Antibiotic Use in Broiler Poultry Farms in Kathmandu Valley of Nepal: Which Antibiotics and Why?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. General Setting
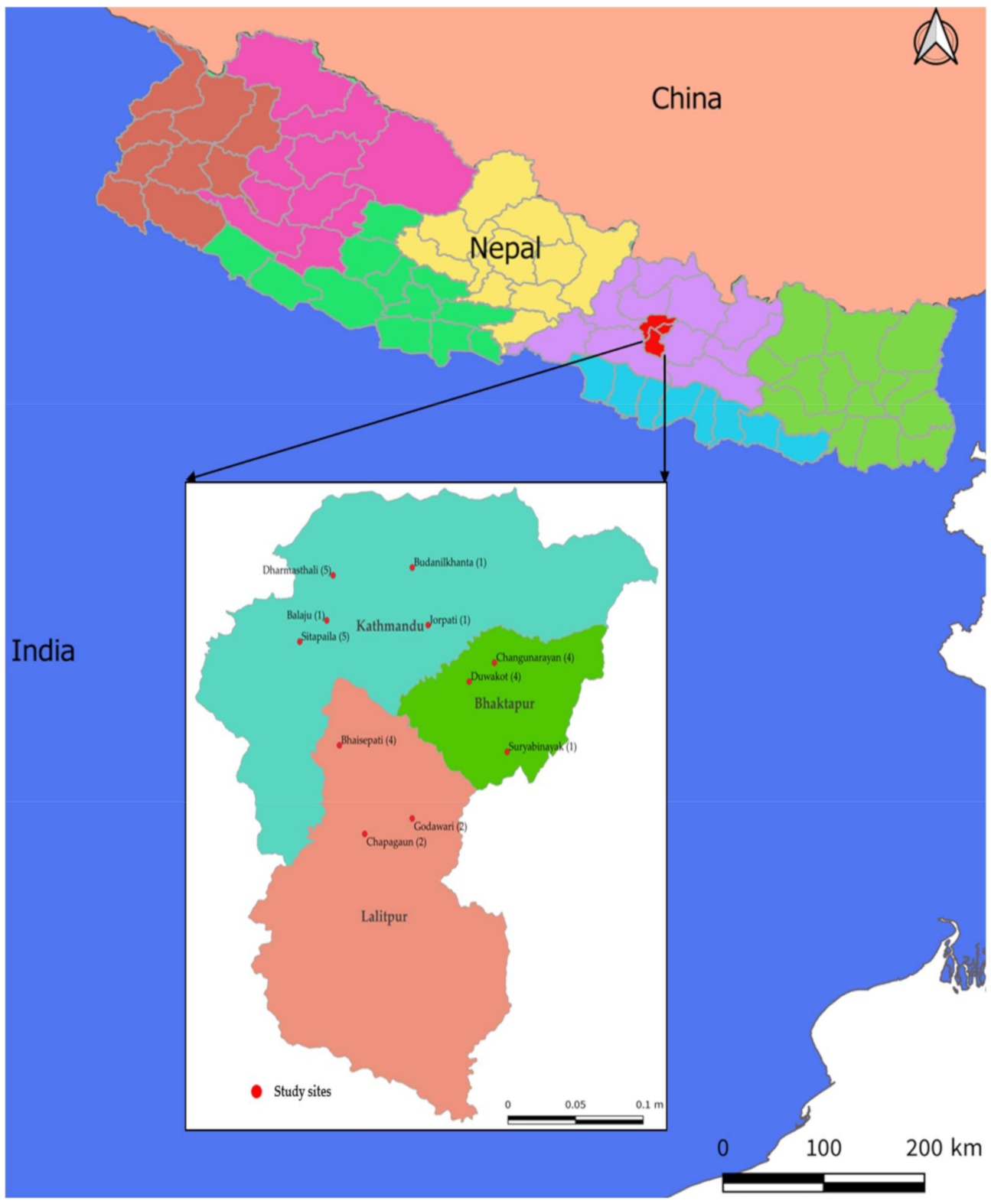
2.3. Specific Setting: Broiler Poultry Farms in the Study Districts of the Kathmandu Valley
2.4. Study Inclusion and Period
2.5. Data Collection, Sources of Data, and Statistical Analysis
3. Results
3.1. Prevalence, Classes, and Types of Antibiotics Used
3.2. Quantities Consumed and Withdrawal Periods
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Open Access Statement and Disclaimer
References
- World Health Organization. Global Action Plan on Antimicrobial Resistance. 2015. Available online: https://www.who.int/antimicrobial-resistance/global-action-plan/en/ (accessed on 24 August 2020).
- Tang, K.L.; Caffrey, N.P.; Nóbrega, D.B.; Cork, S.C.; Ronksley, P.E.; Barkema, H.W.; Polachek, A.J.; Ganshorn, H.; Sharma, N.; Kellner, J.D.; et al. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: A systematic review and meta-analysis. Lancet Planet. Health 2017, 1, e316–e327. [Google Scholar] [CrossRef]
- Zellweger, R.M.; Carrique-Mas, J.; Limmathurotsakul, D.; Day, N.P.J.; Thwaites, G.E.; Baker, S.; Ashley, E.; De Balogh, K.; Baird, K.; Basnyat, B.; et al. A current perspective on antimicrobial resistance in Southeast Asia. J. Antimicrob. Chemother. 2017, 72, 2963–2972. [Google Scholar] [CrossRef] [PubMed]
- Darwish, W.S.; Eldaly, A.E.; El-Abbasy, M.T.; Ikenaka, Y.; Nakayama, S.; Ishizuka, M. Antibiotic residues in food: The African scenario. Jpn. J. Vet. Res. 2013, 61, S13–S22. [Google Scholar] [PubMed]
- World Health Organization. WHO Guidelines on Use of Medically Important Antimicrobials in Food Producing Animals. 2017. Available online: https://apps.who.int/iris/bitstream/handle/10665/259243/WHO-NMH-FOS-FZD-17.5-eng.pdf?sequence=1 (accessed on 25 January 2021).
- World Health Organization. Highest Priority Critically Important Antimicrobials. 2019. Available online: https://www.who.int/foodsafety/cia/en/ (accessed on 25 January 2021).
- Marshall, B.M.; Levy, S.B. Food Animals and Antimicrobials: Impacts on Human Health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, P.M.; Loureiro, L.; Matos, A.J.F. Transfer of Multidrug-Resistant Bacteria between Intermingled Ecological Niches: The Interface between Humans, Animals and the Environment. Int. J. Environ. Res. Public Health 2013, 10, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Lundborg, C.S.; Tamhankar, A.J. Antibiotic residues in the environment of South East Asia. BMJ 2017, 358, j2440. [Google Scholar] [CrossRef] [PubMed]
- Landers, T.F.; Cohen, B.; Wittum, T.E.; Larson, E.L. A Review of Antibiotic Use in Food Animals: Perspective, Policy, and Potential. Public Health Rep. 2012, 127, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Acharya, K.P.; Wilson, R.T. Antimicrobial Resistance in Nepal. Front. Med. 2019, 6, 105. [Google Scholar] [CrossRef] [PubMed]
- Raut, R.; Mandal, R.K.; Kaphle, K.; Pant, D.; Nepali, S.; Shrestha, A. Assessment of antibiotic residues in the marketed meat of Kailali and Kavre of Nepal. Int. J. Appl. Sci. Biotechnol. 2017, 5, 386–389. [Google Scholar] [CrossRef]
- MOAD. Report of the Ministry of Agricultural Development; Government of Nepal: Kathmandu, Nepal, 2015. [Google Scholar]
- World Health Organization. Critically Important Antimicrobials for Human Medicine, 6th Revision. 2019. Available online: https://www.who.int/foodsafety/publications/antimicrobials-sixth/en/ (accessed on 28 January 2021).
- Guo, Y.R.; Cao, Q.D.; Hong, Z.S.; Tan, Y.Y.; Chen, S.D.; Jin, H.J.; Tan, K.S.; Wang, D.Y.; Yan, Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak: An update on the status. Mil. Med. Res. 2020, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Barroga, T.R.M.; Morales, R.G.; Benigno, C.C.; Castro, S.J.M.; Caniban, M.M.; Cabullo, M.F.B.; Agunos, A.; De Balogh, K.; Dorado-Garcia, A. Antimicrobials Used in Backyard and Commercial Poultry and Swine Farms in the Philippines: A Qualitative Pilot Study. Front. Vet. Sci. 2020, 7, 329. [Google Scholar] [CrossRef] [PubMed]
- Van Cuong, N.; Phu, D.H.; Van, N.T.B.; Truong, B.D.; Kiet, B.T.; Hien, B.V.; Thu, H.T.V.; Choisy, M.; Padungtod, P.; Thwaites, G.; et al. High-Resolution Monitoring of Antimicrobial Consumption in Vietnamese Small-Scale Chicken Farms Highlights Discrepancies Between Study Metrics. Front. Vet. Sci. 2019, 6, 174. [Google Scholar] [CrossRef] [PubMed]
- Nhung, N.T.; Cuong, N.V.; Thwaites, G.; Carrique-Mas, J. Antimicrobial Usage and Antimicrobial Resistance in Animal Production in Southeast Asia: A Review. Antibiotics 2016, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Consolidated Guidelines on Drug-Resistant Tuberculosis Treatment. 2019. Available online: https://www.who.int/tb/publications/2019/consolidated-guidelines-drug-resistant-TB-treatment/en/ (accessed on 25 January 2021).
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; Initiative, F.T.S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Bull. World Health Organ. 2007, 85, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Emborg, H.-D.; Ersbøll, A.K.; Heuer, O.E.; Wegener, H.C. The effect of discontinuing the use of antimicrobial growth promoters on the productivity in the Danish broiler production. Prev. Vet. Med. 2001, 50, 53–70. [Google Scholar] [CrossRef]
- Wierup, M. The Swedish Experience of the 1986 Year Ban of Antimicrobial Growth Promoters, with Special Reference to Animal Health, Disease Prevention, Productivity, and Usage of Antimicrobials. Microb. Drug Resist. 2001, 7, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Global Antibiotic Resistance Partnership. Situation Analysis and Recommendations. Antibiotic Use and Resistance in Nepal. 2015. Available online: https://cddep.org/wp-content/uploads/2017/08/garp-nepal_sa.pdf (accessed on 9 February 2021).
| Districts in Kathmandu Valley | p-Value 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Kathmandu | Bhaktapur | Lalitpur | Total | ||||||
| n | (%) | n | (%) | n | (%) | n | (%) | ||
| Total Farms | 13 | 9 | 8 | 30 | |||||
| Farms that used Antibiotics | 10 | (77) | 9 | (100) | 8 | (100) | 27 | (90) | |
| Total Grams of Antibiotics Used 3 | 22,000 | 17,000 | 11,000 | 50,000 | |||||
| Mean Grams per Chicken/45 Days (SD) | 0.6 | (0.5) | 0.6 | (0.2) | 0.4 | (0.4) | 0.5 | (0.3) | ≤0.01 |
| Antibiotics for Prophylaxis | 4 | (44) | 2 | (25) | 6 | (22) | |||
| Fluoroquinolones: Enrofloxacin | - | - | 1 | (11) | - | - | 1 | (4) | |
| Aminoglycosides: Gentamicin | - | - | - | - | 1 | (13) | 1 | (4) | |
| Combination: Neomycin+Doxycycline 4 | - | - | 3 | (33) | 1 | (13) | 4 | (15) | |
| Mean Grams per Chicken/45 Days(SD) | 0.7 | (0.1) | 0.5 | (0.2) | 0.7 | (0.1) | 0.2 | ||
| Antibiotics for treatment 5 | 10 | (100) | 5 | (56) | 6 | (75) | 21 | (78) | |
| Macrolides: Tylosin | 7 | (54) | 3 | (33) | 4 | (50) | 14 | (47) | |
| Polymyxin: Colistin Sulphate | 6 | (46) | 4 | (44) | 4 | (50) | 14 | (47) | |
| Sulphonamides | 1 | (8) | 4 | (44) | - | - | 5 | (17) | |
| Aminoglycosides: Gentamycin | 2 | (15) | - | - | 2 | (25) | 4 | (13) | |
| Fluoroquinolones: Enrofloxacin | 1 | (8) | 1 | (11) | 1 | (13) | 3 | (10) | |
| Fluoroquinolones: Ciprofloxacin | 1 | (8) | - | - | - | - | 1 | (3) | |
| Combination: Neomycin+Doxycycline 6 | 2 | (15) | 5 | (56) | 3 | (38) | 10 | (33) | |
| Mean Grams per Chicken/45 Days(SD) | 0.6 | (0.6) | 0.4 | (0.1) | 0.7 | (0.1) | 0.5 | (0.3) | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koirala, A.; Bhandari, P.; Shewade, H.D.; Tao, W.; Thapa, B.; Terry, R.; Zachariah, R.; Karki, S. Antibiotic Use in Broiler Poultry Farms in Kathmandu Valley of Nepal: Which Antibiotics and Why? Trop. Med. Infect. Dis. 2021, 6, 47. https://doi.org/10.3390/tropicalmed6020047
Koirala A, Bhandari P, Shewade HD, Tao W, Thapa B, Terry R, Zachariah R, Karki S. Antibiotic Use in Broiler Poultry Farms in Kathmandu Valley of Nepal: Which Antibiotics and Why? Tropical Medicine and Infectious Disease. 2021; 6(2):47. https://doi.org/10.3390/tropicalmed6020047
Chicago/Turabian StyleKoirala, Ananta, Priyanka Bhandari, Hemant Deepak Shewade, Wenjing Tao, Badri Thapa, Robert Terry, Rony Zachariah, and Surendra Karki. 2021. "Antibiotic Use in Broiler Poultry Farms in Kathmandu Valley of Nepal: Which Antibiotics and Why?" Tropical Medicine and Infectious Disease 6, no. 2: 47. https://doi.org/10.3390/tropicalmed6020047
APA StyleKoirala, A., Bhandari, P., Shewade, H. D., Tao, W., Thapa, B., Terry, R., Zachariah, R., & Karki, S. (2021). Antibiotic Use in Broiler Poultry Farms in Kathmandu Valley of Nepal: Which Antibiotics and Why? Tropical Medicine and Infectious Disease, 6(2), 47. https://doi.org/10.3390/tropicalmed6020047









