Causes of Phenotypic Variability and Disabilities after Prenatal Viral Infections
Abstract
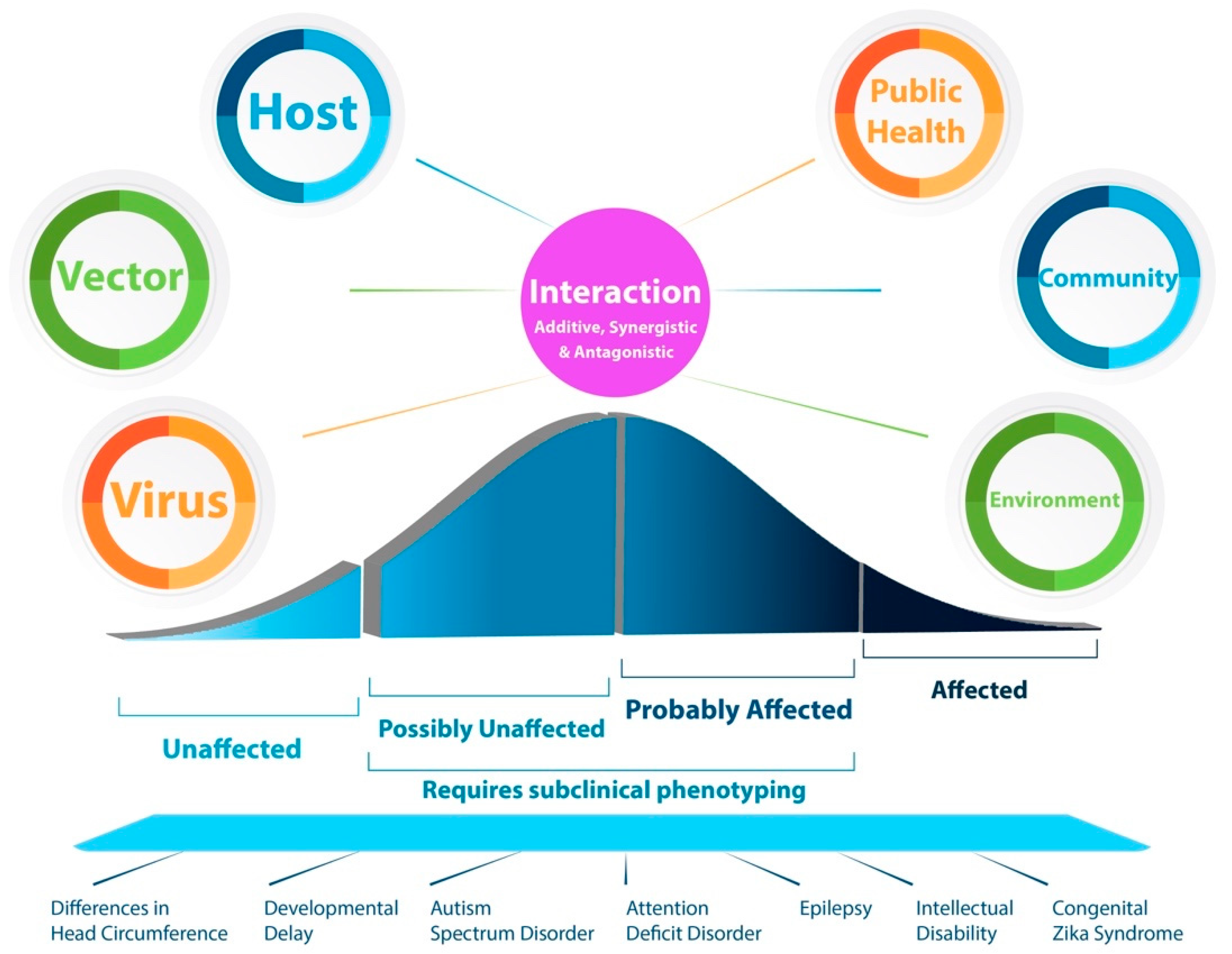
1. Introduction
2. Zika–Mosquito–Host Cycle
3. Virus
4. Viral Evolution Likely Contributed to Pathogenicity
5. Mosquito
6. Host
6.1. Gestational Age Confers Susceptible to Zika Pathogenesis
6.2. Phenotypic Variation: From Congenital Zika Syndrome to Mild, and Subclinical Developmental Delay
6.3. Host Genetic Modifiers after Prenatal Zika Infection
6.4. Differences in Head Circumference after Prenatal Zika Infection Offers a Unique Opportunity to Study a Variable Clinical and Quantitative Phenotype with Impact on Human Development
7. Public Health, Community, and Environmental Factors
7.1. Public Health and Medical Considerations
7.2. Impacts on Transmission
7.3. Interventions to Combat Vector Density
7.4. Socioeconomic Factors as Drivers and Modifiers of Clinical Outcomes
7.5. Global Climate Change
8. A Conclusion and Perspective toward Understanding Phenotypic Variability after Prenatal Viral Infections
Author Contributions
Funding
Conflicts of Interest
References
- Khan, A.M.; Morris, S.K.; Bhutta, Z.A. Neonatal and Perinatal Infections. Pediatr. Clin. N. Am. 2017, 64, 785–798. [Google Scholar] [CrossRef]
- Wu, Y.W.; Escobar, G.J.; Grether, J.K.; Croen, L.A.; Greene, J.D.; Newman, T.B. Chorioamnionitis and cerebral palsy in term and near-term infants. JAMA 2003, 290, 2677–2684. [Google Scholar] [CrossRef] [PubMed]
- Lynch, L.; Ghidini, A. Perinatal infections. Curr. Opin. Obstet. Gynecol. 1993, 5, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.D.; Tan, S. Perinatal infections, prematurity and brain injury. Curr. Opin. Pediatr. 2006, 18, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Rudbeck Roge, H.; Henriques, U. Fetal and perinatal infections. A consecutive study. Pathol. Res. Pract. 1992, 188, 135–140. [Google Scholar] [CrossRef]
- Ludlow, M.; Kortekaas, J.; Herden, C.; Hoffmann, B.; Tappe, D.; Trebst, C.; Griffin, D.E.; Brindle, H.E.; Solomon, T.; Brown, A.S.; et al. Neurotropic virus infections as the cause of immediate and delayed neuropathology. Acta Neuropathol. 2016, 131, 159–184. [Google Scholar] [CrossRef]
- Brasil, P.; Pereira, J.P., Jr.; Moreira, M.E.; Ribeiro Nogueira, R.M.; Damasceno, L.; Wakimoto, M.; Rabello, R.S.; Valderramos, S.G.; Halai, U.A.; Salles, T.S.; et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N. Engl. J. Med. 2016, 375, 2321–2334. [Google Scholar] [CrossRef] [PubMed]
- Nahmias, A.J.; Walls, K.W.; Stewart, J.A.; Herrmann, K.L.; Flynt, W.J., Jr. The ToRCH complex-perinatal infections associated with toxoplasma and rubella, cytomegol- and herpes simplex viruses. Pediatric Res. 1971, 405–406. [Google Scholar] [CrossRef]
- Ford-Jones, E.L. An approach to the diagnosis of congenital infections. Paediatr. Child. Health 1999, 4, 109–112. [Google Scholar] [CrossRef][Green Version]
- Neu, N.; Duchon, J.; Zachariah, P. TORCH infections. Clin. Perinatol. 2015, 42, 77–103. [Google Scholar] [CrossRef]
- Moore, C.A.; Staples, J.E.; Dobyns, W.B.; Pessoa, A.; Ventura, C.V.; Fonseca, E.B.; Ribeiro, E.M.; Ventura, L.O.; Neto, N.N.; Arena, J.F.; et al. Characterizing the Pattern of Anomalies in Congenital Zika Syndrome for Pediatric Clinicians. JAMA Pediatrics 2017, 171, 288–295. [Google Scholar] [CrossRef]
- Cranston, J.S.; Tiene, S.F.; Nielsen-Saines, K.; Vasconcelos, Z.; Pone, M.V.; Pone, S.; Zin, A.; Salles, T.S.; Pereira, J.P., Jr.; Orofino, D.; et al. Association Between Antenatal Exposure to Zika Virus and Anatomical and Neurodevelopmental Abnormalities in Children. JAMA Netw. Open 2020, 3, e209303. [Google Scholar] [CrossRef]
- Mulkey, S.B.; Arroyave-Wessel, M.; Peyton, C.; Bulas, D.I.; Fourzali, Y.; Jiang, J.; Russo, S.; McCarter, R.; Msall, M.E.; du Plessis, A.J.; et al. Neurodevelopmental Abnormalities in Children With In Utero Zika Virus Exposure Without Congenital Zika Syndrome. JAMA Pediatrics 2020, 174, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Mulkey, S.B. Head Circumference as a Measure of In Utero Zika Virus Exposure and Outcomes. JAMA Netw. Open 2020, 3, e209461. [Google Scholar] [CrossRef]
- Mulkey, S.B.; DeBiasi, R.L. Do Not Judge a Book by Its Cover: Critical Need for Longitudinal Neurodevelopmental Assessment of In Utero Zika-Exposed Children. Am. J. Trop. Med. Hyg. 2020, 102, 913–914. [Google Scholar] [CrossRef]
- Coutinho, C.M.; Neg, S.; Araujo, D.; Teixeira, S.R.; Amaral, F.R.; Moro, M.; Fernandes, J.; da Motta, M.; Negrini, B.; Caldas, C.; et al. Early maternal Zika infection predicts severe neonatal neurological damage: Results from the prospective Natural History of Zika Virus Infection in Gestation cohort study. BJOG 2020. [Google Scholar] [CrossRef]
- Cuevas, E.L.; Tong, V.T.; Rozo, N.; Valencia, D.; Pacheco, O.; Gilboa, S.M.; Mercado, M.; Renquist, C.M.; Gonzalez, M.; Ailes, E.C.; et al. Preliminary Report of Microcephaly Potentially Associated with Zika Virus Infection During Pregnancy—Colombia, January-November 2016. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, O.; Beltran, M.; Nelson, C.A.; Valencia, D.; Tolosa, N.; Farr, S.L.; Padilla, A.V.; Tong, V.T.; Cuevas, E.L.; Espinosa-Bode, A.; et al. Zika Virus Disease in Colombia—Preliminary Report. N. Engl. J. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Villa, P.; Puerto, A.K.; Victoria, S.; Gracia, G.; Guasmayan, L.; Arce, P.; Alvarez, G.; Blandon, E.; Rengifo, N.; Holguin, J.A.; et al. Raised Frequency of Central Nervous System Malformations Related to Zika Virus Infection in Two Birth Defects Surveillance Systems in Bogota and Cali, Colombia. Pediatr. Infect. Dis. J. 2017. [Google Scholar] [CrossRef]
- Jaenisch, T.; Rosenberger, K.D.; Brito, C.; Brady, O.; Brasil, P.; Marques, E.T. Risk of microcephaly after Zika virus infection in Brazil, 2015 to 2016. Bull. World Health Organ. 2017, 95, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Adachi, K.; Romero, T.; Nielsen-Saines, K.; Pone, S.; Aibe, M.; Barroso de Aguiar, E.; Sim, M.; Brasil, P.; Zin, A.; Tsui, I.; et al. Early Clinical Infancy Outcomes for Microcephaly and/or Small for Gestational Age Zika-Exposed Infants. Clin. Infect. Dis. 2020, 70, 2663–2672. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.; Tong, V.T.; Rodriguez, H.; Valencia, D.; Acosta, J.; Honein, M.A.; Ospina, M.L.; Team, Z.E.N.S. Cohort Profile: Congenital Zika virus infection and child neurodevelopmental outcomes; Zika en Embarazadas y Ninos (ZEN) cohort study in Colombia. Epidemiol. Health 2020, e2020060. [Google Scholar] [CrossRef] [PubMed]
- Lebov, J.F.; Arias, J.F.; Balmaseda, A.; Britt, W.; Cordero, J.F.; Galvao, L.A.; Garces, A.L.; Hambidge, K.M.; Harris, E.; Ko, A.; et al. International prospective observational cohort study of Zika in infants and pregnancy (ZIP study): Study protocol. BMC Pregnancy Childbirth 2019, 19, 282. [Google Scholar] [CrossRef]
- Oh, Y.; Zhang, F.; Wang, Y.; Lee, E.M.; Choi, I.Y.; Lim, H.; Mirakhori, F.; Li, R.; Huang, L.; Xu, T.; et al. Zika virus directly infects peripheral neurons and induces cell death. Nat. Neurosci. 2017, 20, 1209–1212. [Google Scholar] [CrossRef] [PubMed]
- Miner, J.J.; Diamond, M.S. Zika Virus Pathogenesis and Tissue Tropism. Cell Host Microbe 2017, 21, 134–142. [Google Scholar] [CrossRef]
- Musso, D.; Ko, A.I.; Baud, D. Zika Virus Infection—After the Pandemic. N. Engl. J. Med. 2019, 381, 1444–1457. [Google Scholar] [CrossRef]
- Yuan, L.; Huang, X.Y.; Liu, Z.Y.; Zhang, F.; Zhu, X.L.; Yu, J.Y.; Ji, X.; Xu, Y.P.; Li, G.; Li, C.; et al. A single mutation in the prM protein of Zika virus contributes to fetal microcephaly. Science 2017. [Google Scholar] [CrossRef]
- Petersen, L.R.; Jamieson, D.J.; Powers, A.M.; Honein, M.A. Zika Virus. N. Engl. J. Med. 2016, 374, 1552–1563. [Google Scholar] [CrossRef]
- Ho, C.Y.; Castillo, N.; Encinales, L.; Porras, A.; Mendoza, A.R.; Lynch, R.; Nemirovsky, A.; Mantus, G.; DeBiasi, R.L.; Bethony, J.M.; et al. Second-trimester Ultrasound and Neuropathologic Findings in Congenital Zika Virus Infection. Pediatr. Infect. Dis. J. 2018, 37, 1290–1293. [Google Scholar] [CrossRef]
- Miner, J.J.; Sene, A.; Richner, J.M.; Smith, A.M.; Santeford, A.; Ban, N.; Weger-Lucarelli, J.; Manzella, F.; Ruckert, C.; Govero, J.; et al. Zika Virus Infection in Mice Causes Panuveitis with Shedding of Virus in Tears. Cell Rep. 2016, 16, 3208–3218. [Google Scholar] [CrossRef]
- D’Ortenzio, E.; Matheron, S.; Yazdanpanah, Y.; de Lamballerie, X.; Hubert, B.; Piorkowski, G.; Maquart, M.; Descamps, D.; Damond, F.; Leparc-Goffart, I. Evidence of Sexual Transmission of Zika Virus. N. Engl. J. Med. 2016, 374, 2195–2198. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S.; Schlaberg, R.; Lewis, J.; Hanson, K.E.; Couturier, M.R. Fatal Zika Virus Infection with Secondary Nonsexual Transmission. N. Engl. J. Med. 2016, 375, 1907–1909. [Google Scholar] [CrossRef] [PubMed]
- Newman, C.M.; Dudley, D.M.; Aliota, M.T.; Weiler, A.M.; Barry, G.L.; Mohns, M.S.; Breitbach, M.E.; Stewart, L.M.; Buechler, C.R.; Graham, M.E.; et al. Oropharyngeal mucosal transmission of Zika virus in rhesus macaques. Nat. Commun. 2017, 8, 169. [Google Scholar] [CrossRef] [PubMed]
- Oehler, E.; Watrin, L.; Larre, P.; Leparc-Goffart, I.; Lastere, S.; Valour, F.; Baudouin, L.; Mallet, H.; Musso, D.; Ghawche, F. Zika virus infection complicated by Guillain-Barre syndrome--case report, French Polynesia, December 2013. Eurosurveillance 2014, 19. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, F.; Burns, J.D.; Agameya, A.; Patel, A.; Alfaqih, M.; Small, J.E.; Ooi, W. Case Report: Zika Virus Meningoencephalitis and Myelitis and Associated Magnetic Resonance Imaging Findings. Am. J. Trop. Med. Hyg. 2017, 97, 340–343. [Google Scholar] [CrossRef]
- Joob, B.; Wiwanitkit, V. Zika Virus Meningoencephalitis. Mayo Clin. Proc. 2017, 92, 1313. [Google Scholar] [CrossRef]
- Schwartzmann, P.V.; Ramalho, L.N.; Neder, L.; Vilar, F.C.; Ayub-Ferreira, S.M.; Romeiro, M.F.; Takayanagui, O.M.; Dos Santos, A.C.; Schmidt, A.; Figueiredo, L.T.; et al. Zika Virus Meningoencephalitis in an Immunocompromised Patient. Mayo Clin. Proc. 2017, 92, 460–466. [Google Scholar] [CrossRef]
- Carteaux, G.; Maquart, M.; Bedet, A.; Contou, D.; Brugieres, P.; Fourati, S.; Cleret de Langavant, L.; de Broucker, T.; Brun-Buisson, C.; Leparc-Goffart, I.; et al. Zika Virus Associated with Meningoencephalitis. N. Engl. J. Med. 2016, 374, 1595–1596. [Google Scholar] [CrossRef]
- Nowakowski, T.J.; Pollen, A.A.; Di Lullo, E.; Sandoval-Espinosa, C.; Bershteyn, M.; Kriegstein, A.R. Expression Analysis Highlights AXL as a Candidate Zika Virus Entry Receptor in Neural Stem Cells. CellStemCell 2016, 18, 591–596. [Google Scholar] [CrossRef]
- Lazear, H.M.; Govero, J.; Smith, A.M.; Platt, D.J.; Fernandez, E.; Miner, J.J.; Diamond, M.S. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe 2016, 19, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Aliota, M.T.; Caine, E.A.; Walker, E.C.; Larkin, K.E.; Camacho, E.; Osorio, J.E. Characterization of Lethal Zika Virus Infection in AG129 Mice. PLoS Negl. Trop. Dis. 2016, 10, e0004682. [Google Scholar] [CrossRef]
- Dang, J.; Tiwari, S.K.; Lichinchi, G.; Qin, Y.; Patil, V.S.; Eroshkin, A.M.; Rana, T.M. Zika Virus Depletes Neural Progenitors in Human Cerebral Organoids through Activation of the Innate Immune Receptor TLR3. CellStemCell 2016, 19, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, D.; Ye, Q.; Hong, S.; Jiang, Y.; Liu, X.; Zhang, N.; Shi, L.; Qin, C.F.; Xu, Z. Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. CellStemCell 2016, 19, 120–126. [Google Scholar] [CrossRef]
- Miner, J.J.; Cao, B.; Govero, J.; Smith, A.M.; Fernandez, E.; Cabrera, O.H.; Garber, C.; Noll, M.; Klein, R.S.; Noguchi, K.K.; et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell 2016, 165, 1081–1091. [Google Scholar] [CrossRef]
- Malkki, H. CNS infections: Mouse studies confirm the link between Zika virus infection and microcephaly. Nat. Rev. Neurol. 2016, 12, 369. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Shresta, S. Neuroteratogenic Viruses and Lessons for Zika Virus Models. Trends Microbiol. 2016, 24, 622–636. [Google Scholar] [CrossRef]
- Solomon, I.H.; Milner, D.A.; Folkerth, R.D. Neuropathology of Zika Virus Infection. J. Neuroinfect. Dis. 2016, 7. [Google Scholar] [CrossRef]
- Yockey, L.J.; Varela, L.; Rakib, T.; Khoury-Hanold, W.; Fink, S.L.; Stutz, B.; Szigeti-Buck, K.; Van den Pol, A.; Lindenbach, B.D.; Horvath, T.L.; et al. Vaginal Exposure to Zika Virus during Pregnancy Leads to Fetal Brain Infection. Cell 2016, 166, 1247–1256.e4. [Google Scholar] [CrossRef] [PubMed]
- Govero, J.; Esakky, P.; Scheaffer, S.M.; Fernandez, E.; Drury, A.; Platt, D.J.; Gorman, M.J.; Richner, J.M.; Caine, E.A.; Salazar, V.; et al. Zika virus infection damages the testes in mice. Nature 2016, 540, 438–442. [Google Scholar] [CrossRef]
- Fernandes, N.C.; Nogueira, J.S.; Ressio, R.A.; Cirqueira, C.S.; Kimura, L.M.; Fernandes, K.R.; Cunha, M.S.; Souza, R.P.; Guerra, J.M. Experimental Zika virus infection induces spinal cord injury and encephalitis in newborn Swiss mice. Exp. Toxicol. Pathol. 2017, 69, 63–71. [Google Scholar] [CrossRef] [PubMed]
- van den Pol, A.N.; Mao, G.; Yang, Y.; Ornaghi, S.; Davis, J.N. Zika Virus Targeting in the Developing Brain. J. Neurosci. 2017, 37, 2161–2175. [Google Scholar] [CrossRef]
- Morrison, T.E.; Diamond, M.S. Animal Models of Zika Virus Infection, Pathogenesis, and Immunity. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Shao, Q.; Herrlinger, S.; Yang, S.L.; Lai, F.; Moore, J.M.; Brindley, M.A.; Chen, J.F. Zika virus infection disrupts neurovascular development and results in postnatal microcephaly with brain damage. Development 2016, 143, 4127–4136. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Du, S.; Shan, C.; Nie, K.; Zhang, R.; Li, X.F.; Zhang, R.; Wang, T.; Qin, C.F.; et al. Evolutionary enhancement of Zika virus infectivity in Aedes aegypti mosquitoes. Nature 2017, 545, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Costa, F.; Garcia-Blanco, M.A.; Ko, A.I.; Ribeiro, G.S.; Saade, G.; Shi, P.Y.; Vasilakis, N. Zika virus: History, emergence, biology, and prospects for control. Antiviral. Res. 2016, 130, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.C.; Dombrowski, J.G.; Phelan, J.; Marinho, C.R.F.; Hibberd, M.; Clark, T.G.; Campino, S. Zika might not be acting alone: Using an ecological study approach to investigate potential co-acting risk factors for an unusual pattern of microcephaly in Brazil. PLoS ONE 2018, 13, e0201452. [Google Scholar] [CrossRef] [PubMed]
- Diallo, D.; Sall, A.A.; Diagne, C.T.; Faye, O.; Faye, O.; Ba, Y.; Hanley, K.A.; Buenemann, M.; Weaver, S.C.; Diallo, M. Zika virus emergence in mosquitoes in southeastern Senegal, 2011. PLoS ONE 2014, 9, e109442. [Google Scholar] [CrossRef]
- Buechler, C.R.; Bailey, A.L.; Weiler, A.M.; Barry, G.L.; Jasinska, A.J.; Freimer, N.B.; Apetrei, C.; Phillips-Conroy, J.E.; Jolly, C.J.; Rogers, J.; et al. Prevalence of Zika virus infection in wild African primates. BioRxiv 2016. [Google Scholar] [CrossRef]
- Collette, N.M.; Lao, V.H.I.; Weilhammer, D.R.; Zingg, B.; Cohen, S.D.; Hwang, M.; Coffey, L.L.; Grady, S.L.; Zemla, A.T.; Borucki, M.K. Single Amino Acid Mutations Affect Zika Virus Replication In Vitro and Virulence In Vivo. Viruses 2020, 12, 1295. [Google Scholar] [CrossRef] [PubMed]
- Limonta, D.; Hobman, T.C. Zika Virus and Host Interactions: From the Bench to the Bedside and Beyond. Cells 2020, 9, 2463. [Google Scholar] [CrossRef]
- Cordeiro, C.N.; Bano, R.; Washington Cross, C.I.; Segars, J.H. Zika virus and assisted reproduction. Curr. Opin. Obstet. Gynecol. 2017, 29, 175–179. [Google Scholar] [CrossRef]
- Sirohi, D.; Chen, Z.; Sun, L.; Klose, T.; Pierson, T.C.; Rossmann, M.G.; Kuhn, R.J. The 3.8 A resolution cryo-EM structure of Zika virus. Science 2016, 352, 467–470. [Google Scholar] [CrossRef]
- Mansuy, J.M.; Dutertre, M.; Mengelle, C.; Fourcade, C.; Marchou, B.; Delobel, P.; Izopet, J.; Martin-Blondel, G. Zika virus: High infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect. Dis. 2016, 16. [Google Scholar] [CrossRef]
- Gourinat, A.C.; O’Connor, O.; Calvez, E.; Goarant, C.; Dupont-Rouzeyrol, M. Detection of Zika virus in urine. Emerg. Infect. Dis. 2015, 21, 84–86. [Google Scholar] [CrossRef]
- Nunez, A.I.; Talavera, S.; Birnberg, L.; Rivas, R.; Pujol, N.; Verdun, M.; Aranda, C.; Berdugo, M.; Busquets, N. Evidence of Zika virus horizontal and vertical transmission in Aedes albopictus from Spain but not infectious virus in saliva of the progeny. Emerg. Microbes. Infect. 2020, 1–22. [Google Scholar] [CrossRef]
- Kostyuchenko, V.A.; Lim, E.X.; Zhang, S.; Fibriansah, G.; Ng, T.S.; Ooi, J.S.; Shi, J.; Lok, S.M. Structure of the thermally stable Zika virus. Nature 2016, 533, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Liu, Y.; Liu, J.; Zhao, J.; Champagne, C.; Tong, L.; Zhang, R.; Zhang, F.; Qin, C.F.; Ma, P.; et al. Aedes mosquitoes acquire and transmit Zika virus by breeding in contaminated aquatic environments. Nat. Commun. 2019, 10, 1324. [Google Scholar] [CrossRef] [PubMed]
- Aubry, F.; Dabo, S.; Manet, C.; Filipovic, I.; Rose, N.H.; Miot, E.F.; Martynow, D.; Baidaliuk, A.; Merkling, S.H.; Dickson, L.B.; et al. Enhanced Zika virus susceptibility of globally invasive Aedes aegypti populations. Science 2020, 370, 991–996. [Google Scholar] [CrossRef]
- Brady, O.J.; Hay, S.I. The Global Expansion of Dengue: How Aedes aegypti Mosquitoes Enabled the First Pandemic Arbovirus. Annu. Rev. Entomol. 2020, 65, 191–208. [Google Scholar] [CrossRef] [PubMed]
- McBride, C.S.; Baier, F.; Omondi, A.B.; Spitzer, S.A.; Lutomiah, J.; Sang, R.; Ignell, R.; Vosshall, L.B. Evolution of mosquito preference for humans linked to an odorant receptor. Nature 2014, 515, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Main, B.J.; Lee, Y.; Ferguson, H.M.; Kreppel, K.S.; Kihonda, A.; Govella, N.J.; Collier, T.C.; Cornel, A.J.; Eskin, E.; Kang, E.Y.; et al. The Genetic Basis of Host Preference and Resting Behavior in the Major African Malaria Vector, Anopheles arabiensis. PLoS Genet. 2016, 12, e1006303. [Google Scholar] [CrossRef] [PubMed]
- Ledermann, J.P.; Guillaumot, L.; Yug, L.; Saweyog, S.C.; Tided, M.; Machieng, P.; Pretrick, M.; Marfel, M.; Griggs, A.; Bel, M.; et al. Aedes hensilli as a potential vector of Chikungunya and Zika viruses. PLoS Negl. Trop. Dis. 2014, 8, e3188. [Google Scholar] [CrossRef]
- Lambrechts, L.; Scott, T.W.; Gubler, D.J. Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl. Trop. Dis. 2010, 4, e646. [Google Scholar] [CrossRef] [PubMed]
- Ford, S.A.; Allen, S.L.; Ohm, J.R.; Sigle, L.T.; Sebastian, A.; Albert, I.; Chenoweth, S.F.; McGraw, E.A. Selection on Aedes aegypti alters Wolbachia-mediated dengue virus blocking and fitness. Nat. Microbiol. 2019, 4, 1832–1839. [Google Scholar] [CrossRef] [PubMed]
- Schutte, B.C.; Sander, A.; Malik, M.; Murray, J.C. Refinement of the Van der Woude gene location and construction of a 3.5-Mb YAC contig and STS map spanning the critical region in 1q32-q41. Genomics 1996, 36, 507–514. [Google Scholar] [CrossRef]
- Pool, K.L.; Adachi, K.; Karnezis, S.; Salamon, N.; Romero, T.; Nielsen-Saines, K.; Pone, S.; Boechat, M.; Aibe, M.; Gomes da Silva, T.; et al. Association Between Neonatal Neuroimaging and Clinical Outcomes in Zika-Exposed Infants From Rio de Janeiro, Brazil. JAMA Netw. Open 2019, 2, e198124. [Google Scholar] [CrossRef]
- Abtibol-Bernardino, M.R.; de Almeida Peixoto, L.F.A.; de Oliveira, G.A.; de Almeida, T.F.; Rodrigues, G.R.I.; Otani, R.H.; Soares Chaves, B.C.; de Souza Rodrigues, C.; de Andrade, A.; de Fatima Redivo, E.; et al. Neurological Findings in Children without Congenital Microcephaly Exposed to Zika Virus in Utero: A Case Series Study. Viruses 2020, 12, 1335. [Google Scholar] [CrossRef]
- Vianna, R.A.O.; Rua, E.C.; Fernandes, A.R.; Dos Santos, T.C.S.; Dalcastel, L.A.B.; Dos Santos, M.L.B.; de Paula, P.D.S.; de Carvalho, F.R.; Pache de Faria, A.O.; Almeida, P.L.; et al. Experience in diagnosing congenital Zika syndrome in Brazilian children born to asymptomatic mothers. Acta Trop. 2020, 206, 105438. [Google Scholar] [CrossRef]
- Petzold, S.; Agbaria, N.; Deckert, A.; Dambach, P.; Winkler, V.; Drexler, J.F.; Horstick, O.; Jaenisch, T. Congenital abnormalities associated with Zika virus infection-Dengue as potential co-factor? A systematic review. PLoS Negl. Trop. Dis. 2021, 15, e0008984. [Google Scholar] [CrossRef]
- Peçanha, P.M.; Gomes Junior, S.C.; Pone, S.M.; Pone, M.V.; Vasconcelos, Z.; Zin, A.; Vilibor, R.H.; Costa, R.P.; Meio, M.D.; Nielsen-Saines, K.; et al. Neurodevelopment of children exposed intra-uterus by Zika virus: A case series. PLoS ONE 2020, 15, e0229434. [Google Scholar] [CrossRef]
- Chapman, S.J.; Hill, A.V. Human genetic susceptibility to infectious disease. Nat. Rev. Genet. 2012, 13, 175–188. [Google Scholar] [CrossRef]
- Comstock, G.W. Tuberculosis in twins: A re-analysis of the Prophit survey. Am. Rev. Respir Dis. 1978, 117, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Driss, A.; Hibbert, J.M.; Wilson, N.O.; Iqbal, S.A.; Adamkiewicz, T.V.; Stiles, J.K. Genetic polymorphisms linked to susceptibility to malaria. Malar J. 2011, 10, 271. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.V. Genetic susceptibility to malaria and other infectious diseases: From the MHC to the whole genome. Parasitology 1996, 112, S75–S84. [Google Scholar] [CrossRef]
- Fellay, J.; Shianna, K.V.; Ge, D.; Colombo, S.; Ledergerber, B.; Weale, M.; Zhang, K.; Gumbs, C.; Castagna, A.; Cossarizza, A.; et al. A whole-genome association study of major determinants for host control of HIV-1. Science 2007, 317, 944–947. [Google Scholar] [CrossRef] [PubMed]
- International, H.I.V.C.S.; Pereyra, F.; Jia, X.; McLaren, P.J.; Telenti, A.; de Bakker, P.I.; Walker, B.D.; Ripke, S.; Brumme, C.J.; Pulit, S.L.; et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 2010, 330, 1551–1557. [Google Scholar] [CrossRef]
- Khor, C.C.; Chau, T.N.; Pang, J.; Davila, S.; Long, H.T.; Ong, R.T.; Dunstan, S.J.; Wills, B.; Farrar, J.; Van Tram, T.; et al. Genome-wide association study identifies susceptibility loci for dengue shock syndrome at MICB and PLCE1. Nat. Genet. 2011, 43, 1139–1141. [Google Scholar] [CrossRef]
- Jallow, M.; Teo, Y.Y.; Small, K.S.; Rockett, K.A.; Deloukas, P.; Clark, T.G.; Kivinen, K.; Bojang, K.A.; Conway, D.J.; Pinder, M.; et al. Genome-wide and fine-resolution association analysis of malaria in West Africa. Nat. Genet. 2009, 41, 657–665. [Google Scholar] [CrossRef]
- Blanpain, C.; Libert, F.; Vassart, G.; Parmentier, M. CCR5 and HIV infection. Recept. Channels 2002, 8, 19–31. [Google Scholar] [CrossRef]
- Fellay, J.; Ge, D.; Shianna, K.V.; Colombo, S.; Ledergerber, B.; Cirulli, E.T.; Urban, T.J.; Zhang, K.; Gumbs, C.E.; Smith, J.P.; et al. Common genetic variation and the control of HIV-1 in humans. PLoS Genet. 2009, 5, e1000791. [Google Scholar] [CrossRef]
- Limou, S.; Coulonges, C.; Herbeck, J.T.; van Manen, D.; An, P.; Le Clerc, S.; Delaneau, O.; Diop, G.; Taing, L.; Montes, M.; et al. Multiple-cohort genetic association study reveals CXCR6 as a new chemokine receptor involved in long-term nonprogression to AIDS. J. Infect. Dis. 2010, 202, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Trachtenberg, E.; Bhattacharya, T.; Ladner, M.; Phair, J.; Erlich, H.; Wolinsky, S. The HLA-B/-C haplotype block contains major determinants for host control of HIV. Genes Immun. 2009, 10, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Pelak, K.; Goldstein, D.B.; Walley, N.M.; Fellay, J.; Ge, D.; Shianna, K.V.; Gumbs, C.; Gao, X.; Maia, J.M.; Cronin, K.D.; et al. Host determinants of HIV-1 control in African Americans. J. Infect. Dis. 2010, 201, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Troyer, J.L.; Nelson, G.W.; Lautenberger, J.A.; Chinn, L.; McIntosh, C.; Johnson, R.C.; Sezgin, E.; Kessing, B.; Malasky, M.; Hendrickson, S.L.; et al. Genome-wide association study implicates PARD3B-based AIDS restriction. J. Infect. Dis. 2011, 203, 1491–1502. [Google Scholar] [CrossRef]
- Limou, S.; Le Clerc, S.; Coulonges, C.; Carpentier, W.; Dina, C.; Delaneau, O.; Labib, T.; Taing, L.; Sladek, R.; Deveau, C.; et al. Genomewide association study of an AIDS-nonprogression cohort emphasizes the role played by HLA genes (ANRS Genomewide Association Study 02). J. Infect. Dis. 2009, 199, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Ciborowski, P.; Gendelman, H.E. Human immunodeficiency virus-mononuclear phagocyte interactions: Emerging avenues of biomarker discovery, modes of viral persistence and disease pathogenesis. Curr. HIV Res. 2006, 4, 279–291. [Google Scholar] [CrossRef]
- Kraft-Terry, S.D.; Stothert, A.R.; Buch, S.; Gendelman, H.E. HIV-1 neuroimmunity in the era of antiretroviral therapy. Neurobiol. Dis. 2010, 37, 542–548. [Google Scholar] [CrossRef]
- Sillman, B.; Woldstad, C.; McMillan, J.; Gendelman, H.E. Neuropathogenesis of human immunodeficiency virus infection. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 152, pp. 21–40. [Google Scholar] [CrossRef]
- Haig, D. Genetic conflicts in human pregnancy. Q. Rev. Biol. 1993, 68, 495–532. [Google Scholar] [CrossRef]
- Paixao, E.S.; Leong, W.Y.; Rodrigues, L.C.; Wilder-Smith, A. Asymptomatic Prenatal Zika Virus Infection and Congenital Zika Syndrome. Open Forum. Infect. Dis. 2018, 5, ofy073. [Google Scholar] [CrossRef]
- Santos, C.N.O.; Ribeiro, D.R.; Cardoso Alves, J.; Cazzaniga, R.A.; Magalhães, L.S.; de Souza, M.S.F.; Fonseca, A.B.L.; Bispo, A.J.B.; Porto, R.L.S.; Santos, C.A.d.; et al. Association Between Zika Virus Microcephaly in Newborns With the rs3775291 Variant in Toll-Like Receptor 3 and rs1799964 Variant at Tumor Necrosis Factor-α Gene. J. Infect. Dis. 2019, 220, 1797–1801. [Google Scholar] [CrossRef]
- Mercado, M.; Daza, M.; Moore, C.A.; Valencia, D.; Rico, A.; Alvarez-Diaz, D.A.; Brault, A.C.; Fitzpatrick, K.; Mulkey, S.B. Discordant Clinical Outcomes in a Monozygotic Dichorionic-Diamniotic Twin Pregnancy with Probable Zika Virus Exposure. Case Report. Trop. Med. Infect. Dis. 2020, 5, 188. [Google Scholar] [CrossRef]
- Caires-Junior, L.C.; Goulart, E.; Melo, U.S.; Araujo, B.H.S.; Alvizi, L.; Soares-Schanoski, A.; de Oliveira, D.F.; Kobayashi, G.S.; Griesi-Oliveira, K.; Musso, C.M.; et al. Discordant congenital Zika syndrome twins show differential in vitro viral susceptibility of neural progenitor cells. Nat. Commun. 2018, 9, 475. [Google Scholar] [CrossRef] [PubMed]
- Linden, V.V.; Linden, H.V.J.; Leal, M.C.; Rolim, E.L.F.; Linden, A.V.; Aragao, M.; Brainer-Lima, A.M.; Cruz, D.; Ventura, L.O.; Florencio, T.L.T.; et al. Discordant clinical outcomes of congenital Zika virus infection in twin pregnancies. Arq. Neuropsiquiatr. 2017, 75, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Chavali, P.L.; Stojic, L.; Meredith, L.W.; Joseph, N.; Nahorski, M.S.; Sanford, T.J.; Sweeney, T.R.; Krishna, B.A.; Hosmillo, M.; Firth, A.E.; et al. Neurodevelopmental protein Musashi-1 interacts with the Zika genome and promotes viral replication. Science 2017, 357, 83–88. [Google Scholar] [CrossRef]
- Hamel, R.; Dejarnac, O.; Wichit, S.; Ekchariyawat, P.; Neyret, A.; Luplertlop, N.; Perera-Lecoin, M.; Surasombatpattana, P.; Talignani, L.; Thomas, F.; et al. Biology of Zika Virus Infection in Human Skin Cells. J. Virol. 2015, 89, 8880–8896. [Google Scholar] [CrossRef]
- Barreto, M.L.; Barral-Netto, M.; Stabeli, R.; Almeida-Filho, N.; Vasconcelos, P.F.C.; Teixeira, M.; Buss, P.; Gadelha, P.E. Zika virus and microcephaly in Brazil: A scientific agenda. Lancet 2016, 387, 919–921. [Google Scholar] [CrossRef]
- Devakumar, D.; Bamford, A.; Ferreira, M.U.; Broad, J.; Rosch, R.E.; Groce, N.; Breuer, J.; Cardoso, M.A.; Copp, A.J.; Alexandre, P.; et al. Infectious causes of microcephaly: Epidemiology, pathogenesis, diagnosis, and management. Lancet Infect. Dis. 2017. [Google Scholar] [CrossRef]
- Graham, K.A.; Fox, D.J.; Talati, A.; Pantea, C.; Brady, L.; Carter, S.L.; Friedenberg, E.; Vora, N.M.; Browne, M.L.; Lee, C.T. Prevalence and Clinical Attributes of Congenital Microcephaly—New York, 2013–2015. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 125–129. [Google Scholar] [CrossRef]
- Cragan, J.D.; Isenburg, J.L.; Parker, S.E.; Alverson, C.J.; Meyer, R.E.; Stallings, E.B.; Kirby, R.S.; Lupo, P.J.; Liu, J.S.; Seagroves, A.; et al. Population-based microcephaly surveillance in the United States, 2009 to 2013: An analysis of potential sources of variation. Birth Defects Res. A Clin. Mol. Teratol. 2016, 106, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Mai, C.T.; Kucik, J.E.; Isenburg, J.; Feldkamp, M.L.; Marengo, L.K.; Bugenske, E.M.; Thorpe, P.G.; Jackson, J.M.; Correa, A.; Rickard, R.; et al. Selected birth defects data from population-based birth defects surveillance programs in the United States, 2006 to 2010: Featuring trisomy conditions. Birth Defects Res. A Clin. Mol. Teratol. 2013, 97, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Whelan, M.A. Practice parameter: Evaluation of the child with microcephaly (an evidence-based review): Report of the quality standards subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2010, 74, 1079, author reply 1079 (2010). [Google Scholar] [CrossRef]
- Faheem, M.; Naseer, M.I.; Rasool, M.; Chaudhary, A.G.; Kumosani, T.A.; Ilyas, A.M.; Pushparaj, P.; Ahmed, F.; Algahtani, H.A.; Al-Qahtani, M.H.; et al. Molecular genetics of human primary microcephaly: An overview. BMC Med. Genomics 2015, 8 (Suppl. 1), S4. [Google Scholar] [CrossRef] [PubMed]
- Ashwal, S.; Michelson, D.; Plawner, L.; Dobyns, W.B.; Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: Evaluation of the child with microcephaly (an evidence-based review)—report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2009, 73, 887–897. [Google Scholar] [CrossRef]
- Schwartz, D.A. Autopsy and Postmortem Studies Are Concordant: Pathology of Zika Virus Infection Is Neurotropic in Fetuses and Infants with Microcephaly Following Transplacental Transmission. Arch. Pathol. Lab. Med. 2017, 141, 68–72. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, V.; Pessoa, A.; Dobyns, W.; Barkovich, A.J.; Junior, H.V.; Filho, E.L.; Ribeiro, E.M.; Leal, M.C.; Coimbra, P.P.; Aragao, M.F.; et al. Description of 13 Infants Born during October 2015–January 2016 With Congenital Zika Virus Infection Without Microcephaly at Birth—Brazil. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 1343–1348. [Google Scholar] [CrossRef]
- Mlakar, J.; Korva, M.; Tul, N.; Popovic, M.; Poljsak-Prijatelj, M.; Mraz, J.; Kolenc, M.; Resman Rus, K.; Vesnaver Vipotnik, T.; Fabjan Vodusek, V.; et al. Zika Virus Associated with Microcephaly. N. Engl. J. Med. 2016, 374, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Driggers, R.W.; Ho, C.Y.; Korhonen, E.M.; Kuivanen, S.; Jaaskelainen, A.J.; Smura, T.; Rosenberg, A.; Hill, D.A.; DeBiasi, R.L.; Vezina, G.; et al. Zika Virus Infection with Prolonged Maternal Viremia and Fetal Brain Abnormalities. N. Engl. J. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.A.; Jamieson, D.J.; Honein, M.A.; Petersen, L.R. Zika Virus and Birth Defects--Reviewing the Evidence for Causality. N. Engl. J. Med. 2016, 374, 1981–1987. [Google Scholar] [CrossRef]
- Villamil-Gomez, W.E.; Mendoza-Guete, A.; Villalobos, E.; Gonzalez-Arismendy, E.; Uribe-Garcia, A.M.; Castellanos, J.E.; Rodriguez-Morales, A.J. Diagnosis, management and follow-up of pregnant women with Zika virus infection: A preliminary report of the ZIKERNCOL cohort study on Sincelejo, Colombia. Travel Med. Infect. Dis. 2016, 14, 155–158. [Google Scholar] [CrossRef]
- Silva, N.M.; Santos, N.C.; Martins, I.C. Dengue and Zika Viruses: Epidemiological History, Potential Therapies, and Promising Vaccines. Trop. Med. Infect. Dis. 2020, 5, 150. [Google Scholar] [CrossRef]
- Abrams, R.P.M.; Yasgar, A.; Teramoto, T.; Lee, M.H.; Dorjsuren, D.; Eastman, R.T.; Malik, N.; Zakharov, A.V.; Li, W.; Bachani, M.; et al. Therapeutic candidates for the Zika virus identified by a high-throughput screen for Zika protease inhibitors. Proc. Natl. Acad. Sci. USA 2020, 117, 31365–31375. [Google Scholar] [CrossRef]
- Bouattour, A.; Khrouf, F.; Rhim, A.; M’Ghirbi, Y. First Detection of the Asian Tiger Mosquito, Aedes (Stegomyia) albopictus (Diptera: Culicidae), in Tunisia. J. Med. Entomol. 2019, 56, 1112–1115. [Google Scholar] [CrossRef]
- Tsuzuki, A.; Vu, T.D.; Higa, Y.; Nguyen, T.Y.; Takagi, M. High potential risk of dengue transmission during the hot-dry season in Nha Trang City, Vietnam. Acta Trop. 2009, 111, 325–329. [Google Scholar] [CrossRef]
- Bohers, C.; Mousson, L.; Madec, Y.; Vazeille, M.; Rhim, A.; M’Ghirbi, Y.; Bouattour, A.; Failloux, A.B. The recently introduced Aedes albopictus in Tunisia has the potential to transmit chikungunya, dengue and Zika viruses. PLoS Negl. Trop. Dis. 2020, 14, e0008475. [Google Scholar] [CrossRef]
- Nakhapakorn, K.; Tripathi, N.K. An information value based analysis of physical and climatic factors affecting dengue fever and dengue haemorrhagic fever incidence. Int. J. Health Geogr. 2005. [Google Scholar] [CrossRef] [PubMed]
- Alomar, A.A.; Eastmond, B.H.; Alto, B.W. The effects of exposure to pyriproxyfen and predation on Zika virus infection and transmission in Aedes aegypti. PLoS Negl. Trop. Dis. 2020, 14, e0008846. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Garcia, D.; Lochner, K.A.; Osypuk, T.L.; Subramanian, S.V. Future directions in residential segregation and health research: A multilevel approach. Am. J. Public Health 2003, 93, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Power, G.M.; Francis, S.C.; Sanchez Clemente, N.; Vasconcelos, Z.; Brasil, P.; Nielsen-Saines, K.; Brickley, E.B.; Moreira, M.E. Examining the Association of Socioeconomic Position with Microcephaly and Delayed Childhood Neurodevelopment among Children with Prenatal Zika Virus Exposure. Viruses 2020, 12, 1342. [Google Scholar] [CrossRef] [PubMed]
- Musoke, D.; Boynton, P.; Butler, C.; Musoke, M.B. Health seeking behaviour and challenges in utilising health facilities in Wakiso district, Uganda. Afr. Health Sci. 2014, 14, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.J.; Carlson, C.J.; Tesla, B.; Bonds, M.H.; Ngonghala, C.N.; Mordecai, E.A.; Johnson, L.R.; Murdock, C.C. Warming temperatures could expose more than 1.3 billion new people to Zika virus risk by 2050. Glob. Chang. Biol. 2020, 27, 84–93. [Google Scholar] [CrossRef]
- Ryan, S.J.; Carlson, C.J.; Mordecai, E.A.; Johnson, L.R. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Negl. Trop. Dis. 2019, 13, e0007213. [Google Scholar] [CrossRef] [PubMed]

| CMV 1 | HIV | HSV | LCMV 2 | Rubella | VZV 3 | Zika | |
|---|---|---|---|---|---|---|---|
| Calcifications | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| Cerebral Palsy/ Motor Delay | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| Cerebellar Hypoplasia | ✔ | ✔ | ✔ | ||||
| Chorioretinitis/Blindness | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Cortical Malformation | ✔ | ✔ | ✔ | ✔ | ✔ | ||
| Epilepsy/Seizures | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| Hearing Loss | ✔ | ✔ | ✔ | ✔ | |||
| Intellectual/ Learning Disability | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Intraventricular Hemorrhage | ✔ | ✔ | |||||
| Meningoencephalitis | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Microcephaly | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Myelination Disorder | ✔ | ✔ | ✔ | ||||
| Neuropathies | ✔ | ||||||
| Vasculopathy/ Porencephaly | ✔ | ✔ | ✔ | ✔ | |||
| Ventriculomegaly/ Hydrocephalus | ✔ | ✔ | ✔ | ✔ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kousa, Y.A.; Hossain, R.A. Causes of Phenotypic Variability and Disabilities after Prenatal Viral Infections. Trop. Med. Infect. Dis. 2021, 6, 95. https://doi.org/10.3390/tropicalmed6020095
Kousa YA, Hossain RA. Causes of Phenotypic Variability and Disabilities after Prenatal Viral Infections. Tropical Medicine and Infectious Disease. 2021; 6(2):95. https://doi.org/10.3390/tropicalmed6020095
Chicago/Turabian StyleKousa, Youssef A., and Reafa A. Hossain. 2021. "Causes of Phenotypic Variability and Disabilities after Prenatal Viral Infections" Tropical Medicine and Infectious Disease 6, no. 2: 95. https://doi.org/10.3390/tropicalmed6020095
APA StyleKousa, Y. A., & Hossain, R. A. (2021). Causes of Phenotypic Variability and Disabilities after Prenatal Viral Infections. Tropical Medicine and Infectious Disease, 6(2), 95. https://doi.org/10.3390/tropicalmed6020095







