A Case of Trauma-Induced Falciformispora lignatilis Eumycetoma in a Renal Transplant Recipient
Abstract
:1. Introduction
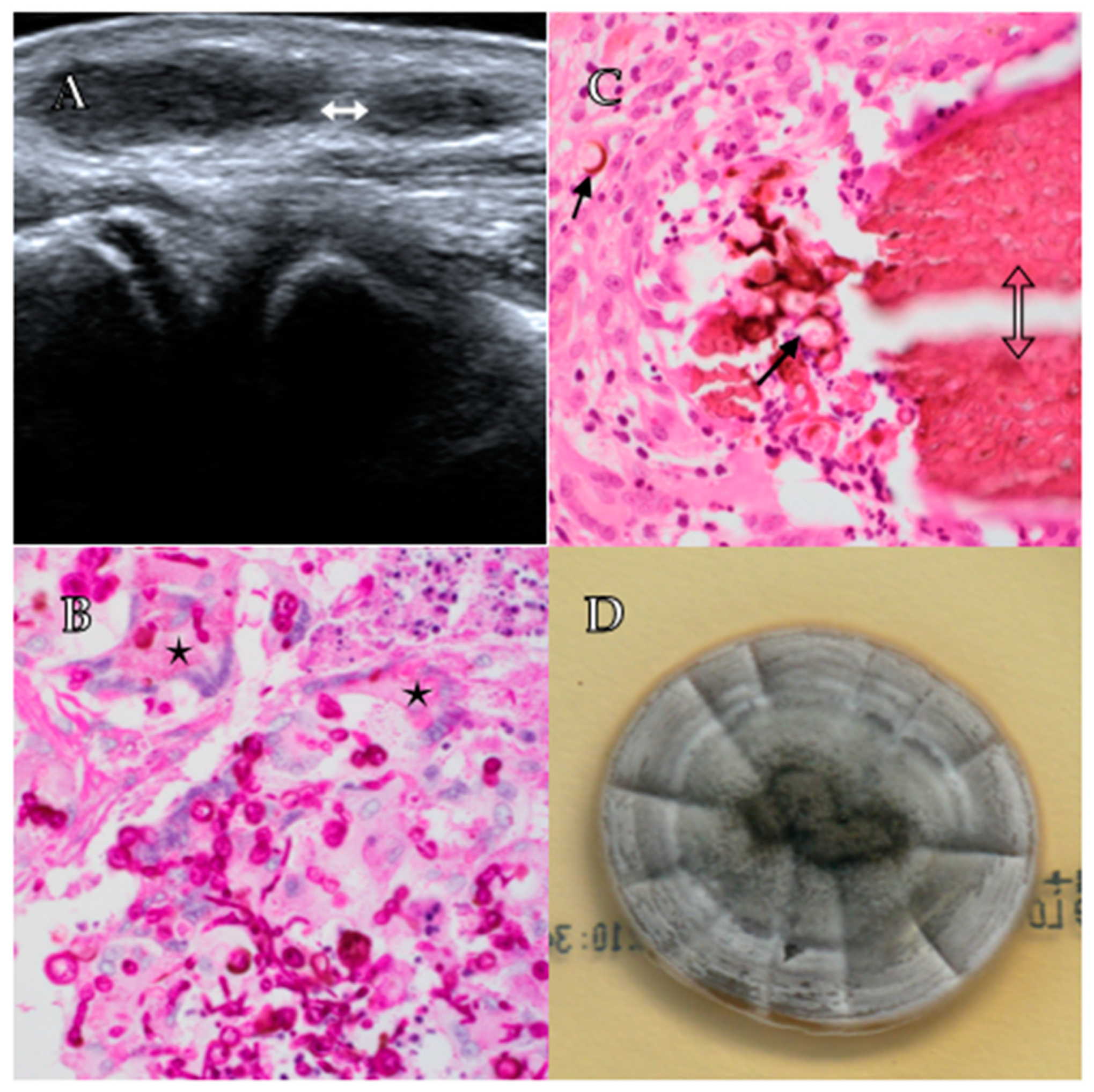
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Centre for Disease Control. Mycetoma | Fungal Diseases. Available online: https://www.cdc.gov/fungal/diseases/mycetoma/index.html (accessed on 20 July 2020).
- Ahmed, A.O.A.; van de Sande, W.W.J.; De Hoog, G.S. Fungi Causing Eumycotic Mycetoma*. In Manual of Clinical Microbiology, 12th ed.; American Society of Microbiology: Washington, DC, USA, 2019; pp. 2261–2277. [Google Scholar]
- van de Sande, W.W.J. Global Burden of Human Mycetoma: A Systematic Review and Meta-analysis. PLoS Negl. Trop. Dis. 2013, 7, e2550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalatharan, L.; Kelley, P. Eumycetoma diagnosed in urban Australia. Med. J. Aust. 2020, 212, 107–107.e1. [Google Scholar] [CrossRef] [PubMed]
- Kidd, S.; Crawford, L.; Halliday, C. Antifungal Susceptibility Testing and Identification. Infect. Dis. Clin. N. Am. 2021, 35, 313–339. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.; de Hoog, G.S.; Stevens, D.A.; Fahal, A.H.; van de Sande, W.W.J. In vitro antifungal susceptibility of coelomycete agents of black grain eumycetoma to eight antifungals. Med. Mycol. 2015, 53, 295–301. [Google Scholar] [CrossRef]
- Ahmed, A.A.; van de Sande, W.; Fahal, A.H. Mycetoma laboratory diagnosis: Review article. PLoS Negl. Trop. Dis. 2017, 11, e0005638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, S.A.; van de Sande, W.W.J.; Stevens, D.A.; Fahal, A.; van Diepeningen, A.D.; Menken, S.B.J.; De Hoog, G. Revision of agents of black-grain eumycetoma in the order Pleosporales. Persoonia Mol. Phylogeny Evol. Fungi 2014, 33, 141–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyde, K.D. Intertidal mangrove fungi from the west coast of Mexico, including one new genus and two new species. Mycol. Res. 1992, 96, 25–30. [Google Scholar] [CrossRef]
- De Hoog, G.S.; Van Diepeningen, A.D.; Mahgoub, E.S.; Van De Sande, W.W.J. New species of Madurella, causative agents of black-grain mycetoma. J. Clin. Microbiol. 2011, 50, 988–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhary, A.; Meis, J.F.; Guarro, J.; de Hoog, G.S.; Kathuria, S.; Arendrup, M.C.; Arikan-Akdagli, S.; Akova, M.; Boekhout, T.; Caira, M.; et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of systemic phaeohyphomycosis: Diseases caused by black fungi. Clin. Microbiol. Infect. 2014, 20, 47–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamoth, F.; Kontoyiannis, D.P. Therapeutic challenges of non-Aspergillus invasive mold infections in immunosuppressed patients. Antimicrob. Agents Chemother. 2019, 63, e01244-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crabol, Y.; Poiree, S.; Bougnoux, M.E.; Maunoury, C.; Barete, S.; Zeller, V.; Arvieux, C.; Pineau, S.; Amazzough, K.; Lecuit, M.; et al. Last Generation Triazoles for Imported Eumycetoma in Eleven Consecutive Adults. PLoS Negl. Trop. Dis. 2014, 8, e3232. [Google Scholar] [CrossRef] [PubMed]
- Meis, J.F.G.M.; Schouten, R.A.; Verweij, P.E.; Dolmans, W.; Wetzels, J.F.M. Atypical presentation of Madurella mycetomatis mycetoma in a renal transplant patient. Transpl. Infect. Dis. 2000, 2, 96–98. [Google Scholar] [CrossRef] [PubMed]
- Mcgrogan, D.; David, M.D.; Roberts, C.; Borman, A.M.; Nath, J.; Inston, N.G.; Mellor, S. Pseudotumoral presentation of fungating mycetoma caused by Phaeoacremonium fuscum in a renal transplant patient. Transpl. Infect. Dis. 2015, 17, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Estrada, R.; Chávez-López, G.; Estrada-Chávez, G.; López-Martínez, R.; Welsh, O. Eumycetoma. Clin. Dermatol. 2012, 30, 389–396. [Google Scholar] [CrossRef] [PubMed]
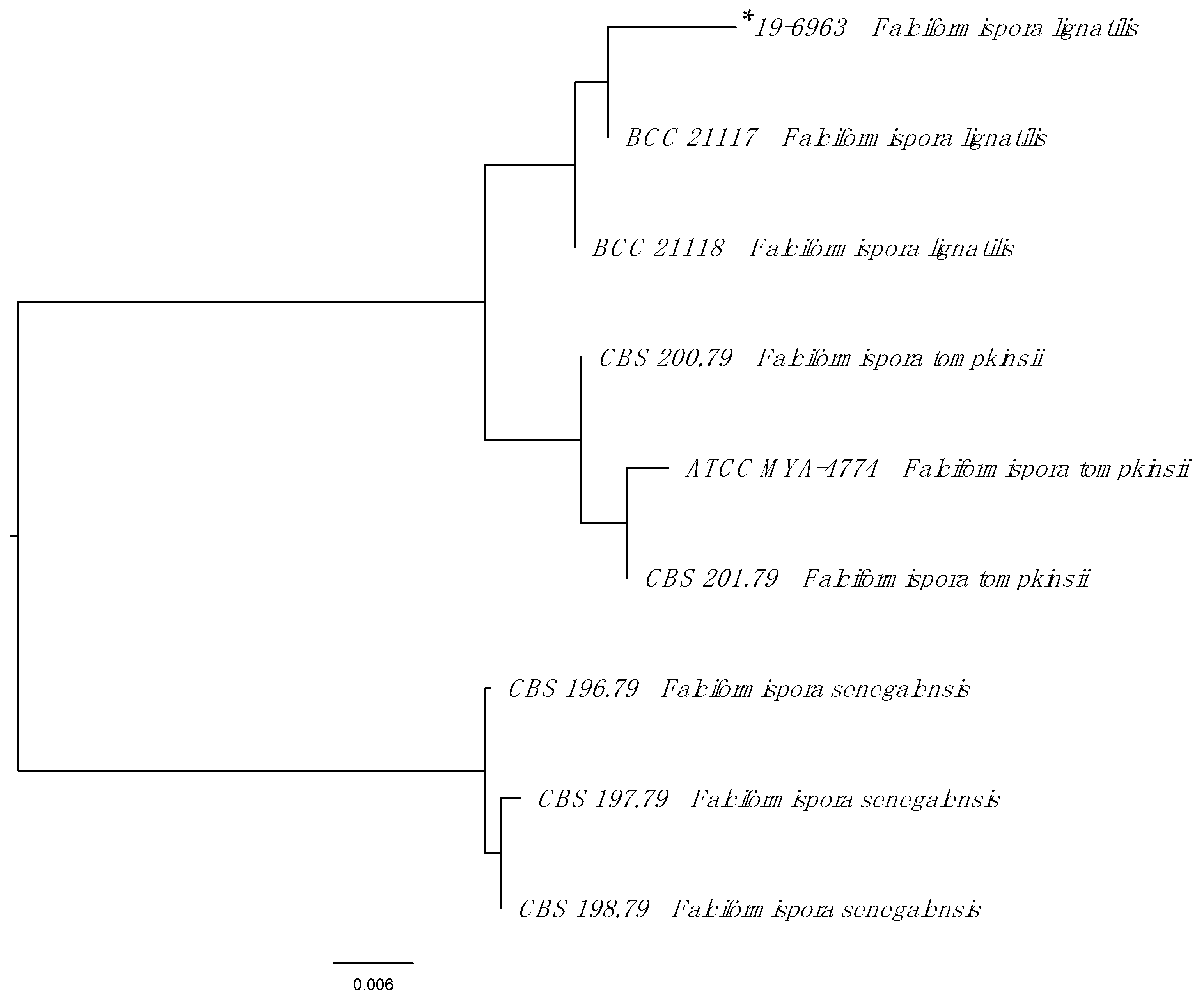
| Grain Colour | Causative Organism |
|---|---|
| Black | Madurella spp. (M. mycetomatis, M. fahali, M. pseudomycetomatis, M. tropicana) |
| Falciformispora (formerly Lepstosphaeria) spp. (F. senegalensis, F. tompkinsi, F. lignatilis) | |
| Curvuralia spp. | |
| Exophiala spp. | |
| Phaeoacremonium spp. | |
| Phialophora verrucosa | |
| Biatriospora mackinnonii (formerly Pyrenochaeta mackinnonii) | |
| Trematosphaeria grisea | |
| Medicopsis romeroi (formerly Pyrenochaeta romeroi) | |
| Pale/White/Yellow | Scedosporium apiospermum (formerly Pseudallescheria apiosperma) |
| Acremonium spp. | |
| Aspergillus spp. | |
| Fusarium spp. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olenski, M.; Halliday, C.; Gullifer, J.; Martinez, E.; Crowe, A.; Sheorey, H.; Darby, J. A Case of Trauma-Induced Falciformispora lignatilis Eumycetoma in a Renal Transplant Recipient. Trop. Med. Infect. Dis. 2021, 6, 144. https://doi.org/10.3390/tropicalmed6030144
Olenski M, Halliday C, Gullifer J, Martinez E, Crowe A, Sheorey H, Darby J. A Case of Trauma-Induced Falciformispora lignatilis Eumycetoma in a Renal Transplant Recipient. Tropical Medicine and Infectious Disease. 2021; 6(3):144. https://doi.org/10.3390/tropicalmed6030144
Chicago/Turabian StyleOlenski, Maxwell, Catriona Halliday, James Gullifer, Elena Martinez, Amy Crowe, Harsha Sheorey, and Jonathan Darby. 2021. "A Case of Trauma-Induced Falciformispora lignatilis Eumycetoma in a Renal Transplant Recipient" Tropical Medicine and Infectious Disease 6, no. 3: 144. https://doi.org/10.3390/tropicalmed6030144
APA StyleOlenski, M., Halliday, C., Gullifer, J., Martinez, E., Crowe, A., Sheorey, H., & Darby, J. (2021). A Case of Trauma-Induced Falciformispora lignatilis Eumycetoma in a Renal Transplant Recipient. Tropical Medicine and Infectious Disease, 6(3), 144. https://doi.org/10.3390/tropicalmed6030144






