Observational Characterization of the Ecological and Environmental Features Associated with the Presence of Oropouche Virus and the Primary Vector Culicoides paraensis: Data Synthesis and Systematic Review
Abstract
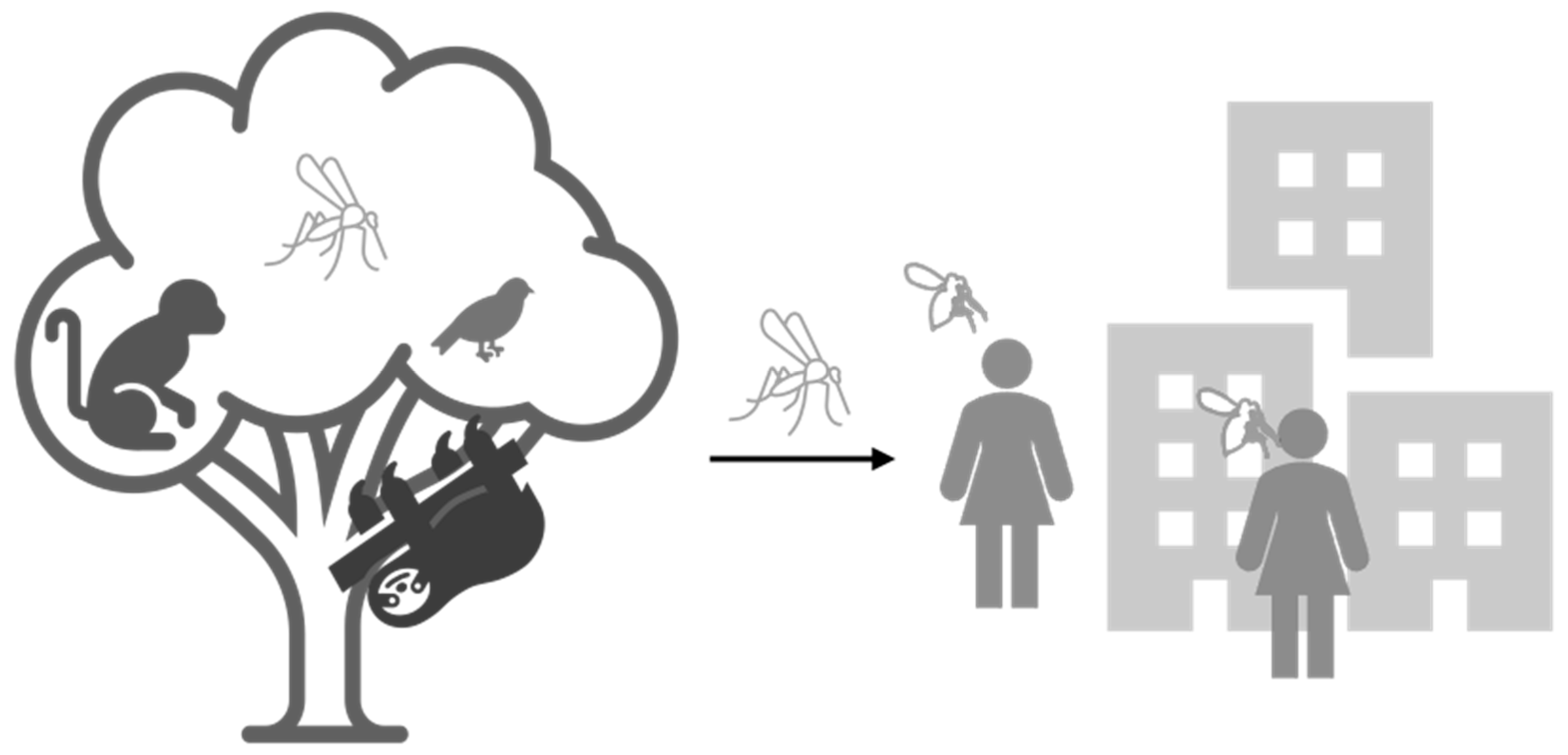
1. Introduction
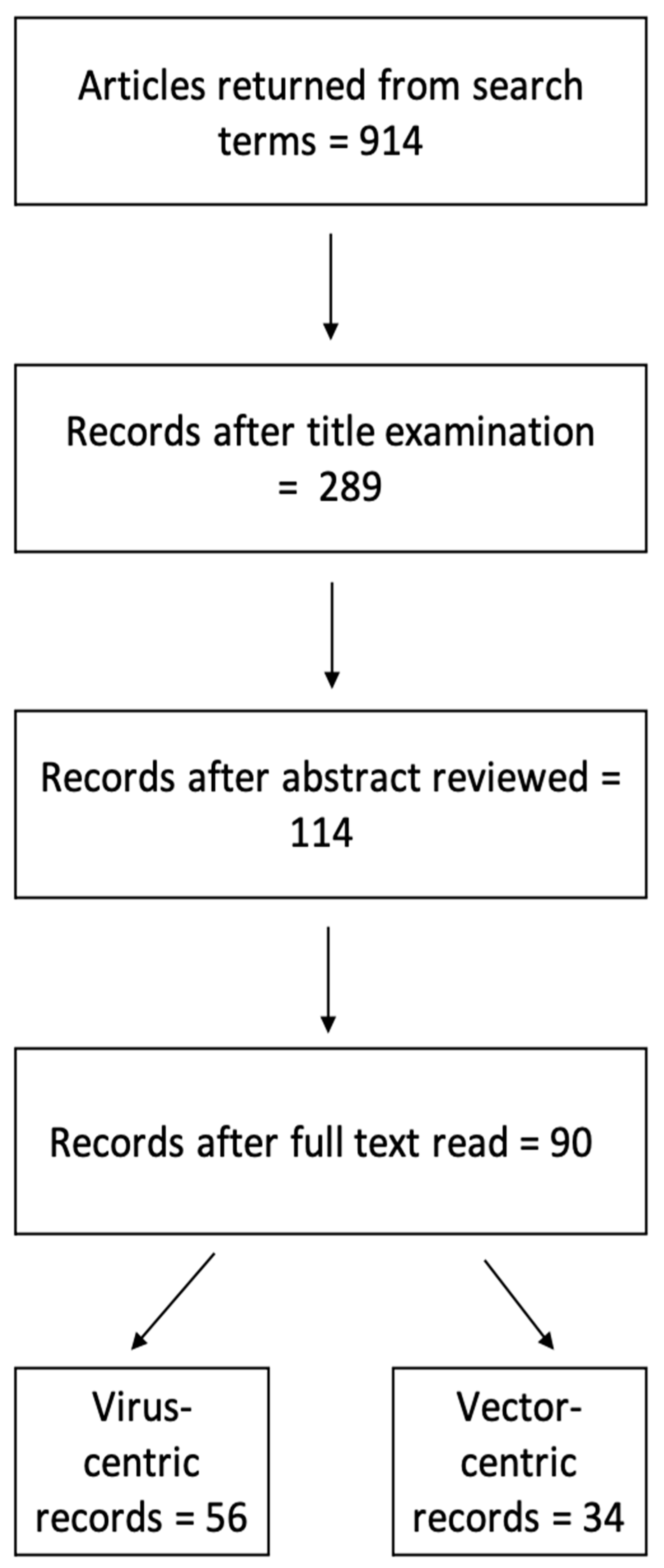
2. Material and Methods
2.1. Study Selection
2.2. Data Extraction and Synthesis
2.3. Classification Models
- 1.
- All eco-environmental variables were run as independent variables.
- 2.
- Reduced models whereby several variables were combined as below and run as a uni-, bi-, and tri-variate models for acute and past infections:
- a.
- “livestock”, “military”, “crops”, and “mining” were combined into a single “anthropological” variable.
- b.
- “river”, “dam”, and “pantanal” were combined into a single “water” variable.
- c.
- “primary” or “secondary” forests were combined into a single “forest” variable.
- 3.
- Reduced models whereby the several variables were combined as below and run as a uni-, bi-, and tri-variate models for the presence of the vector:
- a.
- “livestock”, “military”, and “crops”, were combined into a single “anthropological” variable.
- b.
- “river”, “dam”, “pantanal”, “swamp”, “streams”, “springs”, and “lakes” were combined into a single “water” variable.
- c.
- “primary”, “secondary”, “mangrove”, and “restinga” forests were combined into a single “forest” variable.
2.4. Correlation Analyses
3. Results
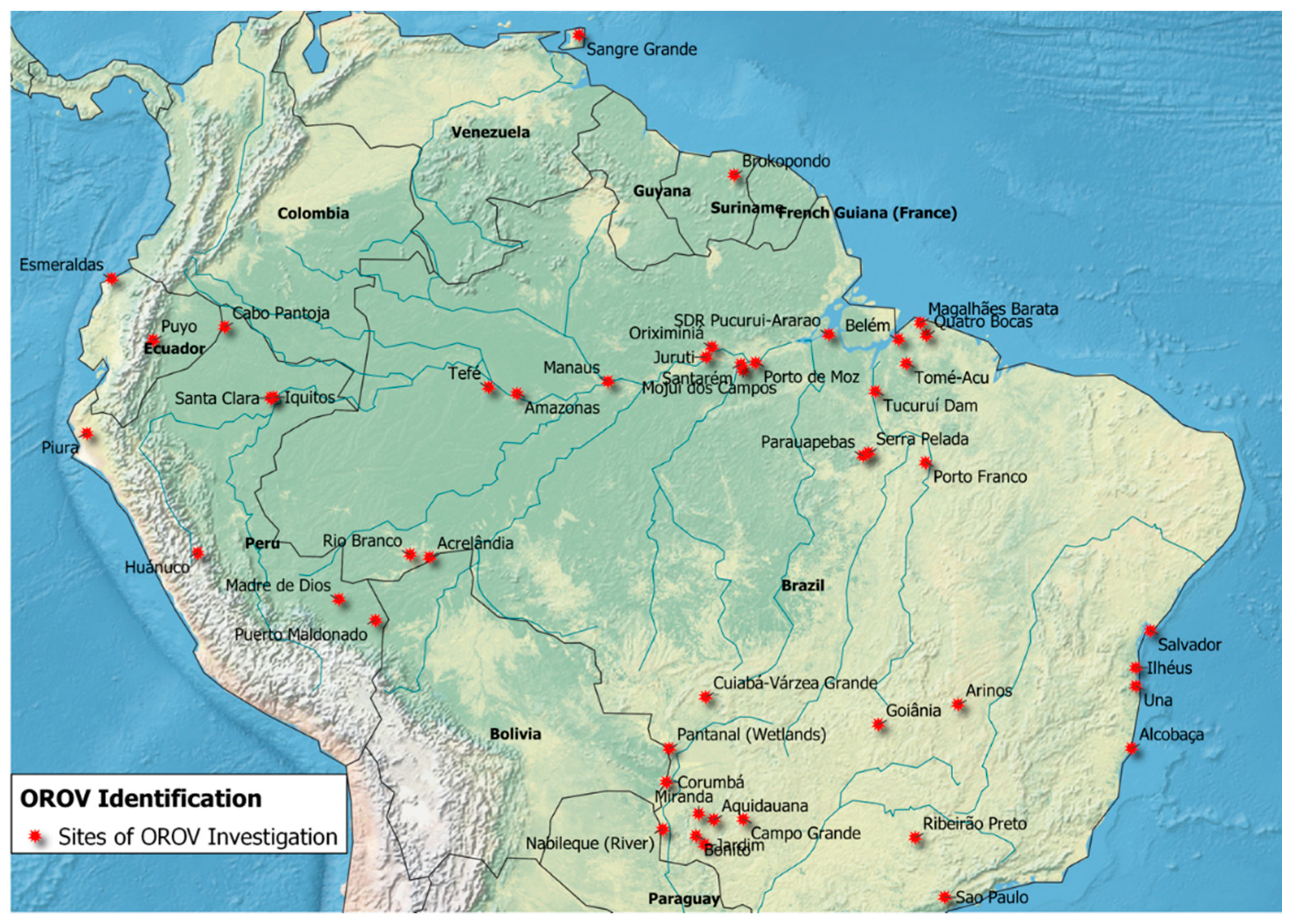
3.1. OROV Surveillance
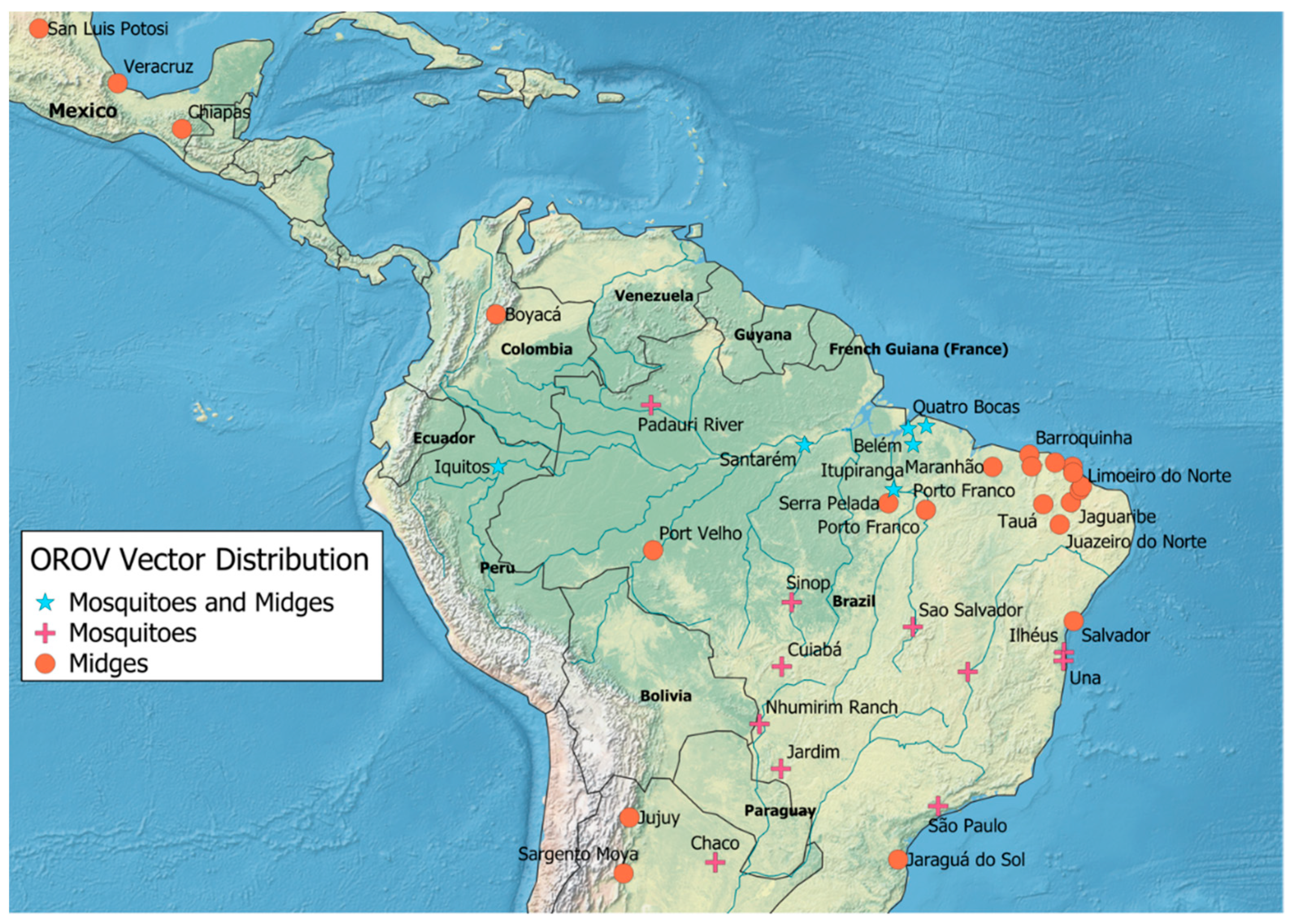
3.2. Arthropod Vector Surveillance
3.3. OROV Detection
4. Ecological Factors Associated with OROV Presence
4.1. Vector Presence and Diversity
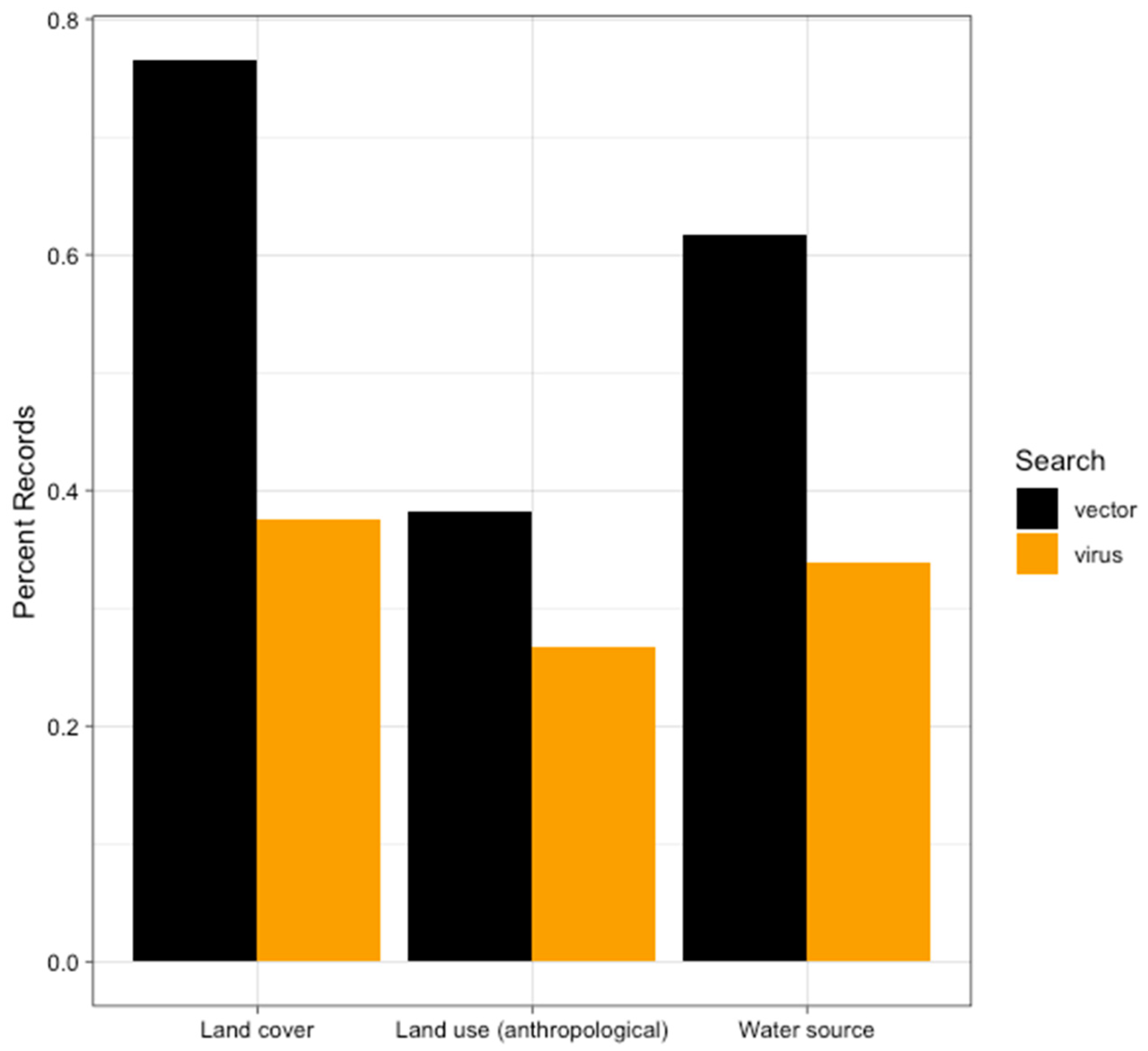
4.2. Anthropogenic Land Use
4.3. Water Source
4.4. Land Cover
4.5. Odds of Detecting Acute and Past Infections
4.6. Odds of Vector Presence
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hughes, H.R.; Adkins, S.; Alkhovskiy, S.; Beer, M.; Blair, C.; Calisher, C.H.; Drebot, M.; Lambert, A.J.; de Souza, W.M.; Marklewitz, M.; et al. ICTV Virus Taxonomy Profile: Peribunyaviridae. J. Gen. Virol 2020, 101, 1–2. [Google Scholar] [CrossRef]
- Pauvolid-Correa, A.; Campos, Z.; Soares, R.; Nogueira, R.M.R.; Komar, N. Neutralizing antibodies for orthobunyaviruses in Pantanal, Brazil. PLoS Negl. Trop. Dis. 2017, 11, e0006014. [Google Scholar] [CrossRef] [PubMed]
- Wise, E.L.; Marquez, S.; Mellors, J.; Paz, V.; Atkinson, B.; Gutierrez, B.; Zapata, S.; Coloma, J.; Pybus, O.G.; Jackson, S.K.; et al. Oropouche virus cases identified in Ecuador using an optimised qRT-PCR informed by metagenomic sequencing. PLoS Negl. Trop. Dis. 2020, 14, e0007897. [Google Scholar] [CrossRef]
- Anderson, C.R.; Spence, L.; Downs, W.G.; Aitken, T.H. Oropouche virus: A new human disease agent from Trinidad, West Indies. Am. J. Trop. Med. Hyg. 1961, 10, 574–578. [Google Scholar] [CrossRef]
- Mouraao, M.P.; Bastos, M.S.; Gimaqu, J.B.; Mota, B.R.; Souza, G.S.; Grimmer, G.H.; Galusso, E.S.; Arruda, E.; Figueiredo, L.T. Oropouche fever outbreak, Manaus, Brazil, 2007–2008. Emerg. Infect. Dis. 2009, 15, 2063–2064. [Google Scholar] [CrossRef] [PubMed]
- Silva-Caso, W.; Aguilar-Luis, M.A.; Palomares-Reyes, C.; Mazulis, F.; Weilg, C.; Del Valle, L.J.; Espejo-Evaristo, J.; Soto-Febres, F.; Martins-Luna, J.; Del Valle-Mendoza, J. First outbreak of Oropouche Fever reported in a non-endemic western region of the Peruvian Amazon: Molecular diagnosis and clinical characteristics. Int. J. Infect. Dis. 2019, 83, 139–144. [Google Scholar] [CrossRef]
- Vasconcelos, H.B.; Azevedo, R.S.; Casseb, S.M.; Nunes-Neto, J.P.; Chiang, J.O.; Cantuaria, P.C.; Segura, M.N.; Martins, L.C.; Monteiro, H.A.; Rodrigues, S.G.; et al. Oropouche fever epidemic in Northern Brazil: Epidemiology and molecular characterization of isolates. J. Clin. Virol. 2009, 44, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Izurieta, R.O.; Macaluso, M.; Watts, D.M.; Tesh, R.B.; Guerra, B.; Cruz, L.M.; Galwankar, S.; Vermund, S.H. Assessing yellow Fever risk in the ecuadorian Amazon. J. Glob. Infect. Dis. 2009, 1, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Groot, H. Estudios sobre virus transmitidos por arthropodos en Colombia. Rev. Acad. Colomb. Cienc. Exactas Físicas Nat. 1964, 12, 46. [Google Scholar]
- Rogers, M.B.; Gulino, K.M.; Tesh, R.B.; Cui, L.; Fitch, A.; Unnasch, T.R.; Popov, V.L.; Travassos da Rosa, A.P.A.; Guzman, H.; Carrera, J.P.; et al. Characterization of five unclassified orthobunyaviruses (Bunyaviridae) from Africa and the Americas. J. Gen. Virol. 2017, 98, 2258–2266. [Google Scholar] [CrossRef]
- Saeed, M.F.; Wang, H.; Nunes, M.; Vasconcelos, P.F.; Weaver, S.C.; Shope, R.E.; Watts, D.M.; Tesh, R.B.; Barrett, A.D. Nucleotide sequences and phylogeny of the nucleocapsid gene of Oropouche virus. J. Gen. Virol. 2000, 81, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.M. Orthobunyaviruses: Recent genetic and structural insights. Nat. Rev. Microbiol. 2014, 12, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Li, P.; Elliott, R.M.; Dong, C. Structure of Schmallenberg orthobunyavirus nucleoprotein suggests a novel mechanism of genome encapsidation. J. Virol. 2013, 87, 5593–5601. [Google Scholar] [CrossRef] [PubMed]
- Dutuze, M.F.; Nzayirambaho, M.; Mores, C.N.; Christofferson, R.C. A Review of Bunyamwera, Batai, and Ngari Viruses: Understudied Orthobunyaviruses With Potential One Health Implications. Front. Vet. Sci. 2018, 5, 69. [Google Scholar] [CrossRef]
- Manock, S.R.; Jacobsen, K.H.; de Bravo, N.B.; Russell, K.L.; Negrete, M.; Olson, J.G.; Sanchez, J.L.; Blair, P.J.; Smalligan, R.D.; Quist, B.K.; et al. Etiology of acute undifferentiated febrile illness in the Amazon basin of Ecuador. Am. J. Trop. Med. Hyg. 2009, 81, 146–151. [Google Scholar] [CrossRef]
- LeDuc, J.W.; Hoch, A.L.; Pinheiro, F.P.; da Rosa, A.P. Epidemic Oropouche virus disease in northern Brazil. Bull. Pan Am. Health Organ. 1981, 15, 97–103. [Google Scholar]
- Pinheiro, F.P.; Travassos da Rosa, A.P.; Travassos da Rosa, J.F.; Bensabath, G. An outbreak of Oropouche virus diease in the vicinity of santarem, para, barzil. Tropenmed. Parasitol. 1976, 27, 213–223. [Google Scholar]
- Watts, D.M.; Lavera, V.; Callahan, J.; Rossi, C.; Oberste, M.S.; Roehrig, J.T.; Cropp, C.B.; Karabatsos, N.; Smith, J.F.; Gubler, D.J.; et al. Venezuelan equine encephalitis and Oropouche virus infections among Peruvian army troops in the Amazon region of Peru. Am. J. Trop. Med. Hyg. 1997, 56, 661–667. [Google Scholar] [CrossRef]
- Tilston-Lunel, N.L.; Acrani, G.O.; Randall, R.E.; Elliott, R.M. Generation of Recombinant Oropouche Viruses Lacking the Nonstructural Protein NSm or NSs. J. Virol. 2015, 90, 2616–2627. [Google Scholar] [CrossRef]
- Gutierrez, B.; Wise, E.L.; Pullan, S.T.; Logue, C.H.; Bowden, T.A.; Escalera-Zamudio, M.; Trueba, G.; Nunes, M.R.T.; Faria, N.R.; Pybus, O.G. Evolutionary Dynamics of Oropouche Virus in South America. J. Virol. 2020, 94, 5. [Google Scholar] [CrossRef]
- Naveca, F.G.; Nascimento, V.A.D.; Souza, V.C.; Nunes, B.T.D.; Rodrigues, D.S.G.; Vasconcelos, P. Multiplexed reverse transcription real-time polymerase chain reaction for simultaneous detection of Mayaro, Oropouche, and Oropouche-like viruses. Mem Inst. Oswaldo Cruz 2017, 112, 510–513. [Google Scholar] [CrossRef]
- Proenca-Modena, J.L.; Sesti-Costa, R.; Pinto, A.K.; Richner, J.M.; Lazear, H.M.; Lucas, T.; Hyde, J.L.; Diamond, M.S. Oropouche virus infection and pathogenesis are restricted by MAVS, IRF-3, IRF-7, and type I interferon signaling pathways in nonmyeloid cells. J. Virol. 2015, 89, 4720–4737. [Google Scholar] [CrossRef]
- Sakkas, H.; Bozidis, P.; Franks, A.; Papadopoulou, C. Oropouche Fever: A Review. Viruses 2018, 10, 175. [Google Scholar] [CrossRef]
- Vernal, S.; Martini, C.C.R.; da Fonseca, B.A.L. Oropouche Virus-Associated Aseptic Meningoencephalitis, Southeastern Brazil. Emerg. Infect. Dis. 2019, 25, 380–382. [Google Scholar] [CrossRef]
- Travassos da Rosa, J.F.; de Souza, W.M.; Pinheiro, F.P.; Figueiredo, M.L.; Cardoso, J.F.; Acrani, G.O.; Nunes, M.R.T. Oropouche Virus: Clinical, Epidemiological, and Molecular Aspects of a Neglected Orthobunyavirus. Am. J. Trop. Med. Hyg. 2017, 96, 1019–1030. [Google Scholar] [CrossRef]
- Weaver, S.C. Urbanization and geographic expansion of zoonotic arboviral diseases: Mechanisms and potential strategies for prevention. Trends Microbiol. 2013, 21, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Karesh, W.B.; Dobson, A.; Lloyd-Smith, J.O.; Lubroth, J.; Dixon, M.A.; Bennett, M.; Aldrich, S.; Harrington, T.; Formenty, P.; Loh, E.H.; et al. Ecology of zoonoses: Natural and unnatural histories. Lancet 2012, 380, 1936–1945. [Google Scholar] [CrossRef]
- Pinheiro, F.P.; Travassos da Rosa, A.P.; Gomes, M.L.; LeDuc, J.W.; Hoch, A.L. Transmission of Oropouche virus from man to hamster by the midge Culicoides paraensis. Science 1982, 215, 1251–1253. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, F.P.; Hoch, A.L.; Gomes, M.L.; Roberts, D.R. Oropouche virus. IV. Laboratory transmission by Culicoides paraensis. Am. J. Trop. Med. Hyg. 1981, 30, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, E.; Cabrera, O.L.; Zipa, Y.; Ferro, C.; Ahumada, M.L.; Pardo, R.H. Preliminary evaluation of the Culicoides biting nuisance (Diptera: Ceratopogonidae) in the province of Boyaca, Colombia. Biomedica 2008, 28, 497–509. [Google Scholar] [PubMed]
- Hoch, A.L.; Pinheiro, F.P.; Roberts, D.R.; Gomes, M.L. Laboratory transmission of Oropouche virus by Culex Quinquefasciatus Say. Bull. Pan Am. Health Organ. 1987, 21, 55–61. [Google Scholar]
- Plnheiro, F.; Plnheiro, M.; Bensabath, G.; Causey, O.R.; Shope, R. Epidemic of Oropouche VIrus in Belém. Rev. Serv. Espec. Saude Publica 1962, 12, 15–23. [Google Scholar]
- Figueiredo, L.T. Emergent arboviruses in Brazil. Rev. Soc. Bras. Med. Trop. 2007, 40, 224–229. [Google Scholar] [CrossRef]
- Martins-Luna, J.; Del Valle-Mendoza, J.; Silva-Caso, W.; Sandoval, I.; Del Valle, L.J.; Palomares-Reyes, C.; Carrillo-Ng, H.; Pena-Tuesta, I.; Aguilar-Luis, M.A. Oropouche infection a neglected arbovirus in patients with acute febrile illness from the Peruvian coast. BMC Res. Notes 2020, 13, 67. [Google Scholar] [CrossRef] [PubMed]
- Romero-Alvarez, D.; Escobar, L.E. Oropouche fever, an emergent disease from the Americas. Microbes Infect. Inst. Pasteur 2018, 20, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Mattar, S. Oropuche virus: A virus present but ignored. Revista MVZ Cordoba 2015, 30, 3. [Google Scholar] [CrossRef]
- QGIS.org, QGIS Geographic Information System, Q. Association, 2021. Available online: https://qgis.org/en/site/ (accessed on 30 June 2021).
- Dixon, K.E.; Travassos da Rosa, A.P.; Travassos da Rosa, J.F.; Llewellyn, C.H. Oropouche virus. II. Epidemiological observations during an epidemic in Santarem, Para, Brazil in 1975. Am. J. Trop. Med. Hyg. 1981, 30, 161–164. [Google Scholar] [CrossRef]
- Tavares-Neto, J.; Freitas-Carvalho, J.; Nunes, M.R.; Rocha, G.; Rodrigues, S.G.; Damasceno, E.; Darub, R.; Viana, S.; Vasconcelos, P.F. Serologic survey for yellow fever and other arboviruses among inhabitants of Rio Branco, Brazil, before and three months after receiving the yellow fever 17D vaccine. Rev. Soc. Bras. Med. Trop. 2004, 37, 1–6. [Google Scholar] [CrossRef] [PubMed]
- De Figueiredo, R.M.; Thatcher, B.D.; de Lima, M.L.; Almeida, T.C.; Alecrim, W.D.; Guerra, M.V. Exanthematous diseases and the first epidemic of dengue to occur in Manaus, Amazonas State, Brazil, during 1998–1999. Rev. Soc. Bras. Med. Trop. 2004, 37, 476–479. [Google Scholar] [CrossRef]
- Azevedo, R.S.; Nunes, M.R.; Chiang, J.O.; Bensabath, G.; Vasconcelos, H.B.; Pinto, A.Y.; Martins, L.C.; Monteiro, H.A.; Rodrigues, S.G.; Vasconcelos, P.F. Reemergence of Oropouche fever, northern Brazil. Emerg. Infect. Dis. 2007, 13, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Bernardes-Terzian, A.C.; de-Moraes-Bronzoni, R.V.; Drumond, B.P.; Da Silva-Nunes, M.; da-Silva, N.S.; Urbano-Ferreira, M.; Speranca, M.A.; Nogueira, M.L. Sporadic oropouche virus infection, acre, Brazil. Emerg. Infect. Dis. 2009, 15, 348–350. [Google Scholar] [CrossRef]
- Terzian, A.C.; Mondini, A.; Bronzoni, R.V.; Drumond, B.P.; Ferro, B.P.; Cabrera, E.M.; Figueiredo, L.T.; Chiaravalloti-Neto, F.; Nogueira, M.L. Detection of Saint Louis encephalitis virus in dengue-suspected cases during a dengue 3 outbreak. Vector Borne Zoonotic Dis. 2011, 11, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Bastos Mde, S.; Figueiredo, L.T.; Naveca, F.G.; Monte, R.L.; Lessa, N.; Pinto de Figueiredo, R.M.; Gimaque, J.B.; Pivoto Joao, G.; Ramasawmy, R.; Mourao, M.P. Identification of Oropouche Orthobunyavirus in the cerebrospinal fluid of three patients in the Amazonas, Brazil. Am. J. Trop. Med. Hyg. 2012, 86, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Batista, P.M.; Andreotti, R.; Chiang, J.O.; Ferreira, M.S.; Vasconcelos, P.F. Seroepidemiological monitoring in sentinel animals and vectors as part of arbovirus surveillance in the state of Mato Grosso do Sul, Brazil. Rev. Soc. Bras. Med. Trop. 2012, 45, 168–173. [Google Scholar] [CrossRef]
- Batista, P.M.; Andreotti, R.; Almeida, P.S.; Marques, A.C.; Rodrigues, S.G.; Chiang, J.O.; Vasconcelos, P.F. Detection of arboviruses of public health interest in free-living New World primates (Sapajus spp.; Alouatta caraya) captured in Mato Grosso do Sul, Brazil. Rev. Soc. Bras. Med. Trop. 2013, 46, 684–690. [Google Scholar] [CrossRef]
- Bastos, M.S.; Lessa, N.; Naveca, F.G.; Monte, R.L.; Braga, W.S.; Figueiredo, L.T.; Ramasawmy, R.; Mourao, M.P. Detection of Herpesvirus, Enterovirus, and Arbovirus infection in patients with suspected central nervous system viral infection in the Western Brazilian Amazon. J. Med. Virol. 2014, 86, 1522–1527. [Google Scholar] [CrossRef]
- Martins Vdo, C.; Bastos Mde, S.; Ramasawmy, R.; de Figueiredo, R.P.; Gimaque, J.B.; Braga, W.S.; Nogueira, M.L.; Nozawa, S.; Naveca, F.G.; Figueiredo, L.T.; et al. Clinical and virological descriptive study in the 2011 outbreak of dengue in the Amazonas, Brazil. PLoS ONE 2014, 9, e100535. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, B.F.; Serra, O.P.; Heinen, L.B.; Zuchi, N.; Souza, V.C.; Naveca, F.G.; Santos, M.A.; Slhessarenko, R.D. Detection of Oropouche virus segment S in patients and inCulex quinquefasciatus in the state of Mato Grosso, Brazil. Mem Inst. Oswaldo Cruz 2015, 110, 745–754. [Google Scholar] [CrossRef]
- Gibrail, M.M.; Fiaccadori, F.S.; Souza, M.; Almeida, T.N.; Chiang, J.O.; Martins, L.C.; Ferreira, M.S.; Cardoso, D. Detection of antibodies to Oropouche virus in non-human primates in Goiania City, Goias. Rev. Soc. Bras. Med. Trop. 2016, 49, 357–360. [Google Scholar] [CrossRef] [PubMed]
- de Souza Luna, L.K.; Rodrigues, A.H.; Santos, R.I.; Sesti-Costa, R.; Criado, M.F.; Martins, R.B.; Silva, M.L.; Delcaro, L.S.; Proenca-Modena, J.L.; Figueiredo, L.T.; et al. Oropouche virus is detected in peripheral blood leukocytes from patients. J. Med. Virol. 2017, 89, 1108–1111. [Google Scholar] [CrossRef]
- da Costa, V.G.; de Rezende Feres, V.C.; Saivish, M.V.; de Lima Gimaque, J.B.; Moreli, M.L. Silent emergence of Mayaro and Oropouche viruses in humans in Central Brazil. Int. J. Infect. Dis. 2017, 62, 84–85. [Google Scholar] [CrossRef] [PubMed]
- Naveca, F.G.; Nascimento, V.A.; Souza, V.C.; de Figueiredo, R.M.P. Human Orthobunyavirus Infections, Tefe, Amazonas, Brazil. PLoS Curr. 2018, 10. [Google Scholar] [CrossRef]
- Fonseca, L.; Carvalho, R.H.; Bandeira, A.C.; Sardi, S.I.; Campos, G.S. Oropouche Virus Detection in Febrile Patients’ Saliva and Urine Samples in Salvador, Bahia, Brazil. Jpn. J. Infect. Dis. 2020, 73, 164–165. [Google Scholar] [CrossRef]
- Nascimento, V.A.D.; Santos, J.H.A.; Monteiro, D.; Pessoa, K.P.; Cardoso, A.J.L.; Souza, V.C.; Abdalla, L.F.; Naveca, F.G. Oropouche virus detection in saliva and urine. Mem Inst. Oswaldo Cruz 2020, 115, e190338. [Google Scholar] [CrossRef]
- Vasconcelos, P.F.; Travassos Da Rosa, J.F.; Guerreiro, S.C.; Degallier, N.; Travassos Da Rosa, E.S.; Travassos Da Rosa, A.P. 1st register of an epidemic caused by Oropouche virus in the states of Maranhao and Goias, Brazil. Rev. Inst. Med. Trop. Sao Paulo 1989, 31, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.P.; Rodrigues, S.G.; Nunes, M.R.; Magalhaes, M.T.; Rosa, J.F.; Vasconcelos, P.F. Outbreak of oropouche virus fever in Serra Pelada, municipality of Curionopolis, Para, 1994. Rev. Soc. Bras. Med. Trop. 1996, 29, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.C.; Prazeres Ado, S.; Gama, E.C.; Lima, M.F.; Azevedo Rdo, S.; Casseb, L.M.; Nunes Neto, J.P.; Martins, L.C.; Chiang, J.O.; Rodrigues, S.G.; et al. Serological survey for arboviruses in Juruti, Para State, Brazil. Cad. Saude Publica 2009, 25, 2517–2523. [Google Scholar] [CrossRef] [PubMed]
- Santana Vdos, S.; Lavezzo, L.C.; Mondini, A.; Terzian, A.C.; Bronzoni, R.V.; Rossit, A.R.; Machado, R.L.; Rahal, P.; Nogueira, M.C.; Nogueira, M.L. Concurrent Dengue and malaria in the Amazon region. Rev. Soc. Bras. Med. Trop. 2010, 43, 508–511. [Google Scholar] [CrossRef]
- Nunes, M.R.; Martins, L.C.; Rodrigues, S.G.; Chiang, J.O.; Azevedo Rdo, S.; da Rosa, A.P.; Vasconcelos, P.F. Oropouche virus isolation, southeast Brazil. Emerg. Infect. Dis. 2005, 11, 1610–1613. [Google Scholar] [CrossRef]
- Moreli, M.L.; Aquino, V.H.; Cruz, A.C.; Figueiredo, L.T. Diagnosis of Oropouche virus infection by RT-nested-PCR. J. Med. Virol. 2002, 66, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Lavezzo, L.C.; Dos Santos Santana, V.; Terzian, A.C.; Mondini, A.; de Moraes Bronzoni, R.V.; Speranca, M.; Dias, S.M.; Rossit, A.R.; Machado, R.L.; Nogueira, M.L. Arboviruses in blood donors: A study in the Amazon region and in a small city with a dengue outbreak. Transfus Med. 2010, 20, 278–279. [Google Scholar] [CrossRef]
- Silva-Nunes, M.; Malafronte Rdos, S.; Luz Bde, A.; Souza, E.A.; Martins, L.C.; Rodrigues, S.G.; Chiang, J.O.; Vasconcelos, P.F.; Muniz, P.T.; Ferreira, M.U. The Acre Project: The epidemiology of malaria and arthropod-borne virus infections in a rural Amazonian population. Cad. Saúde Pública 2006, 22, 1325–1334. [Google Scholar] [CrossRef]
- Brito, M.T.F.M.D.; Aarão, T.L.D.S.; Pinto, D.D.S. Seroepidemiology of arbovirus in communities living under the influence of the lake of a hydroelectric dam in Brazil. Cad. Saúde Coletiva 2018, 26, 1–6. [Google Scholar] [CrossRef]
- Catenacci, L.S.; Ferreira, M.; Martins, L.C.; De Vleeschouwer, K.M.; Cassano, C.R.; Oliveira, L.C.; Canale, G.; Deem, S.L.; Tello, J.S.; Parker, P.; et al. Surveillance of Arboviruses in Primates and Sloths in the Atlantic Forest, Bahia, Brazil. Ecohealth 2018, 15, 777–791. [Google Scholar] [CrossRef]
- de Souza Costa, M.C.; Siqueira Maia, L.M.; Costa de Souza, V.; Gonzaga, A.M.; Correa de Azevedo, V.; Ramos Martins, L.; Chavez Pavoni, J.H.; Gomes Naveca, F.; Dezengrini Slhessarenko, R. Arbovirus investigation in patients from Mato Grosso during Zika and Chikungunya virus introdution in Brazil, 2015–2016. Acta Trop. 2019, 190, 395–402. [Google Scholar] [CrossRef]
- Almeida, M.A.B.; Santos, E.D.; Cardoso, J.D.C.; Noll, C.A.; Lima, M.M.; Silva, F.A.E.; Ferreira, M.S.; Martins, L.C.; Vasconcelos, P.; Bicca-Marques, J.C. Detection of antibodies against Icoaraci, Ilheus, and Saint Louis Encephalitis arboviruses during yellow fever monitoring surveillance in non-human primates (Alouatta caraya) in southern Brazil. J. Med. Primatol. 2019, 48, 211–217. [Google Scholar] [CrossRef]
- Watts, D.M.; Phillips, I.; Callahan, J.D.; Griebenow, W.; Hyams, K.C.; Hayes, C.G. Oropouche virus transmission in the Amazon River basin of Peru. Am. J. Trop. Med. Hyg. 1997, 56, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Baisley, K.J.; Watts, D.M.; Munstermann, L.E.; Wilson, M.L. Epidemiology of endemic Oropouche virus transmission in upper Amazonian Peru. Am. J. Trop. Med. Hyg. 1998, 59, 710–716. [Google Scholar] [CrossRef]
- Forshey, B.M.; Guevara, C.; Laguna-Torres, V.A.; Cespedes, M.; Vargas, J.; Gianella, A.; Vallejo, E.; Madrid, C.; Aguayo, N.; Gotuzzo, E.; et al. Arboviral etiologies of acute febrile illnesses in Western South America, 2000–2007. PLoS Negl. Trop. Dis. 2010, 4, e787. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, P.V.; Barrett, A.D.; Saeed, M.F.; Watts, D.M.; Russell, K.; Guevara, C.; Ampuero, J.S.; Suarez, L.; Cespedes, M.; Montgomery, J.M.; et al. Iquitos virus: A novel reassortant Orthobunyavirus associated with human illness in Peru. PLoS Negl. Trop. Dis. 2011, 5, e1315. [Google Scholar] [CrossRef] [PubMed]
- Alva-Urcia, C.; Aguilar-Luis, M.A.; Palomares-Reyes, C.; Silva-Caso, W.; Suarez-Ognio, L.; Weilg, P.; Manrique, C.; Vasquez-Achaya, F.; Del Valle, L.J.; Del Valle-Mendoza, J. Emerging and reemerging arboviruses: A new threat in Eastern Peru. PLoS ONE 2017, 12, e0187897. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.G.; Del Valle Mendoza, J.; Sadeghi, M.; Altan, E.; Deng, X.; Delwart, E. Sera of Peruvians with fever of unknown origins include viral nucleic acids from non-vertebrate hosts. Virus Genes 2018, 54, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.P.; Merino, N.S.; Figueroa, D.; Marcelo, A.; Tineo, V.E.; Manrique, C.; Donaires, F.; Cespedes, M.; Cabrera, R.; Cabezas, C. Detection of Oropouche viral circulation in Madre de Dios region, Peru (December 2015 to January 2016). Rev. Peru Med. Exp. Salud Publica 2016, 33, 380–381. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Turell, M.J.; Gozalo, A.S.; Guevara, C.; Schoeler, G.B.; Carbajal, F.; Lopez-Sifuentes, V.M.; Watts, D.M. Lack of Evidence of Sylvatic Transmission of Dengue Viruses in the Amazon Rainforest Near Iquitos, Peru. Vector Borne Zoonotic Dis. 2019, 19, 685–689. [Google Scholar] [CrossRef]
- Wise, E.L.; Pullan, S.T.; Marquez, S.; Paz, V.; Mosquera, J.D.; Zapata, S.; Jackson, S.K.; Fejer, G.; Trueba, G.; Logue, C.H. Isolation of Oropouche Virus from Febrile Patient, Ecuador. Emerg. Infect. Dis. 2018, 24, 935–937. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Stittleburg, V.; Cardozo, F.; Bopp, N.; Cantero, C.; Lopez, S.; Bernal, C.; Mendoza, L.; Aguilar, P.; Pinsky, B.A.; et al. Real-time RT-PCR for the detection and quantitation of Oropouche virus. Diagn. Microbiol. Infect. Dis. 2020, 96, 114894. [Google Scholar] [CrossRef]
- van Tongeren, H.A. Occurance of arboviruses belonging to the C, Bunyamwera and Guama groups, and of Oropouche, Junin, Tacaiuma and Kwatta viruses in man in the province of Brokopondo, Surinam. Trop. Geogr. Med. 1967, 19, 309–325. [Google Scholar] [PubMed]
- Medlin, S.; Deardorff, E.R.; Hanley, C.S.; Vergneau-Grosset, C.; Siudak-Campfield, A.; Dallwig, R.; da Rosa, A.T.; Tesh, R.B.; Martin, M.P.; Weaver, S.C.; et al. Serosurvey of Selected Arboviral Pathogens in Free-Ranging, Two-Toed Sloths (Choloepus Hoffmanni) and Three-Toed Sloths (Bradypus Variegatus) in Costa Rica, 2005–2007. J. Wildl. Dis. 2016, 52, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Felippe-Bauer, M.L.; Sternheim, U.S. Culicoides paraensis (Diptera: Ceratopogonidae) infesta- tions in cities of the Itapocú River Valley, southern Brazil. Entomol. News 2008, 119, 185–192. [Google Scholar] [CrossRef]
- Costa, J.C. Culicoides species (Diptera; Ceratopogonidae) and potential hosts in ecotourism area of Lencois Maranhensis National Park, Brazil. Pan-Amazon. J. Health 2013, 4, 11–18. [Google Scholar]
- Alencar, J.; Pacheco, J.B.; Correa, F.F.; Silva Jdos, S.; Guimaraes, A.E. New report on the bionomics of Coquillettidia venezuelensis in temporary breeding sites (Diptera: Culicidae). Rev. Soc. Bras. Med. Trop. 2011, 44, 247–248. [Google Scholar] [CrossRef]
- Pauvolid-Correa, A.; Tavares, F.N.; Alencar, J.; Silva Jdos, S.; Murta, M.; Serra-Freire, N.M.; Pellegrin, A.O.; Gil-Santana, H.; Guimaraes, A.E.; Silva, E.E. Preliminary investigation of Culicidae species in South Pantanal, Brazil and their potential importance in arbovirus transmission. Rev. Inst. Med. Trop. Sao Paulo 2010, 52, 17–24. [Google Scholar] [CrossRef][Green Version]
- Santos, C.F.; Silva, A.C.; Rodrigues, R.A.; de Jesus, J.S.; Borges, M.A. Inventory of Mosquitoes (Diptera: Culicidae) in Conservation Units in Brazilian Tropical Dry Forests. Rev. Inst. Med. Trop. Sao Paulo 2015, 57, 227–232. [Google Scholar] [CrossRef]
- Hutchings, R.S.; Hutchings, R.W.; Menezes, I.S.; Motta, M.A.; Sallum, M.A. Mosquitoes (Diptera: Culicidae) From the Northwestern Brazilian Amazon: Padauari River. J. Med. Entomol. 2016, 53, 1330–1347. [Google Scholar] [CrossRef]
- Carvalho, G.C.D. Composition and diversity of mosquitoes (Diptera: Culicidae) in urban parks in the South region of the city of Sao Paulo, Brazil. Biota Neotrop. 2017, 17, 2. [Google Scholar] [CrossRef]
- Vieira, C.; Thies, S.F.; da Silva, D.J.F.; Kubiszeski, J.R.; Barreto, E.S.; Monteiro, H.A.O.; Mondini, A.; Sao Bernardo, C.S.; Bronzoni, R.V.M. Ecological aspects of potential arbovirus vectors (Diptera: Culicidae) in an urban landscape of Southern Amazon, Brazil. Acta Trop. 2020, 202, 105276. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, L.S.; Nunes-Neto, J.; Deem, S.L.; Palmer, J.L.; Travassos-da Rosa, E.S.; Tello, J.S. Diversity patterns of hematophagous insects in Atlantic forest fragments and human-modified areas of southern Bahia, Brazil. J. Vector Ecol. 2018, 43, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Paula MB, D.; Fernandes, A.; Medeiros-Sousa, A.R.; Ceretti-Júnior, W.; Christe, R.; Stroebel, R.C. Mosquito (Diptera: Culicidae) fauna in parks in greater São Paulo, Brazil. Biota Neotrop. 2015, 15, e20140026. [Google Scholar] [CrossRef]
- Carvalho, L.P.; Pereira Junior, A.M.; Farias, E.S.; Almeida, J.F.; Rodrigues, M.S.; Resadore, F.; Pessoa, F.A.; Medeiros, J.F. A study of Culicoides in Rondonia, in the Brazilian Amazon: Species composition, relative abundance and potential vectors. Med. Vet. Entomol. 2017, 31, 117–122. [Google Scholar] [CrossRef]
- Felippe-Bauer, M.L.; Gonzaga, G.P.; Cavalcante, R.C.; Gomes, R.G.; Silva, R.A. Culicoides (Diptera: Ceratopogonidae) from Ceará State, northeastern Brazil: Diversity, new records and bionomic approaches. Cuad. Investig. UNED 2018, 11, 137–144. [Google Scholar] [CrossRef]
- Cardoso Jda, C.; Paula, M.B.; Fernandes, A.; Santos, E.D.; Almeida, M.A.; Fonseca, D.F.; Sallum, M.A. New records and epidemiological potential of certain species of mosquito (Diptera, Culicidae) in the State of Rio Grande do Sul, Brazil. Rev. Soc. Bras. Med. Trop. 2010, 43, 552–556. [Google Scholar] [CrossRef]
- Roberts, D.R.; Hoch, A.L.; Dixon, K.E.; Llewellyn, C.H. Oropouche virus. III. Entomological observations from three epidemics in Para, Brazil, 1975. Am. J. Trop. Med. Hyg. 1981, 30, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Sherlock, I.A.; Guitton, N. Dermatozoonosis by Culicoides’ bite (Diptera, Ceratopogonidae) in Salvador, State of Bahia, Brazil. I. Entomoligical survey. Mem. Inst. Oswaldo Cruz 1964, 62, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Santarém, M.C.A.; Confalonieri, U.E.C.; Felippe-Bauer, M.L. Diversity of Culicoides (Diptera: Ceratopogonidae) in the National Forest of Caxiuanã, Melgaço, Pará State, Brazil. Rev. Pan-Amaz. Saúde 2010, 1, 29–33. [Google Scholar] [CrossRef]
- Farias, E.d.S.; Almeida, J.F.; Pessoa, F.A.C. List of Culicoides biting midges (Diptera: Ceratopogonidae) from the state of Amazonas, Brazil, including new records. Check List 2016, 12, 6. [Google Scholar] [CrossRef]
- Carvalho, L.P.; Silva, F.S. Seasonal abundance of livestock-associated Culicoides species in northeastern Brazil. Med. Vet. Entomol. 2014, 28, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, M.C.A.; Da Penha, A.; Moraes, J.L.P.; Brito, G.A.; Rebelo, J.M.M. Infestation of Brazilian Peridomiciliary Areas by Culicoides (Diptera: Ceratopogonidae) in Humid and Semihumid Climates. J. Med. Entomol. 2016, 53, 1163–1168. [Google Scholar] [CrossRef]
- Leal-Santos, F.A. Species composition and fauna distribution of mosquitoes and its importance for vector-borne diseases in a rural area of central western Mato Grosso, Brazil. EntomoBrazilis 2017, 10, 2. [Google Scholar] [CrossRef]
- Bandeira, M.; Brito, G.A.; da Penha, A.; Santos, C.L.C.; Rebelo, J.M.M. The influence of environmental management and animal shelters in vector control of Culicoides (Diptera, Ceratopogonidae) in northeastern Brazil. J. Vector Ecol. 2017, 42, 113–119. [Google Scholar] [CrossRef]
- Andrews, E.S.; Schoeler, G.B.; Gozalo, A.S.; Carbajal, F.; Lopez-Sifuentes, V.; Turell, M.J. Species Diversity, Seasonal, and Spatial Distribution of Mosquitoes (Diptera: Culicidae) Captured in Aotus Monkey-Baited Traps in a Forested Site Near Iquitos, Peru. J. Med. Entomol. 2014, 51, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.W.; Turell, M.J.; Sardelis, M.R.; Watts, D.M.; Coleman, R.E.; Fernandez, R.; Carbajal, F.; Pecor, J.E.; Calampa, C.; Klein, T.A. Seasonal distribution, biology, and human attraction patterns of culicine mosquitoes (Diptera: Culicidae) in a forest near Puerto Almendras, Iquitos, Peru. J. Med. Entomol. 2004, 41, 349–360. [Google Scholar] [CrossRef]
- Mercer, D.R.; Castillo-Pizango, M.J. Changes in relative species compositions of biting midges (Diptera: Ceratopogonidae) and an outbreak of Oropouche virus in Iquitos, Peru. J. Med. Entomol. 2005, 42, 554–558. [Google Scholar] [CrossRef]
- Mercer, D.R.; Spinelli, G.R.; Watts, D.M.; Tesh, R.B. Biting rates and developmental substrates for biting midges (Diptera: Ceratopogonidae) in Iquitos, Peru. J. Med. Entomol. 2003, 40, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Felippe-Bauer, M.L.; Cáceres, A.; da Silva, C.S.; Valderrama-Bazan, W.; Gonzales-Perez, A.; Costa, J.M. New records of Culicoides Latreille (Diptera: Ceratopogonidae) from Peruvian Amazonian region. Biota Neotrop. 2008, 8, 33–38. [Google Scholar] [CrossRef][Green Version]
- Turell, M.J.; O’Guinn, M.L.; Jones, J.W.; Sardelis, M.R.; Dohm, D.J.; Watts, D.M.; Fernandez, R.; Travassos da Rosa, A.; Guzman, H.; Tesh, R.; et al. Isolation of viruses from mosquitoes (Diptera: Culicidae) collected in the Amazon Basin region of Peru. J. Med. Entomol. 2005, 42, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.; Zalazar, L.; Willener, J.A.; Almeida, F.L.; Almiron, W.R. Culicidae (Diptera) selection of humans, chickens and rabbits in three different environments in the province of Chaco, Argentina. Mem. Inst. Oswaldo Cruz 2013, 108, 563–571. [Google Scholar] [CrossRef]
- Aybar, V. Spatial and Temporal Distribution of Culicoides insignis and Culicoides paraensis in the Subtropical Mountain Forest of Tucuman, Northwestern Argentina. Fla. Entomol. 2011, 94, 1018–1025. [Google Scholar] [CrossRef]
- Ronderos, M.M.; Greco, N.M.; Spinelli, G.R. Diversity of biting midges of the genus Culicoides Latreille (Diptera: Ceratopogonidae) in the area of the Yacyreta Dam Lake between Argentina and Paraguay. Mem. Inst. Oswaldo Cruz 2003, 98, 19–24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shelley, A.J.; Coscaron, S. Simuliid blackflies (Diptera: Simuliidae) and ceratopogonid midges (Diptera: Ceratopogonidae) as vectors of Mansonella ozzardi (Nematoda: Onchocercidae) in northern Argentina. Mem. Inst. Oswaldo Cruz 2001, 96, 451–458. [Google Scholar] [CrossRef]
- Huerta, H. New records of biting midges of the genus Culicoides Latreille from Mexico (Diptera: Ceratopogonidae). Insecta Mundi 2012, 1–20. [Google Scholar]
- Hudson, J.E. Annotated list of the Ceratopogonidae (Diptera) of Suriname. Ent. Méd. Parasitol. 1986, 24, 293–301. [Google Scholar]
- Mayton, E.H.; Tramonte, A.R.; Wearing, H.J.; Christofferson, R.C. Age-structured vectorial capacity reveals timing, not magnitude of within-mosquito dynamics is critical for arbovirus fitness assessment. Parasit Vectors 2020, 13, 310. [Google Scholar] [CrossRef]
- Liang, G.; Gao, X.; Gould, E.A. Factors responsible for the emergence of arboviruses; strategies, challenges and limitations for their control. Emerg. Microbes Infect. 2015, 4, e18. [Google Scholar] [CrossRef]
- Huang, Y.S.; Higgs, S.; Vanlandingham, D.L. Arbovirus-Mosquito Vector-Host Interactions and the Impact on Transmission and Disease Pathogenesis of Arboviruses. Front. Microbiol. 2019, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Lambin, E.F.; Tran, A.; Vanwambeke, S.O.; Linard, C.; Soti, V. Pathogenic landscapes: Interactions between land, people, disease vectors, and their animal hosts. Int. J. Health Geogr. 2010, 9, 54. [Google Scholar] [CrossRef]
- Purse, B.V.; Carpenter, S.; Venter, G.J.; Bellis, G.; Mullens, B.A. Bionomics of temperate and tropical Culicoides midges: Knowledge gaps and consequences for transmission of Culicoides-borne viruses. Annu. Rev. Entomol. 2015, 60, 373–392. [Google Scholar] [CrossRef] [PubMed]
- Aide, T.M.; Grau, H.R. Ecology. Globalization, migration, and Latin American ecosystems. Science 2004, 305, 1915–1916. [Google Scholar] [CrossRef] [PubMed]
- Laurance, W.F.; Sayer, J.; Cassman, K.G. Agricultural expansion and its impacts on tropical nature. Trends Ecol. Evol. 2014, 29, 107–116. [Google Scholar] [CrossRef]
- Drewry, J.; Shandro, J.; Winkler, M.S. The extractive industry in Latin America and the Caribbean: Health impact assessment as an opportunity for the health authority. Int. J. Public Health 2017, 62, 253–262. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walsh, C.E.S.; Robert, M.A.; Christofferson, R.C. Observational Characterization of the Ecological and Environmental Features Associated with the Presence of Oropouche Virus and the Primary Vector Culicoides paraensis: Data Synthesis and Systematic Review. Trop. Med. Infect. Dis. 2021, 6, 143. https://doi.org/10.3390/tropicalmed6030143
Walsh CES, Robert MA, Christofferson RC. Observational Characterization of the Ecological and Environmental Features Associated with the Presence of Oropouche Virus and the Primary Vector Culicoides paraensis: Data Synthesis and Systematic Review. Tropical Medicine and Infectious Disease. 2021; 6(3):143. https://doi.org/10.3390/tropicalmed6030143
Chicago/Turabian StyleWalsh, Christine E. S., Michael A. Robert, and Rebecca C. Christofferson. 2021. "Observational Characterization of the Ecological and Environmental Features Associated with the Presence of Oropouche Virus and the Primary Vector Culicoides paraensis: Data Synthesis and Systematic Review" Tropical Medicine and Infectious Disease 6, no. 3: 143. https://doi.org/10.3390/tropicalmed6030143
APA StyleWalsh, C. E. S., Robert, M. A., & Christofferson, R. C. (2021). Observational Characterization of the Ecological and Environmental Features Associated with the Presence of Oropouche Virus and the Primary Vector Culicoides paraensis: Data Synthesis and Systematic Review. Tropical Medicine and Infectious Disease, 6(3), 143. https://doi.org/10.3390/tropicalmed6030143





