Y.; Chuang, Y.C.; Su, Y.C.; Liu, W.C.; Hung, C.C.; Chang, S.C. Virological Response to Tenofovir Disoproxil Fumarate in HIV-Positive Patients with Lamivudine-Resistant Hepatitis B Virus Coinfection in an Area Hyperendemic for Hepatitis B Virus Infection. PLoS ONE 2016, 11, e0169228. [Google Scholar] [CrossRef][Green Version]
- Lam, Y.F.; Seto, W.K.; Wong, D.; Cheung, K.S.; Fung, J.; Mak, L.Y.; Yuen, J.; Chong, C.K.; Lai, C.L.; Yuen, M.F. Seven-Year Treatment Outcome of Entecavir in a Real-World Cohort: Effects on Clinical Parameters, HBsAg and HBcrAg Levels. Clin. Transl. Gastroenterol. 2017, 8, e125. [Google Scholar] [CrossRef]
- Deval, J.; Symons, J.A.; Beigelman, L. Inhibition of viral RNA polymerases by nucleoside and nucleotide analogs: Therapeutic applications against positive-strand RNA viruses beyond hepatitis C virus. Curr. Opin. Virol. 2014, 9, 1–7. [Google Scholar] [CrossRef]
- Behnam, M.A.; Nitsche, C.; Boldescu, V.; Klein, C.D. The Medicinal Chemistry of Dengue Virus. J. Med. Chem. 2016, 59, 5622–5649. [Google Scholar] [CrossRef]
- Boldescu, V.; Behnam, M.A.M.; Vasilakis, N.; Klein, C.D. Broad-spectrum agents for flaviviral infections: Dengue, Zika and beyond. Nat. Rev. Drug Discov. 2017, 16, 565–586. [Google Scholar] [CrossRef]
- Choi, K.H.; Rossmann, M.G. RNA-dependent RNA polymerases from Flaviviridae. Curr. Opin. Struct. Biol. 2009, 19, 746–751. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E.; Neyts, J. Antiviral agents acting as DNA or RNA chain terminators. Handb. Exp. Pharmacol. 2009, 189, 53–84. [Google Scholar] [CrossRef]
- De Clercq, E. Antivirals and antiviral strategies. Nat. Rev. Microbiol. 2004, 2, 704–720. [Google Scholar] [CrossRef]
- Cihlar, T.; Ray, A.S. Nucleoside and nucleotide HIV reverse transcriptase inhibitors: 25 years after zidovudine. Antiviral Res. 2010, 85, 39–58. [Google Scholar] [CrossRef]
- De Clercq, E. Recent highlights in the development of new antiviral drugs. Curr. Opin. Microbiol. 2005, 8, 552–560. [Google Scholar] [CrossRef]
- Bartholomeusz, A.; Tomlinson, E.; Wright, P.J.; Birch, C.; Locarnini, S.; Weigold, H.; Marcuccio, S.; Holan, G. Use of a flavivirus RNA-dependent RNA polymerase assay to investigate the antiviral activity of selected compounds. Antiviral Res. 1994, 24, 341–350. [Google Scholar] [CrossRef]
- Herve, M.; Sinoussi-Barre, F.; Chermann, J.C.; Herve, G.; Jasmin, C. Correlation between structure of polyoxotungstates and their inhibitory activity on polymerases. Biochem. Biophys. Res. Commun. 1983, 116, 222–229. [Google Scholar] [CrossRef]
- Caillet-Saguy, C.; Simister, P.C.; Bressanelli, S. An objective assessment of conformational variability in complexes of hepatitis C virus polymerase with non-nucleoside inhibitors. J. Mol. Biol. 2011, 414, 370–384. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.P.; Noble, C.G.; Nilar, S.; Shi, P.Y.; Yokokawa, F. Discovery of Potent Non-nucleoside Inhibitors of Dengue Viral RNA-Dependent RNA Polymerase from Fragment Screening and Structure-Guided Design. Adv. Exp. Med. Biol. 2018, 1062, 187–198. [Google Scholar] [CrossRef]
- Muller, D.A.; Young, P.R. The flavivirus NS1 protein: Molecular and structural biology, immunology, role in pathogenesis and application as a diagnostic biomarker. Antiviral Res. 2013, 98, 192–208. [Google Scholar] [CrossRef]
- Mackenzie, J.M.; Jones, M.K.; Young, P.R. Immunolocalization of the dengue virus nonstructural glycoprotein NS1 suggests a role in viral RNA replication. Virology 1996, 220, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Heinz, F.X.; Stiasny, K. Flaviviruses and flavivirus vaccines. Vaccine 2012, 30, 4301–4306. [Google Scholar] [CrossRef]
- Libraty, D.H.; Young, P.R.; Pickering, D.; Endy, T.P.; Kalayanarooj, S.; Green, S.; Vaughn, D.W.; Nisalak, A.; Ennis, F.A.; Rothman, A.L. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J. Infect. Dis. 2002, 186, 1165–1168. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.; Khaliq, M.; Kuhn, R.J. Closing the door on flaviviruses: Entry as a target for antiviral drug design. Antiviral Res. 2008, 80, 11–22. [Google Scholar] [CrossRef]
- Zhou, Z.; Khaliq, M.; Suk, J.E.; Patkar, C.; Li, L.; Kuhn, R.J.; Post, C.B. Antiviral compounds discovered by virtual screening of small-molecule libraries against dengue virus E protein. ACS Chem. Biol. 2008, 3, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Byrd, C.M.; Dai, D.; Grosenbach, D.W.; Berhanu, A.; Jones, K.F.; Cardwell, K.B.; Schneider, C.; Wineinger, K.A.; Page, J.M.; Harver, C.; et al. A novel inhibitor of dengue virus replication that targets the capsid protein. Antimicrob. Agents Chemother. 2013, 57, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Sung, C.; Kumar, G.S.; Vasudevan, S.G. Dengue Drug Development. In Dengue and Dengue Hemorrhagic Fever, 2nd ed.; CABI: Wallingford, UK, 2014. [Google Scholar]
- Chen, Y.L.; Abdul Ghafar, N.; Karuna, R.; Fu, Y.; Lim, S.P.; Schul, W.; Gu, F.; Herve, M.; Yokohama, F.; Wang, G.; et al. Activation of peripheral blood mononuclear cells by dengue virus infection depotentiates balapiravir. J. Virol. 2014, 88, 1740–1747. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J. The global pandemic of dengue/dengue haemorrhagic fever: Current status and prospects for the future. Ann. Acad. Med. Singap. 1998, 27, 227–234. [Google Scholar] [PubMed]
- Halstead, S.B.; Deen, J. The future of dengue vaccines. Lancet 2002, 360, 1243–1245. [Google Scholar] [CrossRef]
- Utarini, A.; Indriani, C.; Ahmad, R.A.; Tantowijoyo, W.; Arguni, E.; Ansari, M.R.; Supriyati, E.; Wardana, D.S.; Meitika, Y.; Ernesia, I.; et al. Efficacy of Wolbachia-Infected Mosquito Deployments for the Control of Dengue. N. Engl. J. Med. 2021, 384, 2177–2186. [Google Scholar] [CrossRef]
- Bertsy, G.; Saleh, M.; Giner, A.; Lopez-Moya, J.J.; Lakatos, L.; Tanguy, M.; Pfeffer, S.; Haasnoot, J.; Berkhout, B.; Fuchs, G.; et al. RNA Interference and Viruses: Current Innovations and Future Trends; Caister Academic Press: Wallingford, UK, 2010. [Google Scholar]
- Noble, C.G.; Shi, P.Y. Structural biology of dengue virus enzymes: Towards rational design of therapeutics. Antiviral Res. 2012, 96, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, P.; Liu, Y.; Roskin, K.M.; Jackson, K.K.; Dixit, V.P.; Lee, J.Y.; Artiles, K.L.; Zompi, S.; Vargas, M.J.; Simen, B.B.; et al. Convergent antibody signatures in human dengue. Cell Host Microbe 2013, 13, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Liberis, E.; Velickovic, P.; Sormanni, P.; Vendruscolo, M.; Liò, P. Parapred: Antibody paratope prediction using convolutional and recurrent neural networks. Bioinformatics 2018, 34, 2944–2950. [Google Scholar] [CrossRef] [PubMed]
- Deac, A.; VeliČković, P.; Sormanni, P. Attentive Cross-Modal Paratope Prediction. J. Comput. Biol. 2019, 26, 536–545. [Google Scholar] [CrossRef] [PubMed]
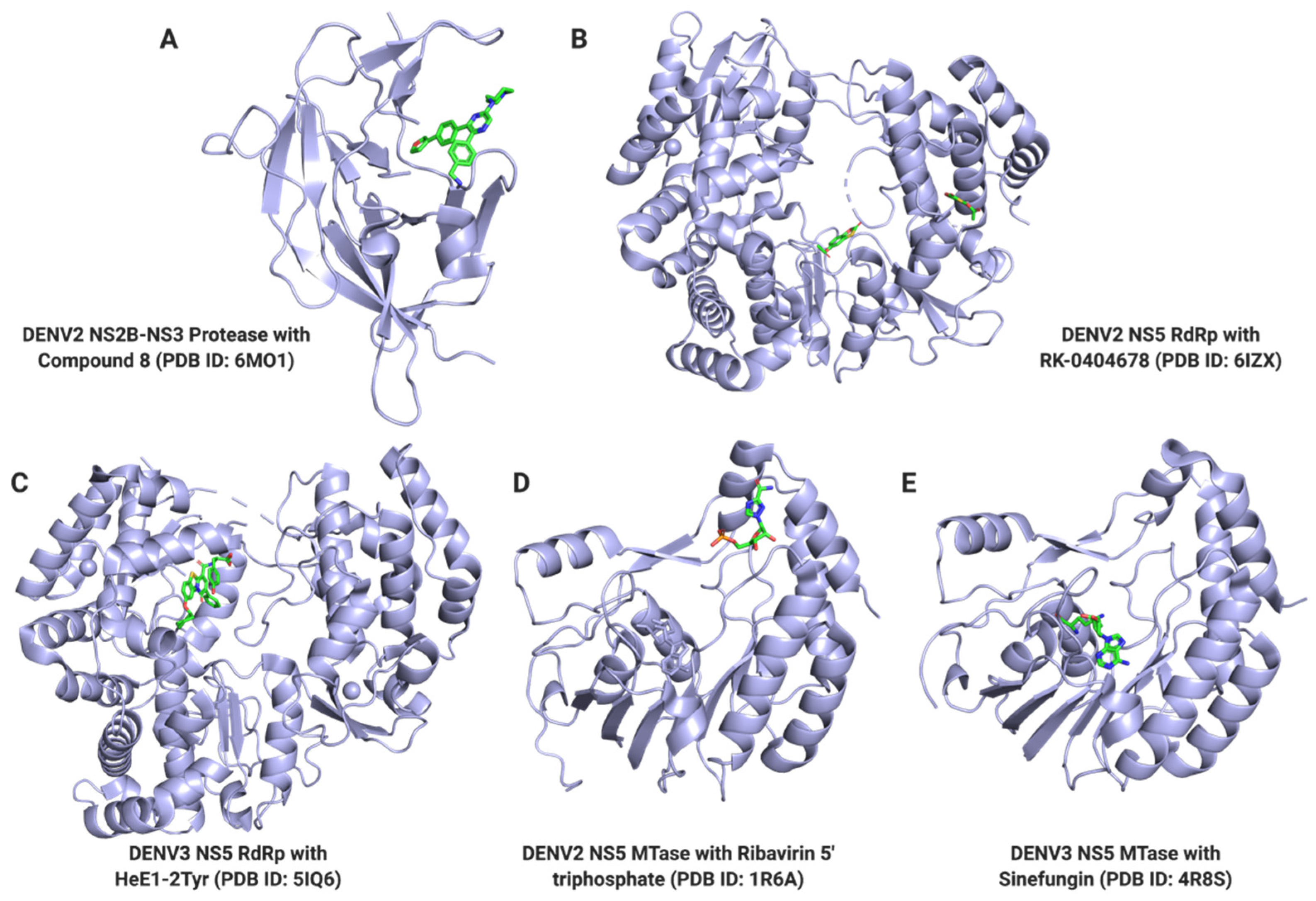
| Target | Function | PDB ID | Compound Name | Ki or KD (μM) | IC50 (μM) | References |
|---|---|---|---|---|---|---|
| NS5 | MTase | 3P8Z | Compound 10 | 0.82 (N7), 0.17 (2′-O) | na | [73] |
| 1R6A | Ribavirin 5′-triphosphate | 55 * | 100 | [74] | ||
| 5EHI | BF287 | na | 452 | [75] | ||
| 5EKX | NB2E11 | na | >1000 | [75] | ||
| 5EHG | BF341 | na | 369 | [75] | ||
| 5EIF | NB2C3 | na | >1000 | [75] | ||
| 5EC8 | BF175 | na | na | [75] | ||
| 5EIW | NB3C2 | na | >1000 | [75] | ||
| 5E9Q | BF174 | na | na | [75] | ||
| 4R8S | Sinefungin | 0.136 * | 0.03 (N7), 0.04 (2′-O) | [76,77,78] | ||
| na | 1-TP | 22 | 8.4 | [79] | ||
| na | 2-TP | na | 1.1 | [79] | ||
| RdRp | 5K5M | Compound 27 | na | 0.173 | [80] | |
| 5I3P | Compound 27 | na | 0.048 | [80] | ||
| 5I3Q | Compound 29 | na | 0.016 | [80] | ||
| 5IQ6 | HeE1-2Tyr | 1.96 | 1.5 | [81] | ||
| 5HMW | Compound 5 | 154 * | 177 | [82] | ||
| 5HMY | Compound 15 | 1.4 * | 1.7 | [82] | ||
| 6IZX | RK-0404678 | na | 201 | [83] | ||
| 5HMX | Compound 10 | 28 * | 15 | [82] | ||
| 5HN0 | Compound 4 | >200 * | 769 | [82] | ||
| 5HMZ | Compound 23 | 0.12 * | 0.34 | [82] | ||
| 3VWS | NITD-107 | 225 * | 113 | [84] | ||
| 5F3Z | PC-79-SH52 | 29 * | 140 | [85] | ||
| 5F3T | JF-31-MG46 | 210 | 730 | [85] | ||
| 5F41 | FD-83-KI26 | 67 * | 210 | [85] | ||
| 6IZZ | RK-0404678 | na | 287 | [83] | ||
| NS3 | Protease (NS2B-NS3) | 4M9T | DTNB | na | na | [86] |
| 3U1J | Aprotinin | 0.026 | na | [87,88] | ||
| 6MO1 | Compound 8 | na | 0.29 | [89] | ||
| 6MO2 | Compound 9 | na | 0.59 | [89] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obi, J.O.; Gutiérrez-Barbosa, H.; Chua, J.V.; Deredge, D.J. Current Trends and Limitations in Dengue Antiviral Research. Trop. Med. Infect. Dis. 2021, 6, 180. https://doi.org/10.3390/tropicalmed6040180
Obi JO, Gutiérrez-Barbosa H, Chua JV, Deredge DJ. Current Trends and Limitations in Dengue Antiviral Research. Tropical Medicine and Infectious Disease. 2021; 6(4):180. https://doi.org/10.3390/tropicalmed6040180
Chicago/Turabian StyleObi, Juliet O., Hernando Gutiérrez-Barbosa, Joel V. Chua, and Daniel J. Deredge. 2021. "Current Trends and Limitations in Dengue Antiviral Research" Tropical Medicine and Infectious Disease 6, no. 4: 180. https://doi.org/10.3390/tropicalmed6040180
APA StyleObi, J. O., Gutiérrez-Barbosa, H., Chua, J. V., & Deredge, D. J. (2021). Current Trends and Limitations in Dengue Antiviral Research. Tropical Medicine and Infectious Disease, 6(4), 180. https://doi.org/10.3390/tropicalmed6040180






