Hantaviruses in Agricultural and Forestry Workers: Knowledge, Attitudes and Practices in Italian Physicians
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Questionnaire
- (a)
- Any knowledge that hantavirus infections may occur in Italy (yes vs. no);
- (b)
- Any personal and/or professional interaction with hantavirus infections (i.e., managing of infected patient(s), personal infection, infection in friends, relatives, etc.).
2.3. Data Analysis
2.4. Ethical Considerations
3. Results
3.1. Descriptive Analysis: General Characteristics of the Sample
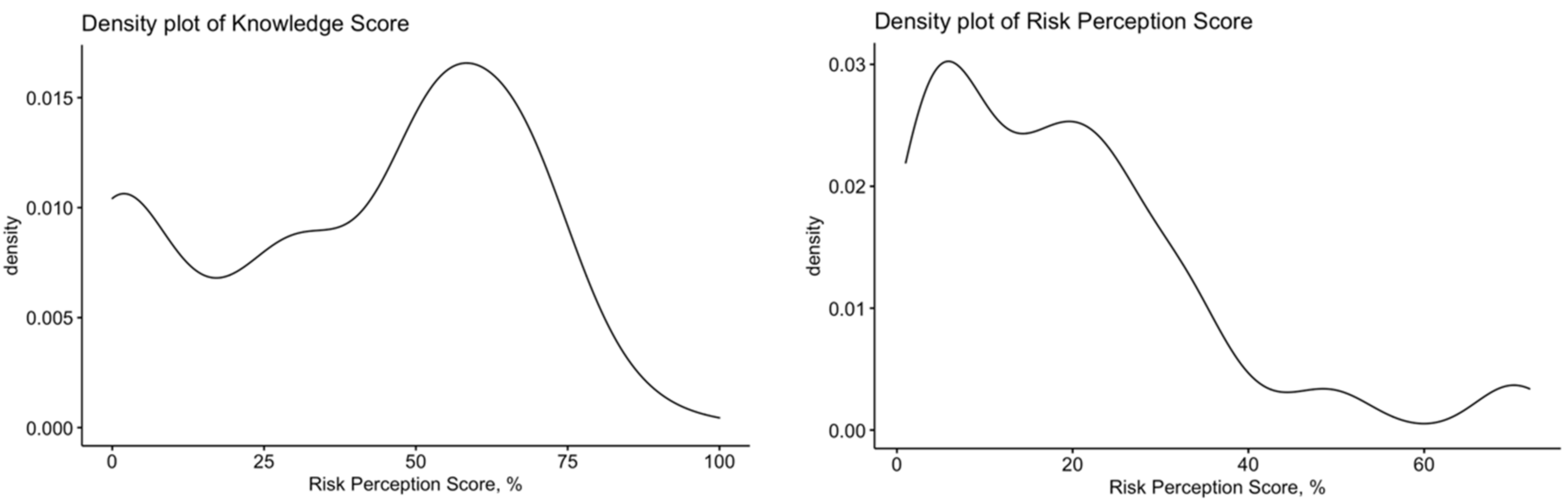
3.2. Assessment of the Risk Perception
3.3. Assessment of Knowledge about Human Hantavirus Infections
3.4. Univariate Analysis
3.5. Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Avšič-Županc, T.; Saksida, A.; Korva, M. Hantavirus Infections. Clin. Microbiol. Infect. 2019, 21, e6–e16. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Ma, H.; Shu, J.; Zhang, Q.; Han, M.; Liu, Z.; Jin, X.; Zhang, F.; Wu, X. Vaccines and Therapeutics Against Hantaviruses. Front. Microbiol. 2020, 10, 2989. [Google Scholar] [CrossRef] [PubMed]
- Castel, G.; Chevenet, F.; Razzauti, M.; Murri, S.; Marianneau, P.; Cosson, J.F.; Tordo, N.; Plyusnin, A. Phylogeography of Puumala Orthohantavirus in Europe. Viruses 2019, 11, 679. [Google Scholar] [CrossRef] [PubMed]
- Heyman, P.; Vaheri, A.; Lundkvist, Å.; Avsic-Zupanc, T. Hantavirus Infections in Europe: From Virus Carriers to a Major Public-Health Problem. Expert Rev. Anti-Infect. Ther. 2009, 7, 205–217. [Google Scholar] [CrossRef]
- Schöffel, N.; Braun, M.; Bendels, M.H.K.; Brüggmann, D.; Groneberg, D.A. Die Humane Hantavirus-Infektion: Eine Literaturübersicht. Zentralbl. Arbeitsmed. Arb. Ergon. 2018, 68, 94–97. [Google Scholar] [CrossRef]
- Hjertqvist, M.; Klein, S.L.; Ahlm, C.; Klingström, J. Mortality Rate Patterns for Hemorrhagic Fever with Renal Syndrome Caused by Puumala Virus. Emerg. Infect. Dis. 2010, 16, 1584–1586. [Google Scholar] [CrossRef]
- Heyman, P.; Ceianu, C.S.; Christova, I.; Tordo, N.; Beersma, M.; Alves, M.J.; Lundkvist, A.; Hukic, M.; Papa, A.; Tenorio, A.; et al. A Five-Year Perspective on the Situation of Haemorrhagic Fever with Renal Syndrome and Status of the Hantavirus Reservoirs in Europe, 2005–2010. Eurosurveillance 2011, 16, 19961. [Google Scholar] [CrossRef] [PubMed]
- Laenen, L.; Vergote, V.; Calisher, C.H.; Klempa, B.; Klingström, J.; Kuhn, J.H.; Maes, P. Hantaviridae: Current Classification and Future Perspectives. Viruses 2019, 11, 788. [Google Scholar] [CrossRef]
- Lledó, L.; Giménez-Pardo, C.; Gegúndez, M.I. Screening of Forestry Workers in Guadalajara Province (Spain) for Antibodies to Lymphocytic Choriomeningitis Virus, Hantavirus, Rickettsia Spp. and Borrelia Burgdorferi. Int. J. Environ. Res. Public Health 2019, 16, 4500. [Google Scholar] [CrossRef]
- Mertens, M.; Hofmann, J.; Petraityte-Burneikiene, R.; Ziller, M.; Sasnauskas, K.; Friedrich, R.; Niederstrasser, O.; Krüger, D.H.; Groschup, M.H.; Petri, E.; et al. Seroprevalence Study in Forestry Workers of a Non-Endemic Region in Eastern Germany Reveals Infections by Tula and Dobrava-Belgrade Hantaviruses. Med. Microbiol. Immunol. 2011, 200, 263–268. [Google Scholar] [CrossRef]
- Jurke, A.; Bannert, N.; Brehm, K.; Fingerle, V.; Kempf, V.A.J.; Kömpf, D.; Lunemann, M.; Mayer-Scholl, A.; Niedrig, M.; Nöckler, K.; et al. Serological Survey of Bartonella spp., Borrelia Burgdorferi, Brucella spp., Coxiella Burnetii, Francisella Tularensis, Leptospira spp., Echinococcus, Hanta-, TBE- and XMR-Virus Infection in Employees of Two Forestry Enterprises in North Rhine-Westphalia. Int. J. Med. Microbiol. 2015, 305, 652–662. [Google Scholar] [CrossRef]
- Groen, J.; Gerding, M.N.; Jordans, J.G.M.; Clement, J.P.; Nieuwenhuijs, J.H.M.; Osterhaus, A.D.M.E. Hantavirus Infections in The Netherlands: Epidemiology and Disease. Epidemiol. Infect. 1995, 114, 373–383. [Google Scholar] [CrossRef]
- Oldal, M.; Németh, V.; Madai, M.; Pintér, R.; Kemenesi, G.; Dallos, B.; Kutas, A.; Sebők, J.; Horváth, G.; Bányai, K.; et al. Serosurvey of Pathogenic Hantaviruses among Forestry Workers in Hungary. Int. J. Occup. Med. Environ. Health 2014, 27, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Sibold, C.; Meisel, H.; Lundkvist, Å.; Schulz, A.; Cifire, F.; Ulrich, R.; Kozuch, O.; Labuda, M.; Krüger, D.H. Short Report: Simultaneous Occurrence of Dobrava, Puumala, and Tula Hantaviruses in Slovakia. Am. J. Trop. Med. Hyg. 1999, 61, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Bergstedt Oscarsson, K.; Brorstad, A.; Baudin, M.; Lindberg, A.; Forssén, A.; Evander, M.; Eriksson, M.; Ahlm, C. Human Puumala Hantavirus Infection in Northern Sweden; Increased Seroprevalence and Association to Risk and Health Factors. BMC Infect. Dis. 2016, 16, 566. [Google Scholar] [CrossRef]
- Ahlm, C.; Thelin, A.; Elgh, F.; Juto, P.; Stiernström, E.L.; Holmberg, S.; Tärnvik, A. Prevalence of Antibodies Specific to Puumala Virus among Farmers in Sweden. Scand. J. Work Environ. Health 1998, 24, 104–108. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Traavik, T.; Sommer, A.I.; Mehl, R.; Berdal, B.P.; Stavem, K.; Hunderi, O.H.; Dalrymple, J.M. Nephropathia Epidemica in Norway: Antigen and Antibodies in Rodent Reservoirs and Antibodies in Selected Human Populations. J. Hyg. 1984, 93, 139–146. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Diseases Prevention and Control (ECDC). Hantavirus Infection. In Annual Epidemiological Report for 2019; ECDC: Stockholm, Sweden, 2020; Available online: https://www.ecdc.europa.eu/en/publications-data/hantavirus-infection-annual-epidemiological-report-2019 (accessed on 25 June 2021).
- Nuti, M.; Agostini, M.; Albini, E.; Avsic-Zupanc, T.; Kraigher, A. Hantaan Antibody in Italian Ex-Soldiers Who Served in the Balkans. Lancet 1991, 338, 1277. [Google Scholar] [CrossRef]
- Nuti, M.; Ieradi, L.A.; Cristaldi, M.; Gibbs, C.J. Prevalence of Antibody to Hantaviruses in Humans and Rodentsin Italy. Provisional Evidence of Hantaan-like Virus Infections in Humans and Seoul-like Virus Infections in Rodents. In Hemorrhagic Fever with Renal Syndrome, Tick- and Mosquito-Borne Viruses. Archives of Virology Supplementum; Calisher, C.H., Ed.; Springer: Vienna, Austria, 1990; Volume 1. [Google Scholar] [CrossRef]
- Nuti, M.; Amadeo, D.; Autorino, G.L.; Crovatto, M.; Crucil, C.; Ghionni, A.; Giommi, M.; Salvati, F.; Santini, G.F. Seroprevalence of Antibodies to Hantaviruses and Leptospires in Selected Italian Population Groups. Eur. J. Epidemiol. 1992, 8, 98–102. [Google Scholar] [CrossRef]
- Nuti, M.; Amaddeo, D.; Crovatto, M.; Ghionni, A.; Polato, D.; Lillini, E.; Pitzus, E.; Santini, G.F. Infections in an Alpine Environment: Antibodies to Hantaviruses, Leptospira, Rickettsiae, and Borrelia Burgdorferi in Defined Italian Populations. Am. J. Trop. Med. Hyg. 1993, 48, 20–25. [Google Scholar] [CrossRef]
- Kreidl, P.; Walder, G.; Morosetti, G. Studio TIMO-Sieroprevalenza Di Varie Malattie Trasmissibili Attraverso Zecche, Zanzare e Roditori Nel Tirolo Settentrionale, Orientale e Alto Adige; Bozen/Innsbruck, Italy. 2004. Available online: http://www.provincia.bz.it/salute-benessere/osservatorio-salute/pubblicazioni.asp?publ_page=12#download-area-idx126884 (accessed on 25 June 2021).
- Tagliapietra, V.; Rosà, R.; Rossi, C.; Rosso, F.; Hauffe, H.C.; Tommasini, M.; Versini, W.; Cristallo, A.F.; Rizzoli, A. Emerging Rodent-Borne Viral Zoonoses in Trento, Italy. EcoHealth 2018, 15, 695–704. [Google Scholar] [CrossRef]
- Kallio-Kokko, H.; Laakkonen, J.; Rizzoli, A.; Tagliapietra, V.; Cattadori, I.; Perkins, S.E.; Hudson, P.J.; Cristofolini, A.; Versini, W.; Vapalahti, O.; et al. Hantavirus and Arenavirus Antibody Prevalence in Rodents and Humans in Trentino, Northern Italy. Epidemiol. Infect. 2006, 134, 830–836. [Google Scholar] [CrossRef]
- Manigold, T.; Vial, P. Human Hantavirus Infections: Epidemiology, Clinical Features, Pathogenesis and Immunology. Swiss Med. Wkly. 2014, 144, w13937. [Google Scholar] [CrossRef]
- Riccò, M.; Cattani, S.; Casagranda, F.; Gualerzi, G.; Signorelli, C. Knowledge, Attitudes, Beliefs and Practices of Occupational Physicians towards Vaccinations of Health Care Workers: A Cross Sectional Pilot Study in North-Eastern Italy. Int. J. Occup. Med. Environ. Health 2017, 30, 775–790. [Google Scholar] [CrossRef]
- Yates, F.J.; Stone, E.R. The Risk Construct. In Risk-Taking Behaviour; Yates, F.J., Ed.; John Wiley & Sons: Chichester, UK, 1992; pp. 1–25. ISBN 047-192-2-501. [Google Scholar]
- Betsch, C.; Wicker, S. Personal Attitudes and Misconceptions, Not Official Recommendations Guide Occupational Physicians’ Vaccination Decisions. Vaccine 2014, 32, 4478–4484. [Google Scholar] [CrossRef]
- Ermonval, M.; Baychelier, F.; Tordo, N. What Do We Know about How Hantaviruses Interact with Their Different Hosts? Viruses 2016, 8, 223. [Google Scholar] [CrossRef]
- Vapalahti, O.; Mustonen, J.; Lundkvist, Å.; Henttonen, H.; Plyusnin, A.; Vaheri, A. Hantavirus Infections in Europe. Lancet Infect. Dis. 2003, 3, 653–661. [Google Scholar] [CrossRef]
- Olsson, G.E.; Leirs, H.; Henttonen, H. Hantaviruses and Their Hosts in Europe: Reservoirs Here and There, but Not Everywhere? Vector Borne Zoonotic Dis. 2010, 10, 549–561. [Google Scholar] [CrossRef]
- Vapalahti, O.; Plyusnin, A.; Vaheri, A.; Henttonen, H. Hantavirus Antibodies in European Mammalogists. Lancet 1995, 345, 1569. [Google Scholar] [CrossRef]
- Faulde, M.; Sobe, D.; Kimmig, P.; Scharninghausen, J. Renal Failure and Hantavirus Infection in Europe. Nephrol. Dial. Transplant. 2000, 15, 751–753. [Google Scholar] [CrossRef][Green Version]
- Sin, M.A.; Stark, K.; Treeck, U.; van Dieckmann, H.; Uphoff, H.; Hautmann, W.; Bornhofen, B.; Jensen, E.; Pfaff, G.; Koch, J. Risk Factors for Hantavirus Infection in Germany, 2005. Emerg. Infect. Dis. 2007, 13, 1364–1366. [Google Scholar] [CrossRef]
- Jonsson, C.B.; Figueiredo, L.T.M.; Vapalahti, O. A Global Perspective on Hantavirus Ecology, Epidemiology, and Disease. Clin. Microbiol. Rev. 2010, 23, 412–441. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 23 April 2021).
- Suzuki, K.; Mutinelli, L.E. Knowledge and Practices about Hantavirus Pulmonary Syndrome in a Cluster of Japanese Communities in Argentina. Rev. Panam. Salud Public 2009, 25, 128–133. [Google Scholar] [CrossRef]
- Riccò, M.; Peruzzi, S.; Ranzieri, S.; Balzarini, F.; Valente, M.; Marchesi, F.; Bragazzi, N.L. Hantavirus Infections in Italy: Not Reported Doesn’t Mean Inexistent. Acta Biomed. Atenei Parm. 2020, 92, e2021324. [Google Scholar] [CrossRef]
- Riccò, M.; Gualerzi, G.; Ranzieri, S.; Ferraro, P.; Bragazzi, N.L. Knowledge, Attitudes, Practices (KAP) of Italian Occupational Physicians towards Tick Borne Encephalitis. Trop. Med. Infect. Dis. 2020, 5, 177. [Google Scholar] [CrossRef]
- Riccò, M.; Vezzosi, L.; Gualerzi, G.; Signorelli, C. Knowledge, Attitudes and Practices (KAP) towards Vaccinations in the School Settings: An Explorative Survey. J. Prev. Med. Hyg. 2017, 58, 266–278. [Google Scholar] [CrossRef]
- Riccò, M.; Cattani, S.; Veronesi, L.; Colucci, M.E. Knowledge, Attitudes, Beliefs and Practices of Construction Workers towards Tetanus Vaccine in Northern Italy. Ind. Health 2016, 54, 554–563. [Google Scholar] [CrossRef]
- Riccò, M.; Ranzieri, S.; Balzarini, F.; Vezzosi, L.; Marchesi, F.; Valente, M.; Peruzzi, S. A Pilot Study on Knowledge, Attitudes and Beliefs of Medical Professionals on Invasive Fungal Infections. J. Mycol. Med. 2021, 31, 101103. [Google Scholar] [CrossRef]
- Valdivieso, F.; Gonzalez, C.; Najera, M.; Olea, A.; Cuiza, A.; Aguilera, X.; Mertz, G. Knowledge, Attitudes, and Practices Regarding Hantavirus Disease and Acceptance of a Vaccine Trial in Rural Communities of Southern Chile. Hum. Vaccines Immunother. 2017, 13, 808–815. [Google Scholar] [CrossRef]
- McConnell, M.S. Hantavirus Public Health Outreach Effectiveness in Three Populations: An Overview of Northwestern New Mexico, Los Santos Panama, and Region IX Chile. Viruses 2014, 6, 986–1003. [Google Scholar] [CrossRef]
- Harris, C.; Armién, B. Sociocultural Determinants of Adoption of Preventive Practices for Hantavirus: A Knowledge, Attitudes, and Practices Survey in Tonosí, Panama. PLoS Negl. Trop. Dis. 2020, 14, e0008111. [Google Scholar] [CrossRef] [PubMed]
- Marx, G.; Stinson, K.; Deatrich, M.; Albanese, B. Notes from the Field: Hantavirus Pulmonary Syndrome in a Migrant Farm Worker—Colorado, 2016. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 62–63. [Google Scholar] [CrossRef]
- Tokarska-Rodak, M.; Plewik, D.; Michalski, A.J.; Kolodziej, M.; Melgies, A.; Panczuk, A.; Konon, H.; Niemcewicz, M. Serological Surveillance of Vector-Borne and Zoonotic Diseases among Hunters in Eastern Poland. J. Vector Borne Dis. 2016, 53, 355–361. [Google Scholar] [PubMed]
- Khan, A.; Khan, M.; Ullah, S.; Wei, D.Q. Hantavirus: The Next Pandemic We Are Waiting For? Interdiscip. Sci. 2021, 13, 147–152. [Google Scholar] [CrossRef]
- Ma, C.; Yu, P.; Nawaz, M.; Zuo, S.; Jin, T.; Li, Y.; Li, J.; Li, H.; Xu, J. Hantaviruses in Rodents and Humans, Xi’an, PR China. J. Gen. Virol. 2012, 93, 2227–2236. [Google Scholar] [CrossRef]
- Dheerasekara, K.; Sumathipala, S.; Muthugala, R. Hantavirus Infections—Treatment and Prevention. Curr. Treat. Options Infect. Dis. 2020, 12, 410–421. [Google Scholar] [CrossRef]
- Mitchell, K.C.; Ryan, P.; Howard, D.E.; Feldman, K.A. Understanding Knowledge, Attitudes, and Behaviors Toward West Nile Virus Prevention: A Survey of High-Risk Adults in Maryland. Vector Borne Zoonotic Dis. 2018, 18, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Olsson, G.E.; Dalerum, F.; Hörnfeldt, B.; Elgh, F.; Palo, T.R.; Juto, P.; Ahlm, C. Human Hantavirus Infections, Sweden. Emerg. Infect. Dis. 2003, 9, 1395–1401. [Google Scholar] [CrossRef]
- Leoncini, F.; Bartolozzi, D.; Buonamici, C.; Mazzotta, F.; Paci, P.; Salvadori, M.; Lombardi, M.; Balducci, M.; Verani, P.; Nicoletti, L. New Etiological Agents of Nephropathy in Italy. The Hantavirus. G. Mal. Infett. Parassit. 1989, 41, 68–72. Available online: https://www.researchgate.net/publication/310617677_New_etiological_agents_of_nephropathy_in_Italy_The_Hantavirus (accessed on 25 June 2021). (In Italian).
- Salvadori, M.; Lombardi, M.; Nicoletti, L. Acute Renal Involvement in Hantavirus Infection: First Report in Italy. J. Nephrol. 1989, 1, 17–22. [Google Scholar]
- Durando, P.; Dini, G.; Massa, E.; la Torre, G. Tackling Biological Risk in the Workplace: Updates and Prospects Regarding Vaccinations for Subjects at Risk of Occupational Exposure in Italy. Vaccines 2019, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Dini, G.; Toletone, A.; Sticchi, L.; Orsi, A.; Bragazzi, N.L.; Durando, P. Influenza Vaccination in Healthcare Workers: A Comprehensive Critical Appraisal of the Literature. Hum. Vaccines Immunother. 2018, 14, 772–789. [Google Scholar] [CrossRef] [PubMed]
- Manzoli, L.; Sotgiu, G.; Magnavita, N.; Durando, P.; Barchitta, M.; Carducci, A.; Conversano, M.; de Pasquale, G.; Dini, G.; Firenze, A.; et al. Evidence-Based Approach for Continuous Improvement of Occupational Health. Epidemiol. Prev. 2015, 39, S81–S85. [Google Scholar]
- Heiervang, E.; Goodman, R. Advantages and Limitations of Web-Based Surveys: Evidence from a Child Mental Health Survey. Soc. Psychiat. Epidemiol. 2011, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xu, S.; Lei, W.; Zhao, Y.; Liu, H.; Yao, D.; Xu, Y.; Lv, Q.; Hao, G.; Xu, Y.; et al. Knowledge, Attitudes, and Practices Regarding Zika: Paper and Internet Based Survey in Zhejiang, China. JMIR Public Health Surveill. 2017, 3, e81. [Google Scholar] [CrossRef] [PubMed]
- Moßhammer, D.; Michaelis, M.; Mehne, J.; Wilm, S.; Rieger, M.A. General Practitioners’ and Occupational Health Physicians’ Views on Their Cooperation: A Cross-Sectional Postal Survey. Int. Arch. Occup. Environ. Health 2016, 89, 449–459. [Google Scholar] [CrossRef]
- Betsch Id, C.; Schmid Id, P.; Id, D.H.; Korn, L.; Holtmann, C.; Bö Hm, R. Beyond Confidence: Development of a Measure Assessing the 5C Psychological Antecedents of Vaccination. PLoS ONE 2018, 13, e0208601. [Google Scholar] [CrossRef]
| Variable | No., % | Average ± S.D. |
|---|---|---|
| Age (years) | 44.2 ± 8.2 | |
| Male gender | 95, 42.6% | |
| Working as occupational physician | 94, 42.2% | |
| Residence | ||
| Northern Italy | 93, 41.7% | |
| Central Italy | 114, 51.1% | |
| Southern Italy | 16, 7.2% | |
| Residence in “Triveneto” | 16, 7.2% | |
| Hantavirus infections in Italy acknowledged as possible | 151, 67.7% | |
| Previously managed cases of hantaviruses in the practice | 10, 4.5% | |
| Perceived severity of hantavirus infections in the general population (significant, highly significant) | 85, 38.1% | |
| Perceived frequency of hantavirus infections in the general population (significant, highly significant) | 20, 9.0% | |
| Risk perception score (%) | 19.2 ± 16.0 | |
| Perceived risk of hantavirus infections (significant, highly significant) | ||
| … among agricultural workers | 143, 64.1% | |
| … among forestry workers | 146, 65.5% | |
| … among construction workers | 66, 29.6% | |
| … among food industry workers | 41, 18.4% | |
| … among dairy farmers | 41, 18.4% | |
| General knowledge score (%) | 40.7 ± 26.2 | |
| General knowledge score > median (i.e., 46.7%) | 109, 48.9% |
| Statement | CORRECT ANSWER | TOTAL (No./223, %) | OP (No./94, %) | HCP (No./129, %) | p Value * |
|---|---|---|---|---|---|
| Q01. In the last five years, human cases of hantavirus infections have been officially reported. | FALSE | 8, 3.6% | 7,7.4% | 1, 0.8% | 0.023 |
| Q02. Rodents are the main hosts of hantaviruses. | TRUE | 149, 66.8% | 77, 81.9% | 72, 55.8% | <0.001 |
| Q03. Mosquitos are potential vectors for hantaviruses. | FALSE | 122, 54.7% | 60, 63.8% | 62, 48.1% | 0.028 |
| Q04. Ticks are potential vector for hantaviruses. | FALSE | 90, 40.4% | 48, 51.1% | 42, 32.6% | 0.008 |
| Q05. Hantaviruses are characterized by frequent interhuman spreading. | FALSE | 104, 46.6% | 44, 46.8% | 60, 46.5% | 1.000 |
| Q06. Effective vaccines against hantaviruses are commercially available. | FALSE | 116, 52.0% | 56, 59.6%% | 60, 46.5% | 0.073 |
| Q07. Human hantavirus infections can elicit acute renal failure. | TRUE | 135, 60.5% | 62, 66.0%% | 73, 56.6% | 0.202 |
| Q08. Human hantavirus infections can elicit chronic renal failure. | TRUE | 60, 26.9% | 29, 30.9%% | 31, 24.0% | 0.327 |
| Q09. The majority of human hantavirus infections elicits an Influenza-like illness. | TRUE | 111, 49.8% | 54, 57.4%% | 57, 44.2% | 0.069 |
| Q10. Case fatality ratio of European hantavirus infections is estimated … | 0.006 | ||||
| <1% | FALSE | 25, 11.2% | 14, 14.9%% | 11, 8.5% | |
| 1–10% | TRUE | 25, 11.2% | 14, 14.9%% | 11, 8.5% | |
| 10–20% | FALSE | 9, 4.0% | 5, 5.3% | 4, 3.1% | |
| 20–30% | FALSE | 5, 2.2% | 5, 5.3% | 0, - | |
| >30% | FALSE | 0, - | 0, - | 0, - | |
| Don’t know | - | 159, 71.3% | 56, 59.6% | 103, 79.8% | |
| Q11. Seroprevalence of hantaviruses in Italian general population is estimated to be … | 0.040 | ||||
| <1% | FALSE | 45, 20.2% | 24, 25.5% | 21, 16.3% | |
| 1–5% | TRUE | 35, 15.7% | 20, 21.3% | 15, 11.6% | |
| 5–10% | FALSE | 6, 2.7% | 5, 5.3% | 1, 0.8% | |
| >10% | FALSE | 0, - | 0, - | 0, - | |
| Don’t know | - | 137, 61.4% | 50, 53.2% | 87, 67.4% | |
| Q12. High seroprevalence for hantaviruses in humans and rodents is documented in … | 0.006 | ||||
| Alpe Adria region | TRUE | 71, 31.8% | 35, 37.2% | 36, 27.9% | |
| Western Alps (i.e., Piedmont, Aosta Valley and Lombardy) | FALSE | 13, 5.8% | 2, 2.1% | 11, 8.5% | |
| Apennine mountains between Tuscany and Emilia-Romagna | FALSE | 19, 8.5% | 11, 11.7% | 8, 6.2% | |
| Apennine mountains between Umbria and Latium | FALSE | 26, 11.7% | 6, 6.4% | 20, 15.5% | |
| Po River Valley | FALSE | 42, 18.8% | 14, 14.9% | 28, 21.7% | |
| Tiber River Valley | FALSE | 14, 6.3% | 10, 10.6% | 4, 3.1% | |
| Don’t know | - | 37, 17.0% | 16, 17.0% | 21, 16.3% | |
| Q13. Humans are infected by hantaviruses mainly through … | 0.383 | ||||
| Inhalation of aerosols containing urine and feces of infected rodents | TRUE | 119, 53.4% | 51, 54.3% | 58, 45.0% | |
| Bite of fleas feed up on infected rodents | FALSE | 12, 5.4% | 7, 7.4% | 5, 3.9% | |
| Bite of infected rodents | FALSE | 22, 9.9% | 11, 11.7% | 11, 8.5% | |
| Don’t know | - | 70, 31.4% | 25, 26.6% | 45, 34.9% | |
| Q14. Human hantavirus infections are seasonal ones | 0.180 | ||||
| True, peak during the cold season | FALSE | 6, 2.7% | 1, 1.1% | 5, 3.9% | |
| True, peak during the warm season | TRUE | 75, 33.6% | 36, 38.3% | 39, 30.% | |
| False | FALSE | 29, 13.0% | 15, 16.0% | 14, 10.9% | |
| Don’t know | - | 113, 50.7% | 42, 44.7% | 71, 55.0% | |
| Q15. Official notification of human hantavirus infections is legally required | TRUE | 140, 62.8% | 69, 73.4% | 71, 55.0% | 0.008 |
| Clinical Feature | TOTAL (No./223, %) | OP (No./94, %) | HCP (No./129, %) | p Value * |
|---|---|---|---|---|
| Fever (T >38 °C) | 156, 70.0% | 70, 74.5% | 86, 66.7% | 0.268 |
| Headache | 136, 61.0% | 66, 70.2% | 70, 54.4% | 0.023 |
| Abdominal pain | 122, 54.7% | 50, 53.2% | 72, 55.8% | 0.801 |
| Back pain | 61, 27.4% | 31, 33.0% | 30, 23.3% | 0.145 |
| Nausea, vomiting | 106, 47.5% | 40, 42.6% | 66, 51.2% | 0.256 |
| Petechiae | 74, 33.2% | 32, 34.0% | 42, 32.6% | 0.930 |
| Hypotension | 49, 22.0% | 20, 21.3% | 29, 22.5% | 0.960 |
| Oliguria (<0.5 L/24 h) | 129, 57.8% | 94, 100% | 35, 27.1% | 0.107 |
| Polyuria (>2.0 L/24 h) | 46, 20.6% | 29, 30.9% | 17, 13.2% | 0.002 |
| Leukocytosis | 140, 62.8% | 71, 75.5% | 69, 53.5% | 0.001 |
| Thrombocytopenia | 90, 40.4% | 48, 51.1% | 42, 32.6% | 0.008 |
| Proteinuria | 143, 64.1% | 79, 84.0% | 64, 49.6% | 0.009 |
| Hematuria | 125, 56.1% | 66, 70.2% | 59, 45.7% | 0.001 |
| Variable | TOTAL (No./223) | Perceived Risk of Hantavirus Infections in Agricultural Workers | Perceived Risk of Hantavirus Infections in Forestry Workers | ||
|---|---|---|---|---|---|
| Significant/Very Significant (No.,%) | p Value | Significant/Very Significant (No.,%) | p Value | ||
| Age >50 years | 42, 18.8% | 28, 66.7% | 0.839 | 29, 69.0% | 0.718 |
| Male gender | 95, 42.6% | 55, 57.9% | 0.126 | 60, 63.2% | 0.629 |
| Working as occupational physician | 94, 42.2% | 55, 58.5% | 0.177 | 64, 68.1% | 0.577 |
| Residence in Northern Italy | 94, 42.2% | 57, 60.6% | 0.432 | 58, 61.7% | 0.386 |
| Residence in “Triveneto” | 16, 7.2% | 14, 87.5% | 0.080 | 14, 87.5% | 0.099 |
| Hantavirus infections in Italy acknowledged as possible | 151, 67.7% | 112, 74.2% | <0.001 | 114, 75.5% | <0.001 |
| Previously managed cases of hantaviruses in the practice | 10, 4.5% | 8, 80.0% | 0.463 | 8, 80.0% | 0.517 |
| General knowledge score > median (i.e., 46.7%) | 109, 48.9% | 86, 78.9% | <0.001 | 89, 81.7% | <0.001 |
| Hantavirus diseases acknowledged as severe (in the general population) | 85, 38.1% | 60, 70.6% | 0.151 | 64, 75.3% | 0.023 |
| Hantavirus diseases acknowledged as frequently reported (in the general population) | 20, 9.0% | 13, 65.0% | 1.000 | 13, 65.0% | 1.000 |
| Perceived risk of hantavirus infections | |||||
| among forestry workers | 146, 65.5% | 125, 85.6% | <0.001 | - | - |
| among agricultural workers | 143, 64.1% | - | - | 125, 87.4% | <0.001 |
| among construction workers | 66, 29.6% | 58, 87.9% | <0.001 | 48, 72.7% | 0.186 |
| among food processing workers | 41, 18.4% | 35, 85.4% | 0.003 | 37, 90.2% | <0.001 |
| among dairy farmers | 41, 18.4% | 36, 87.8% | 0.001 | 40, 97.6% | <0.001 |
| Perceived Risk of Being Infected by Hantaviruses among Agricultural Workers | Perceived Risk of Being Infected by Hantaviruses among Forestry Workers | |||
|---|---|---|---|---|
| aOR | 95%CI | aOR | 95% CI | |
| Male gender | 0.585 | 0.225; 1.525 | - | - |
| Working as occupational physician | 1.105 | 0.290; 3.080 | - | - |
| Residence in “Triveneto” | 4.413 | 0.715; 27.251 | 0.637 | 0.068; 5.922 |
| Hantavirus infections in Italy acknowledged as possible | 21.193 | 3.666; 122.505 | 0.481 | 0.129; 1.800 |
| General knowledge score >median | 0.531 | 0.134; 2.106 | 5.880 | 1.620; 21.343 |
| Hantavirus diseases acknowledged as severe | 0.581 | 0.209; 1.612 | 1.319 | 0.511; 3.406 |
| Perceived risk of hantavirus infections | ||||
| … among forestry workers | 34.993 | 11.690; 140.751 | - | - |
| … among agricultural workers | - | - | 33.505 | 10.995; 102.103 |
| … among dairy farmers | 1.496 | 0.436; 5.129 | 26.209 | 2.516; 272.936 |
| … among food processing workers | 1.896 | 0.533; 6.750 | 5.219 | 0.879; 31.000 |
| … among construction workers | 67.915 | 17.551; 262.799 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riccò, M.; Ferraro, P.; Peruzzi, S.; Balzarini, F.; Ranzieri, S. Hantaviruses in Agricultural and Forestry Workers: Knowledge, Attitudes and Practices in Italian Physicians. Trop. Med. Infect. Dis. 2021, 6, 169. https://doi.org/10.3390/tropicalmed6030169
Riccò M, Ferraro P, Peruzzi S, Balzarini F, Ranzieri S. Hantaviruses in Agricultural and Forestry Workers: Knowledge, Attitudes and Practices in Italian Physicians. Tropical Medicine and Infectious Disease. 2021; 6(3):169. https://doi.org/10.3390/tropicalmed6030169
Chicago/Turabian StyleRiccò, Matteo, Pietro Ferraro, Simona Peruzzi, Federica Balzarini, and Silvia Ranzieri. 2021. "Hantaviruses in Agricultural and Forestry Workers: Knowledge, Attitudes and Practices in Italian Physicians" Tropical Medicine and Infectious Disease 6, no. 3: 169. https://doi.org/10.3390/tropicalmed6030169
APA StyleRiccò, M., Ferraro, P., Peruzzi, S., Balzarini, F., & Ranzieri, S. (2021). Hantaviruses in Agricultural and Forestry Workers: Knowledge, Attitudes and Practices in Italian Physicians. Tropical Medicine and Infectious Disease, 6(3), 169. https://doi.org/10.3390/tropicalmed6030169








