Elevated Pediatric Chagas Disease Burden Complicated by Concomitant Intestinal Parasites and Malnutrition in El Salvador
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Population Characteristics
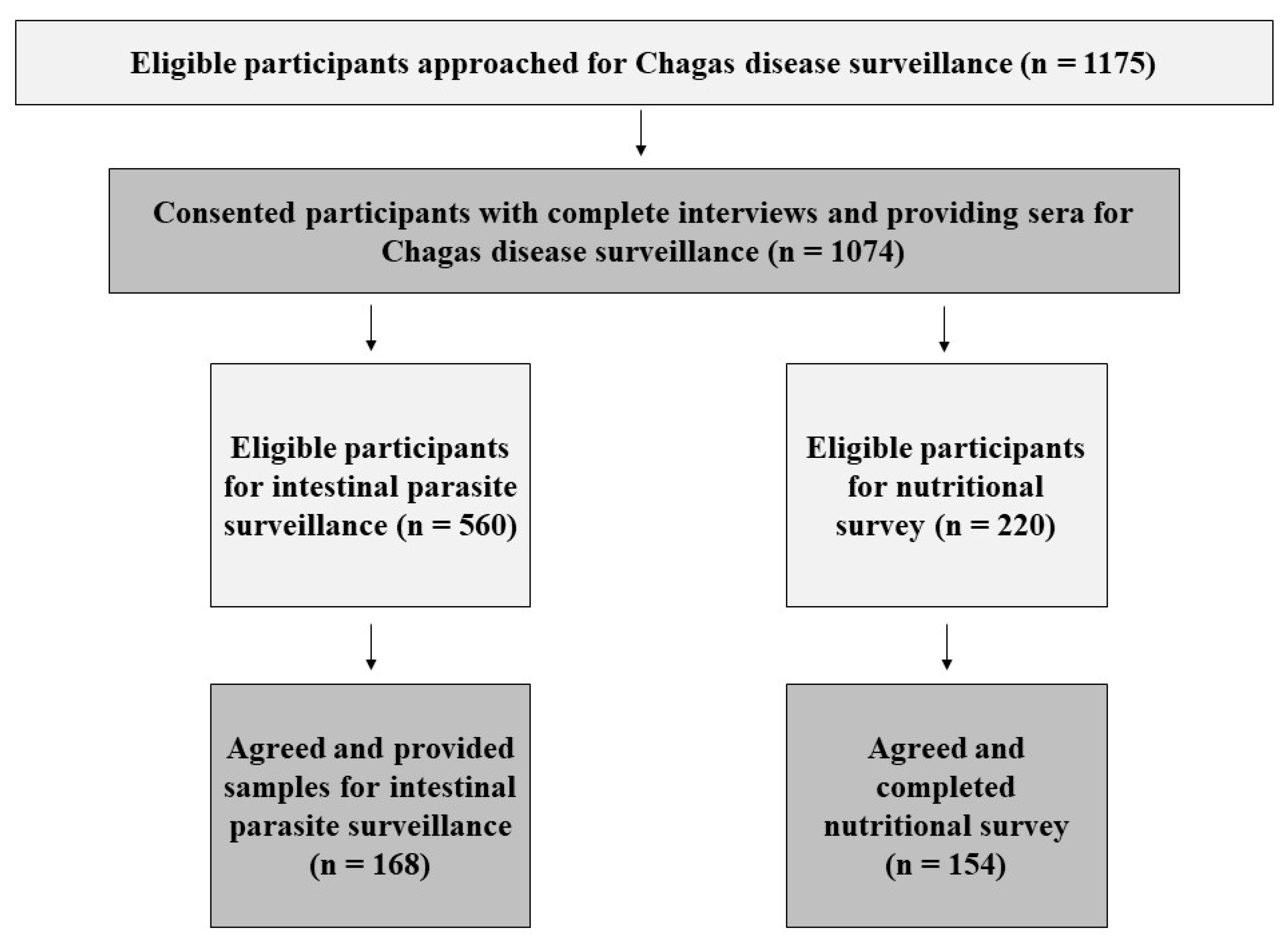
2.2. Study Recruitment and Activities
2.3. Ethics Statement
2.4. Data Analysis
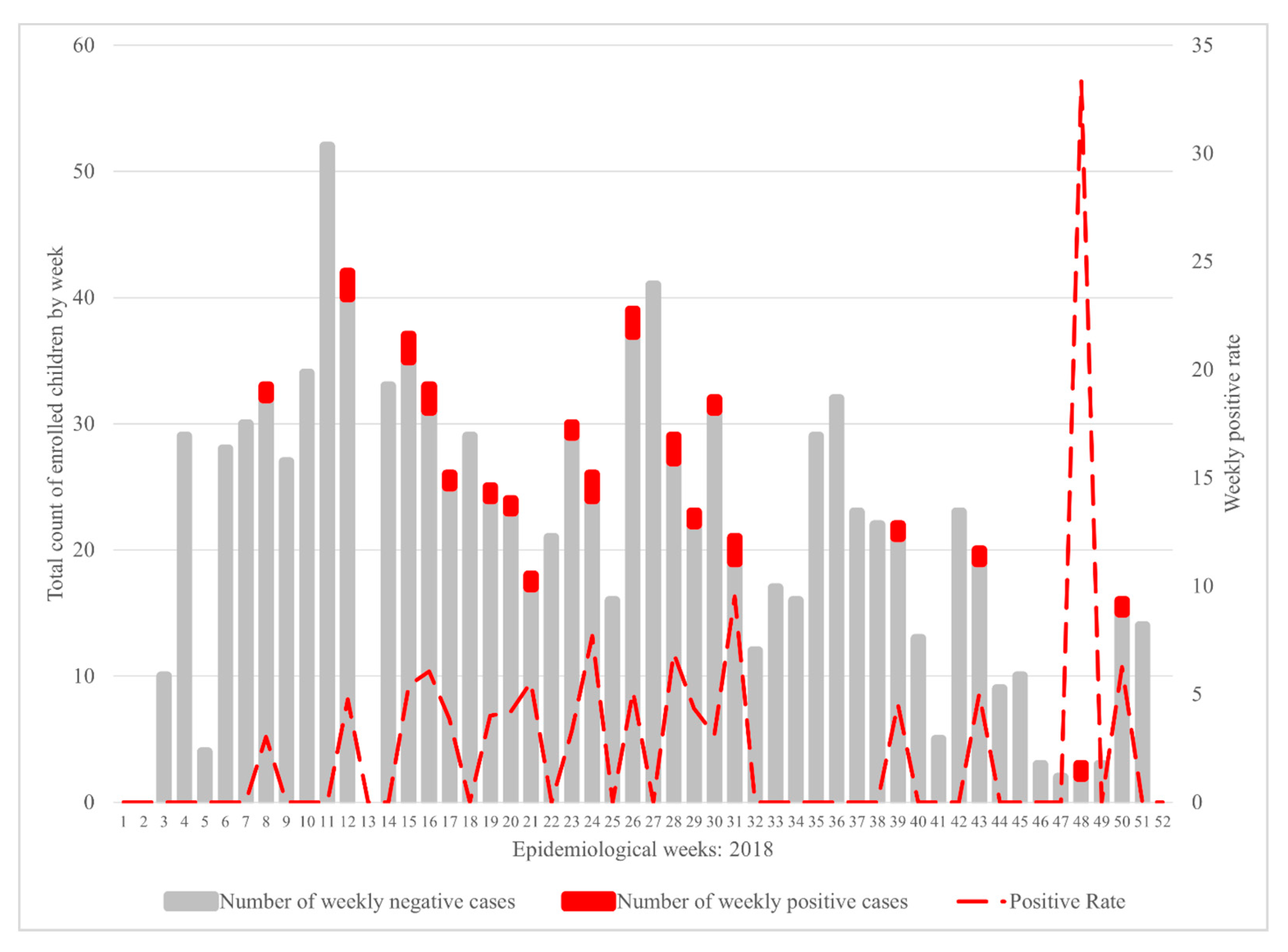
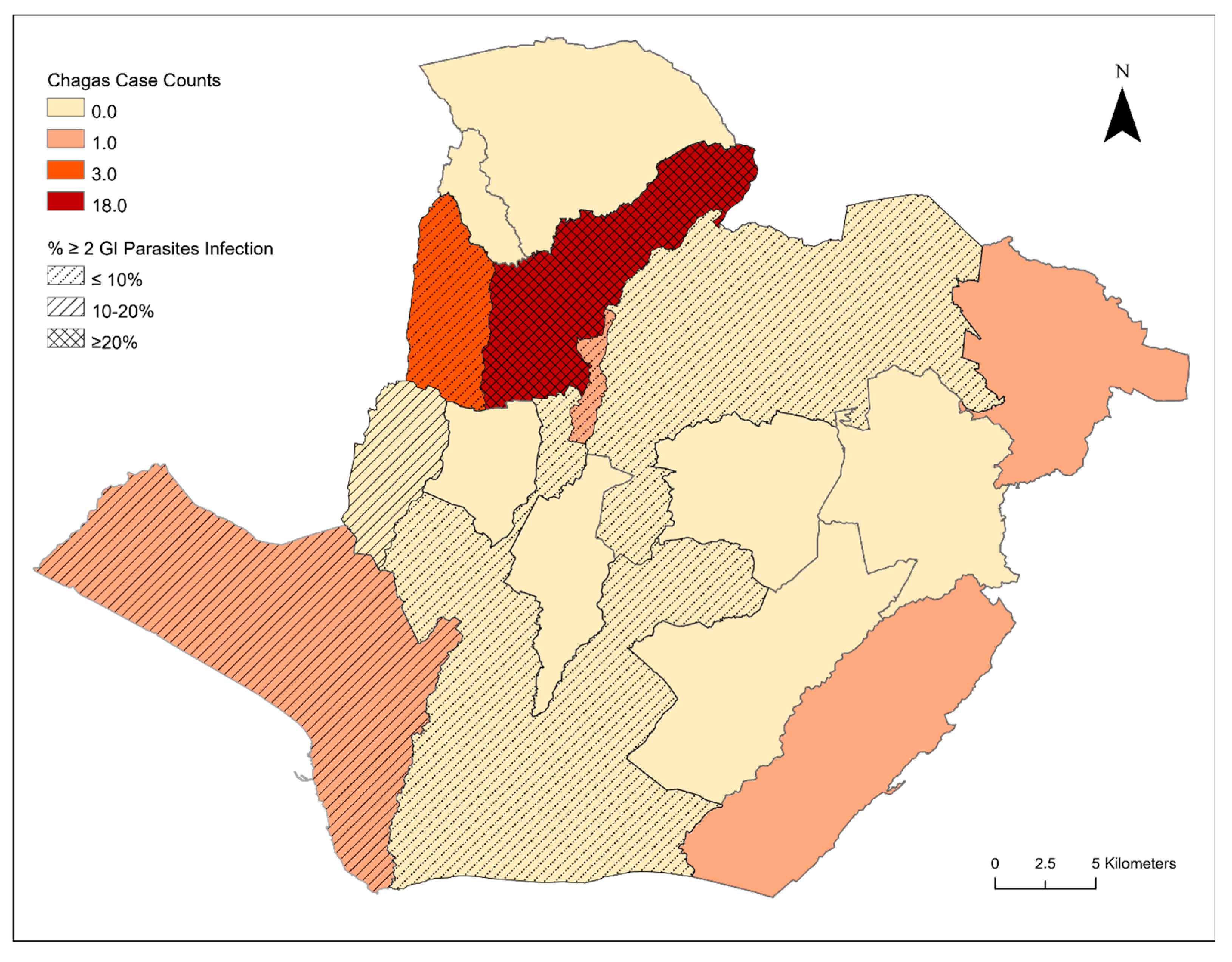
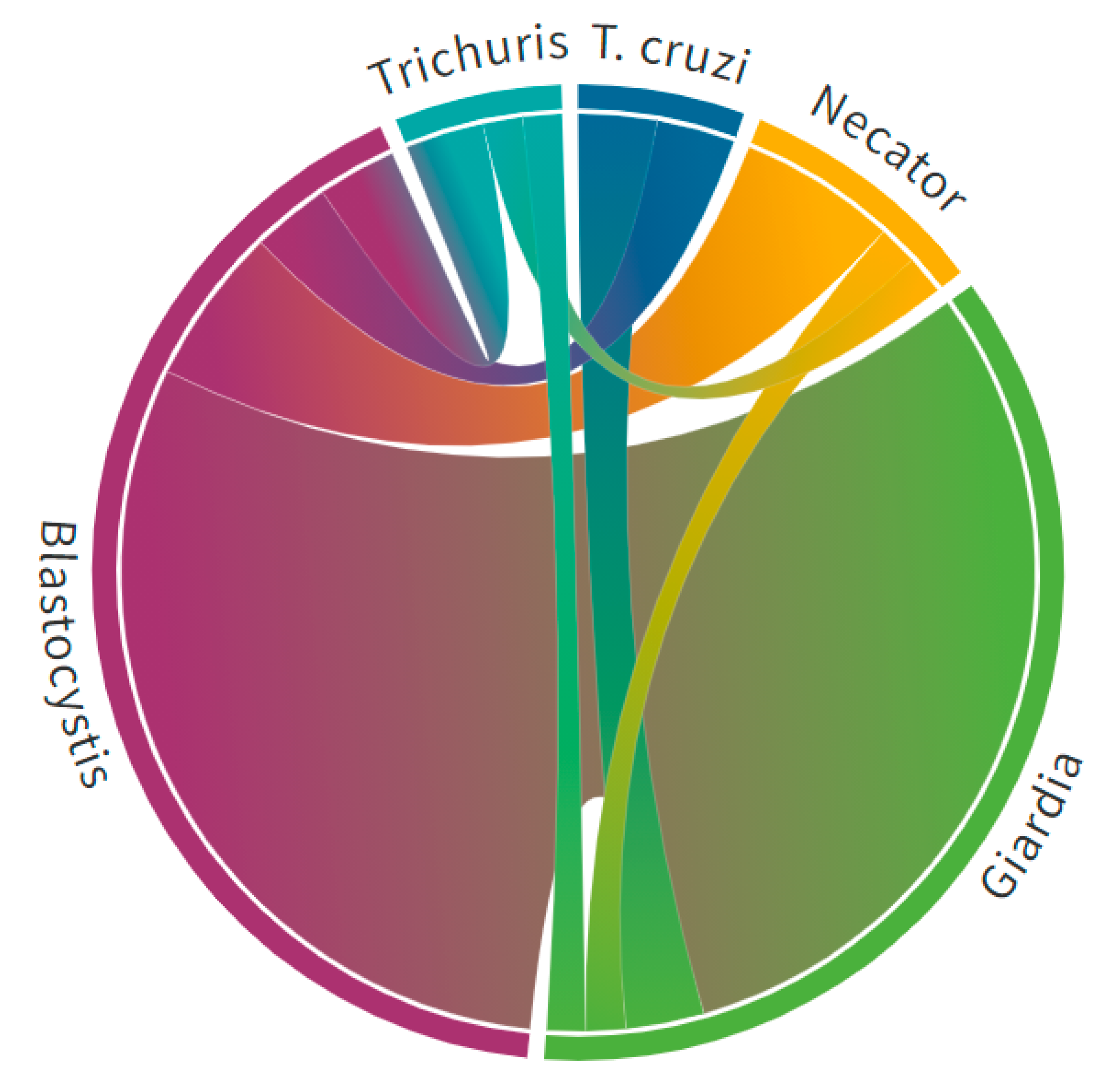
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lee, B.Y.; Bacon, K.M.; Bottazzi, M.E.; Hotez, P.J. Global economic burden of Chagas disease: A computational simulation model. Lancet Infect. Dis. 2013, 13, 342–348. [Google Scholar] [CrossRef]
- Traina, M.I.; Sanchez, D.R.; Hernandez, S.; Bradfield, J.S.; Labedi, M.R.; Ngab, T.A.; Steurer, F.; Montgomery, S.P.; Meymandi, S.K. Prevalence and Impact of Chagas Disease Among Latin American Immigrants with Nonischemic Cardiomyopathy in Los Angeles, California. Circ. Heart Fail 2015, 8, 938–943. [Google Scholar] [CrossRef]
- Herwaldt, B.L.; Dougherty, C.P.; Allen, C.K.; Jolly, J.P.; Brown, M.N.; Yu, P.; Yu, Y. Characteristics of Patients for Whom Benznidazole Was Released Through the CDC-Sponsored Investigational New Drug Program for Treatment of Chagas Disease—United States, 2011–2018. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 803–805. [Google Scholar] [CrossRef]
- Manne-Goehler, J.; Umeh, C.A.; Montgomery, S.P.; Wirtz, V.J. Estimating the Burden of Chagas Disease in the United States. PLoS Negl. Trop. Dis. 2016, 10, e0005033. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, C.; Meymandi, S.; Moss, I.; Cone, J.; Cohen, R.; Batista, C. Proposed multidimensional framework for understanding Chagas disease healthcare barriers in the United States. PLoS Negl. Trop. Dis. 2019, 13, e0007447. [Google Scholar] [CrossRef] [PubMed]
- Sales Junior, P.A.; Molina, I.; Fonseca Murta, S.M.; Sánchez-Montalvá, A.; Salvador, F.; Corrêa-Oliveira, R.; Carneiro, C.M. Experimental and Clinical Treatment of Chagas Disease: A Review. Am. J. Trop. Med. Hyg. 2017, 97, 1289–1303. [Google Scholar] [CrossRef]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Segovia, J. Un caso de tripanosomiasis. Arch. Hosp. Rosales 1913, 8, 249–254. [Google Scholar]
- Cedillos, R.A.; Romero, J.E.; Sasagawa, E. Elimination of Rhodnius prolixus in El Salvador, Central America. Mem. Inst. Oswaldo Cruz 2012, 107, 1068–1069. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sasagawa, E.; Aguilar, A.V.; Ramirez, M.A.; Chevez, J.E.; Nakagawa, J.; Cedillos, R.A.; Kita, K. Acute Chagas disease in El Salvador 2000–2012—need for surveillance and control. Mem. Inst. Oswaldo Cruz 2014, 109, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Sasagawa, E.; Aiga, H.; Corado Soriano, E.Y.; Cuyuch Marroquín, B.L.; Hernández Ramírez, M.A.; Guevara de Aguilar, A.V.; Romero Chévez, J.E.; Ramos Hernández, H.M.; Cedillos, R.A.; Misago, C.; et al. Mother-to-Child Transmission of Chagas Disease in El Salvador. Am. J. Trop. Med. Hyg. 2015, 93, 326–333. [Google Scholar] [CrossRef]
- Sasagawa, E.; Guevara de Aguilar, A.V.; Hernández de Ramírez, M.A.; Romero Chévez, J.E.; Nakagawa, J.; Cedillos, R.A.; Misago, C.; Kita, K. Prevalence of Trypanosoma cruzi infection in blood donors in El Salvador between 2001 and 2011. J. Infect. Dev. Ctries. 2014, 8, 1029–1036. [Google Scholar] [CrossRef][Green Version]
- Sasagawa, E.; Aiga, H.; Corado, E.Y.; Cuyuch, B.L.; Hernández, M.A.; Guevara, A.V.; Romero, J.E.; Ramos, H.M.; Cedillos, R.A.; Misago, C.; et al. Risk factors for Chagas disease among pregnant women in El Salvador. Trop. Med. Int. Health 2015, 20, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Galindo, V.D.; Marín Recinos, M.F.; Gámez Hidalgo, S.A.; Recinos Paredes, G.; Posada Vaquerano, E.E.; Romero Magaña, A.L.; Castillo Ayala, A.K. Morphological variability and ecological characterization of the Chagas disease vector Triatoma dimidiata (Hemiptera: Reduviidae) in El Salvador. Acta Trop. 2020, 205, 105392. [Google Scholar] [CrossRef]
- Yoshioka, K.; Provedor, E.; Manne-Goehler, J. The resilience of Triatoma dimidiata: An analysis of reinfestation in the Nicaraguan Chagas disease vector control program (2010–2016). PLoS ONE 2018, 13, e0202949. [Google Scholar] [CrossRef]
- Censos Nacionales de El Salvador: Censo de Poblacion y Vivienda. Available online: http://www.digestyc.gob.sv/ (accessed on 25 December 2020).
- The World Factbook 2020. Available online: https://www.cia.gov/library/publications/resources/the-world-factbook/index.html (accessed on 26 December 2020).
- Demographic Yearbook 2019. Available online: https://unstats.un.org/unsd/demographic-social/products/dyb/dybsets/2019.pdf (accessed on 25 December 2020).
- Bustamante Zamora, D.M.; Hernández, M.M.; Torres, N.; De Abrego, V.; Monroy Escobar, M.C.; Zúniga, C.; Sosa, W. Information to Act: Household Characteristics are Predictors of Domestic Infestation with the Chagas Vector Triatoma dimidiata in Central America. Am. J. Trop. Med. Hyg. 2015, 93, 97–107. [Google Scholar] [CrossRef]
- Naceanceno, K.S.; Matamoros, G.; Gabrie, J.A.; Bottazzi, M.E.; Sanchez, A.; Mejia, R. Use of Multi-Parallel Real-Time Quantitative PCR to Determine Blastocystis Prevalence and Association with Other Gastrointestinal Parasite Infection in a Rural Honduran Location. Am. J. Trop. Med. Hyg. 2020, 102, 1373–1375. [Google Scholar] [CrossRef] [PubMed]
- Monsalve Álvarez, J.M.; González Zapata, L.I. [Development of questionnaire to assess food intake in the University of Antioquia, Colombia]. Nutr Hosp. 2011, 26, 1333–1344. [Google Scholar] [CrossRef]
- Guía alimentaria para las familias salvadoreñas; Ministerio de Salud de El Salvador: San Salvador, El Salvador, 2012.
- Cedillos, R.A.; Romero Chévez, J.E.; Ramos Hernández, H.M.; Sasagawa, E. La Enfermedad de Chagas en El Salvador, Evolución Histórica y Desafíos para el Control; Organización Panamericana de la Salud (OPS): San Salvador, El Salvador, 2010; p. 64. [Google Scholar]
- Lima-Cordón, R.A.; Stevens, L.; Solórzano Ortíz, E.; Rodas, G.A.; Castellanos, S.; Rodas, A.; Abrego, V.; Zúniga Valeriano, C.; Monroy, M.C. Implementation science: Epidemiology and feeding profiles of the Chagas vector Triatoma dimidiata prior to Ecohealth intervention for three locations in Central America. PLoS Negl. Trop. Dis. 2018, 12, e0006952. [Google Scholar] [CrossRef] [PubMed]
- JA, D.E.F.-V.; Cabrera-Bravo, M.; Enríquez-Vara, J.N.; Bucio-Torres, M.I.; Gutiérrez-Cabrera, A.E.; Vidal-López, D.G.; Martínez-Ibarra, J.A.; Salazar-Schettino, P.M.; Córdoba-Aguilar, A. Relationships between altitude, triatomine (Triatoma dimidiata) immune response and virulence of Trypanosoma cruzi, the causal agent of Chagas’ disease. Med. Vet. Entomol. 2017, 31, 63–71. [Google Scholar] [CrossRef]
- Hashimoto, K.; Zúniga, C.; Romero, E.; Morales, Z.; Maguire, J.H. Determinants of Health Service Responsiveness in Community-Based Vector Surveillance for Chagas Disease in Guatemala, El Salvador, and Honduras. PLoS Negl. Trop. Dis. 2015, 9, e0003974. [Google Scholar] [CrossRef] [PubMed]
- Soto López, J.D.; Monroy, M.C.; Dorn, P.; Castellanos, S.; Lima, R.; Rodas, A. Effect of community education in an integrate control for Triatoma dimidiata (Hemiptera: Reduviidae). Rev. Cuba. Med. Trop. 2019, 71, e380. [Google Scholar]
- Cahan, S.H.; Orantes, L.C.; Wallin, K.F.; Hanley, J.P.; Rizzo, D.M.; Stevens, L.; Dorn, P.L.; Rodas, A.; Monroy, C. Residual survival and local dispersal drive reinfestation by Triatoma dimidiata following insecticide application in Guatemala. Infect. Genet. Evol. 2019, 74, 104000. [Google Scholar] [CrossRef]
- Monroy, C.; Bustamante, D.M.; Pineda, S.; Rodas, A.; Castro, X.; Ayala, V.; Quiñónes, J.; Moguel, B. House improvements and community participation in the control of Triatoma dimidiata re-infestation in Jutiapa, Guatemala. Cad. Saude Publica 2009, 25 (Suppl. 1), S168–S178. [Google Scholar] [CrossRef][Green Version]
- Castro-Arroyave, D.; Monroy, M.C.; Irurita, M.I. Integrated vector control of Chagas disease in Guatemala: A case of social innovation in health. Infect. Dis. Poverty 2020, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Aiga, H.; Chévez, J.E.R.; Zúniga, C.; Nakagawa, J.; Hernández, H.M.R.; Hashimoto, K.; Tabaru, Y.; Sasagawa, E.; Nakamura, J. Chagas Disease: Assessing the Existence of a Threshold for Bug Infestation Rate. Am. J. Trop. Med. Hyg. 2012, 86, 972–979. [Google Scholar] [CrossRef]
- Valeriano, Z.C. Ruta inversa proyecto chagas BID. Status (unpublished). 17 March 2021. [Google Scholar]
- Faria, A.R.; Nunes, J.B.; Leite, A.L.L.; Ramos, A.; Siqueira, R.V.; Nogueira, E.S.C.; Marques, M.J.; Colombo, F.A. Risk of Trypanosoma cruzi transmission in southern Minas Gerais, Brazil—Data from 2014 to 2020. Vet. Parasitol. Reg. Stud. Rep. 2021, 23, 100530. [Google Scholar] [CrossRef]
- Jaimes-Dueñez, J.; Triana-Chávez, O.; Cantillo-Barraza, O.; Hernández, C.; Ramírez, J.D.; Góngora-Orjuela, A. Molecular and serological detection of Trypanosoma cruzi in dogs (Canis lupus familiaris) suggests potential transmission risk in areas of recent acute Chagas disease outbreaks in Colombia. Prev. Vet. Med. 2017, 141, 1–6. [Google Scholar] [CrossRef]
- Klein, M.D.; Proaño, A.; Noazin, S.; Sciaudone, M.; Gilman, R.H.; Bowman, N.M. Risk Factors for Vertical Transmission of Chagas Disease: A Systematic Review and Meta-analysis. Int. J. Infect. Dis. 2021, 105, 357–373. [Google Scholar] [CrossRef]
- Black, R.E. Would control of childhood infectious diseases reduce malnutrition? Acta Paediatr. Scand. Suppl. 1991, 374, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Campisi, S.C.; Cherian, A.M.; Bhutta, Z.A. World Perspective on the Epidemiology of Stunting between 1990 and 2015. Horm. Res. Paediatr. 2017, 88, 70–78. [Google Scholar] [CrossRef]
- De Andrade, A.L.; Zicker, F. Chronic malnutrition and Trypanosoma cruzi infection in children. J. Trop. Pediatr. 1995, 41, 112–115. [Google Scholar] [CrossRef]
- Malafaia, G.; Talvani, A. Nutritional Status Driving Infection by Trypanosoma cruzi: Lessons from Experimental Animals. J. Trop. Med. 2011, 2011, 981879. [Google Scholar] [CrossRef][Green Version]
- Boia, M.N.; Motta, L.P.D.; Salazar, M.D.S.P.; Mutis, M.P.S.; Coutinho, R.B.A.; Coura, J.R. Estudo das parasitoses intestinais e da infecção chagásica no Município de Novo Airão, Estado do Amazonas, Brasil. Cad. Saúde Pública 1999, 15, 497–504. [Google Scholar] [CrossRef]
- Ramos, J.M.; Leon, R.; Andreu, M.; De Las Parras, E.R.; Rodriguez-Diaz, J.C.; Esteban, A.; Saugar, J.M.; Torrus, D. Serological study of Trypanosoma cruzi, Strongyloides stercoralis, HIV, human T cell lymphotropic virus (HTLV) and syphilis infections in asymptomatic Latin-American immigrants in Spain. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Salvador, F.; Sulleiro, E.; Sánchez-Montalvá, A.; Martínez-Gallo, M.; Carrillo, E.; Molina, I. Impact of Helminth Infection on the Clinical and Microbiological Presentation of Chagas Diseases in Chronically Infected Patients. PLoS Negl. Trop. Dis. 2016, 10, e0004663. [Google Scholar] [CrossRef]
- Puerta-Alcalde, P.; Gomez-Junyent, J.; Requena-Mendez, A.; Pinazo, M.J.; Álvarez-Martínez, M.J.; Rodríguez, N.; Gascon, J.; Muñoz, J. High prevalence of S. Stercoralis infection among patients with Chagas disease: A retrospective case-control study. PLoS Negl. Trop. Dis. 2018, 12, e0006199. [Google Scholar] [CrossRef] [PubMed]
- Dopico, E.; Rando-Matos, Y.; Solsona, L.; Almeda, J.; Santos, F.L.N.; Vinuesa, T. Infection by Strongyloides stercoralis in immigrants with Chagas disease: Evaluation of eosinophilia as screening method in primary care. Trop. Med. Int. Health 2020, 25, 467–474. [Google Scholar] [CrossRef]
- Salvador, F.; Sulleiro, E.; Piron, M.; Sánchez-Montalvá, A.; Sauleda, S.; Molina-Morant, D.; Moure, Z.; Molina, I. Strongyloides stercoralis infection increases the likelihood to detect Trypanosoma cruzi DNA in peripheral blood in Chagas disease patients. Trop Med. Int. Health 2017, 22, 1436–1441. [Google Scholar] [CrossRef]
- Chieffi, P.P. Interrelationship between Schistosomiasis and concomitant diseases. Mem Inst. Oswaldo Cruz 1992, 87, 291–296. [Google Scholar] [CrossRef]
- Overgaauw, P.A.; van Knapen, F. Veterinary and public health aspects of Toxocara spp. Vet. Parasitol 2013, 193, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Santarém, V.A.; Franco Eda, C.; Kozuki, F.T.; Fini, D.; Prestes-Carneiro, L.E. Environmental contamination by Toxocara spp. eggs in a rural settlement in Brazil. Rev. Inst. Med. Trop. Sao Paulo 2008, 50, 279–281. [Google Scholar] [CrossRef]
- Simonato, G.; Cassini, R.; Morelli, S.; Di Cesare, A.; La Torre, F.; Marcer, F.; Traversa, D.; Pietrobelli, M.; Frangipane di Regalbono, A. Contamination of Italian parks with canine helminth eggs and health risk perception of the public. Prev. Vet. Med. 2019, 172, 104788. [Google Scholar] [CrossRef]
- Tyungu, D.L.; McCormick, D.; Lau, C.L.; Chang, M.; Murphy, J.R.; Hotez, P.J.; Mejia, R.; Pollack, H. Toxocara species environmental contamination of public spaces in New York City. PLoS Negl. Trop. Dis. 2020, 14, e0008249. [Google Scholar] [CrossRef]
- Kirchheimer, R.; Jacobs, D.E. Toxocara species egg contamination of soil from children’s play areas in southern England. Vet. Rec. 2008, 163, 394–395. [Google Scholar] [CrossRef] [PubMed]
- Harvey, T.V.; Tang, A.M.; da Paixao Sevá, A.; Albano Dos Santos, C.; Santos Carvalho, S.M.; Magalhães da Rocha, C.M.B.; Oliveira, B.C.M.; Albuquerque, G.R. Enteric parasitic infections in children and dogs in resource-poor communities in northeastern Brazil: Identifying priority prevention and control areas. PLoS Negl. Trop. Dis. 2020, 14, e0008378. [Google Scholar] [CrossRef]
- Cociancic, P.; Zonta, M.L.; Navone, G.T. A cross-sectional study of intestinal parasitoses in dogs and children of the periurban area of La Plata (Buenos Aires, Argentina): Zoonotic importance and implications in public health. Zoonoses Public Health 2018, 65, e44–e53. [Google Scholar] [CrossRef] [PubMed]
- Altcheh, J.; Moscatelli, G.; Mastrantonio, G.; Moroni, S.; Giglio, N.; Marson, M.E.; Ballering, G.; Bisio, M.; Koren, G.; García-Bournissen, F. Population pharmacokinetic study of benznidazole in pediatric Chagas disease suggests efficacy despite lower plasma concentrations than in adults. PLoS Negl. Trop. Dis. 2014, 8, e2907. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, N.; Moroni, S.; Moscatelli, G.; Ballering, G.; Jurado, I.; Falk, N.; Bochoeyer, A.; Goldsman, A.; Freilij, H.; Chatelain, E.; et al. Early antiparasitic treatment prevents progression of Chagas disease: Results of a long-term cardiological follow-up in a pediatric population. Am. J. Trop. Med. Hyg. 2020, 103, 110. [Google Scholar]
- Hotez, P.J.; Fenwick, A.; Molyneux, D.H. Collateral Benefits of Preventive Chemotherapy—Expanding the War on Neglected Tropical Diseases. N. Engl. J. Med. 2019, 380, 2389–2391. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J. Could Nitazoxanide Be Added to Other Essential Medicines for Integrated Neglected Tropical Disease Control and Elimination? PLoS Negl. Trop. Dis. 2014, 8, e2758. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.M. Chagas Disease: Epidemiology and Barriers to Treatment. Am. J. Med. 2020, 133, 1262–1265. [Google Scholar] [CrossRef] [PubMed]

| Chagas Disease Seronegative N = 1049 (%) | Chagas Disease Seropositive N = 25 (%) | Univariate Logistic Regression Odds Ratio (95% Confidence Interval) | |
|---|---|---|---|
| Participant characteristics | |||
| Female | 559 (53%) | 11 (44%) | |
| Participant age (median) † | 8 years (range: 9 months to 18 years) | 13 years (range: 5 to 18 years) | 1.17 (1.07 to 1.27) * |
| Pediatric patient was febrile at time of enrollment | 328 (31%) | 8 (32%) | |
| Patient had other acute clinical symptoms | 375 (36%) | 9 (36%) | |
| Household occupancy and composition | |||
| Number living in the household (median) | 5 (range: 2 to 14) | 6 (range: 4 to 31) | 1.10 (1.03 to 1.17) ¶ |
| Number always living in the household (median) | 4 (range: 1 to 14) | 5 (range: 2 to 31) | |
| Number of kids in household (median) † | 2 (range 1 to 8) | 3 (range: 1 to 9) | 1.36 (1.12 to 1.65) ¶ |
| Number of school-aged children | 1 (range 0 to 5) | 2 (range: 0 to 6) | 1.76 (1.32 to 2.35) * |
| Number of women of childbearing age (median) | 1 (range: 0 to 5) | 1 (range: 0 to 7) | |
| Number of currently pregnant women (median) | 0 (range: 0 to 1) | 0 (range: 0 to 1) | |
| Mother’s last year of education (median) | 5 (range: 0 to 15) | 2 (range: 0 to 12) | 0.82 (0.72 to 0.94) ¶ |
| Father’s last year of education (median) | 6 (range: 0 to 15) | 5 (range: 0 to 8) | 0.89 (0.80 to 0.99) § |
| Household poverty indicators | |||
| Agriculture as primary household income source | 613/966 (63%) | 16 (64%) | |
| Household receives remesas | 59 (6%) | 1 (4%) | |
| Number of beds in home (median) | 4 (range: 2 to 14) | 5 (range: 0 to 16) | 1.19 (1.05 to 1.34) ¶ |
| Bare earth floor in bedroom | 820 (78%) | 20 (80%) | |
| Adobe wall material in bedroom | 443/1047 (42%) | 14 (56%) | |
| Leña cooking fuel type | 145/1047 (14%) | 4 (16%) | |
| Potable water in the house | 919 (88%) | 22 (88%) | |
| Family uses outdoor latrine for bathroom | 961 (92%) | 25 (100%) | |
| House has electricity | 656/1035 (64%) | 12 (48%) | |
| House has fowl (chickens or turkeys) | 544 (52%) | 14/24 (58%) | |
| House has dogs † | 533/1011 (53%) | 19/24 (79%) | 3.41 (1.27 to 9.20) § |
| House has fowl and dogs | 281/1011 (28%) | 9/24 (37.5%) | |
| Household triatomine exposure and parent or legal guardian Chagas disease knowledge | |||
| Knows what a “chinche” is | 716 (68%) | 15 (60%) | |
| Has seen chinches inside their house within the past year | 295/716 (41%) | 9/15 (60%) | |
| Someone in house has been bitten by a chinche in the past year | 143/714 (20%) | 3/15 (20%) | |
| Your house has been fumigated in past year | 107/1047 (10%) | 1/25 (4%) | |
| Knows about Chagas disease | 200/1047 (19%) | 4/25 (16%) | |
| Know someone that has or had Chagas disease | 43/200 (22%) | 0/4 (0%) | |
| Other household health concerns | |||
| Someone in your house sought clinical care in the past year | 714/1027 (70%) | 15/23 (65%) | |
| Household member(s) who sought clinic care in the past year went for an infection origin | 404/714 (57%) | 10/15 (67%) | |
| Someone in your household took medicine for intestinal parasites in the past year | 732 (69%) | 21/25 (84%) | |
| Urban N = 58 (%) | Rural N = 96 (%) | Univariate p-Value OR (95% CI) | |
|---|---|---|---|
| Child’s health status and concerns | |||
| Child’s age at time of survey (median) | 9 years (range: 1–18 years) | 8 years (range: 1–18 years) | 0.374 |
| Child’s mother reported prenatal complications | 5 (9%) | 12 (13%) | 0.480 |
| Child was breastfed | 53 (92%) | 85 (89%) | 0.577 |
| Length mother breastfed (median) | 16.5 months (range: 2–48 months) | 17.5 months (range: 2–48 months) | 0.996 |
| Number of times child was sick last year (median) | 0 (range: 0–4 times) | 0 (range: 0–4 times) | 0.812 |
| Child was febrile at time of survey | 14 (24%) | 7 (7%) | 0.005 0.25 (0.09–0.66) |
| Child was Chagas disease seropositive | 2 (3%) | 1 (1%) | 0.332 |
| Child with suspected GI parasitic infection | 11 (19%) | 19 (20%) | 0.586 |
| Mother has health concerns for child | 12 (21%) | 18 (19%) | 0.768 |
| Mother has growth and development concerns for child | 3 (5%) | 3 (3%) | 0.529 |
| Mother has educational concerns for child | 6 (10%) | 14 (15%) | 0.450 |
| Mother has safety concerns for child | 1 (2%) | 4 (4%) | 0.422 |
| Household and participant’s hygienic practices | |||
| Dirt floor in child’s bedroom | 39 (67%) | 59 (61%) | 0.546 |
| Child washes hands with soap before eating * | 58 (100%) | 91 (95%) | - |
| Child washes hands with soap after restroom * | 58 (100%) | 94 (98%) | - |
| Child bathes 3+ times per week | 58 (100%) | 93 (97%) | 0.743 |
| Household uses a refrigerator for food storage | 20 (34%) | 42 (44%) | 0.694 |
| Household uses well water for drinking * | 0 (0%) | 55 (57%) | - |
| Household uses well water for cooking * | 0 (0%) | 56 (58%) | - |
| Household nutrition and food insecurity | |||
| Family eats the same thing each day | 37 (64%) | 58 (60%) | 0.860 |
| Majority of consumed food is grown at home | 5 (9%) | 26 (27%) | 0.011 3.77 (1.35–10.51) |
| Mother familiar with nutritional guidelines | 9 (15%) | 2 (2%) | 0.012 0.13 (0.03–0.64) |
| Child went 1+ day(s) without a meal last week | 4 (7%) | 7 (7%) | 0.022 0.20 (0.05–0.79) |
| Family went 1+ day(s) without a meal last month | 3 (5%) | 13 (14%) | 0.438 |
| Mother worries about family’s next meal | 37 (64%) | 75 (78%) | 0.072 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nolan, M.S.; Murray, K.O.; Mejia, R.; Hotez, P.J.; Villar Mondragon, M.J.; Rodriguez, S.; Palacios, J.R.; Murcia Contreras, W.E.; Lynn, M.K.; Torres, M.E.; et al. Elevated Pediatric Chagas Disease Burden Complicated by Concomitant Intestinal Parasites and Malnutrition in El Salvador. Trop. Med. Infect. Dis. 2021, 6, 72. https://doi.org/10.3390/tropicalmed6020072
Nolan MS, Murray KO, Mejia R, Hotez PJ, Villar Mondragon MJ, Rodriguez S, Palacios JR, Murcia Contreras WE, Lynn MK, Torres ME, et al. Elevated Pediatric Chagas Disease Burden Complicated by Concomitant Intestinal Parasites and Malnutrition in El Salvador. Tropical Medicine and Infectious Disease. 2021; 6(2):72. https://doi.org/10.3390/tropicalmed6020072
Chicago/Turabian StyleNolan, Melissa S., Kristy O. Murray, Rojelio Mejia, Peter J. Hotez, Maria Jose Villar Mondragon, Stanley Rodriguez, Jose Ricardo Palacios, William Ernesto Murcia Contreras, M. Katie Lynn, Myriam E. Torres, and et al. 2021. "Elevated Pediatric Chagas Disease Burden Complicated by Concomitant Intestinal Parasites and Malnutrition in El Salvador" Tropical Medicine and Infectious Disease 6, no. 2: 72. https://doi.org/10.3390/tropicalmed6020072
APA StyleNolan, M. S., Murray, K. O., Mejia, R., Hotez, P. J., Villar Mondragon, M. J., Rodriguez, S., Palacios, J. R., Murcia Contreras, W. E., Lynn, M. K., Torres, M. E., & Monroy Escobar, M. C. (2021). Elevated Pediatric Chagas Disease Burden Complicated by Concomitant Intestinal Parasites and Malnutrition in El Salvador. Tropical Medicine and Infectious Disease, 6(2), 72. https://doi.org/10.3390/tropicalmed6020072








