Abstract
Rickettsia typhi and Bartonella henselae are the causative agents of murine typhus and cat-scratch disease, respectively. A small-scale survey (N = 202) was conducted in the Attica region, Greece, for determining the prevalence rates of IgG antibodies against B. henselae and R. typhi by indirect fluorescence antibody test. IgG against B. henselae and R. typhi were present in 17.8% (36/202) and 4.5% (9/202) of the participants, respectively; co-occurring IgG against both B. henselae and R. typhi were detected in 3.5% (7/202), whereas only anti-B. henselae IgG in 14.3% (29/202), and only anti-R. typhi IgG in 1.0% (2/202). Titres 1/64, 1/128, 1/256, and 1/512, of anti-B. henselae IgG were identified in 6.4%, 4.5%, 4.5%, and 2.4%, whereas titres 1/40 and 1/80 of anti-R. typhi IgG were detected in 4.0%, and 0.5%, respectively. A positive association of anti-B. henselae IgG prevalence with a coastal area featuring a major seaport (p = 0.009) and with younger age (p = 0.046) was identified. The findings of this survey raise concern for exposure of the population of Attica to B. henselae and R. typhi, which should be considered in the differential diagnosis when compatible symptoms are present. Our results also suggest that seaports may represent high-risk areas for exposure to Bartonella spp.
1. Introduction
Among flea-borne pathogens, Rickettsia typhi and Bartonella henselae are causative agents of murine typhus and cat-scratch disease, well described disease entities, for which conventional serological tests are widely available [1].
Murine (endemic) typhus frequently appears as a non-eschar forming malaise, with variable manifestations commonly including fever, headache and rash [2]. Infection is frequently acquired through exposure to rat-fleas (Xenopsylla cheopis) [3,4]. However, an urban transmission cycle of R. typhi, involving companion animals, especially cats, and cosmopolitan pet flea species (Ctenocephalides spp.) has also been recognized [5].
Cat-scratch disease is manifested as a sub-acute febrile regional lymphadenopathy, usually spontaneously resolving from two weeks to four months. Immunocompromised patients may develop severe vasoproliferative tumor-like lesions. Chronic sequelae, such as endocarditis, arthritis, endophthalmitis, neuroretinitis, and neurologic disorders, may occur [6,7]. B. henselae is maintained in asymptomatic bacteremic cats for prolonged period and subsequently acquired by fleas during blood meals [6]. Transmission of B. henselae occurs through direct skin inoculation of the pathogen by cat claws; however, exposure to Ctenocephalides spp. has been strongly suggested as another potential route of infection [6,8,9]. B. henselae may cause chronic infections and the bacterial DNA has been detected in saliva of cats and dogs, nevertheless, other transmission pathways from those animal species to humans have been suggested but not demonstrated [1,8,10,11,12].
In this study, we examined sera of residents of Attica in Greece for IgG antibodies against R. typhi and B. henselae and investigated potential risk factors for association with seropositivity.
2. Materials and Methods
The Attica region comprises eight regional units with a total area of 3808.10 km2, encompasses Athens, the country’s capital and largest city, and has a population of 3,756,453. The participants of the study were recruited among Attica residents visiting primary care biopathology laboratories for routine check-up or referred by a physician, during a 23-months period (March 2017–January 2019). The examinees were informed for the purpose of the study and voluntarily consented in written form for inclusion, completed a questionnaire and provided a blood sample.
The collected information included age, gender, location of residence, profession, farming or gardening activities, subjective perception of insect bites excluding mosquitoes, and contact with pets. Those having contact with pets were additionally surveyed for consistent implementation of flea control regime according to the attending veterinarian and visual detection of fleas on the animals. The purpose of visiting the biopathology laboratory and the health status of the participants, were not surveyed.
Approximately two mL of separated sera from blood drawn from a venipuncture, after centrifugation at 4000 × g for at least five minutes at room temperature, was collected from each participant and was stored at −20 °C. Each sample was tested with two commercial indirect immunofluorescence antibody test (IFAT) for detecting IgG antibodies, against B. henselae and R. typhi, respectively (Vircell™, S.L., Granada, Spain). The tests were manufactured with B. henselae genotype I (ATCC 49882/ Houston-1 strain) and R. typhi (ATCC VR-738/ Philip. Strain 18), grown in Vero cells. Tests were performed according to the manufacturer’s instructions. The examined serum was subjected in two-fold dilutions and inspected under a UV microscope, at 400× magnification; an apple green fluorescence was indicative of a reactive serum dilution. The end point dilution demonstrating fluorescence was the outcome of the assay (titre). Dilutions started from as low as 1/40 and 1/64, reaching a theoretical maximum of 1/640 and 1/1024, for R. typhi and B. henselae, respectively. Positive and negative controls were included in each run. A second independent observer repeated the microscopy examination to minimize bias; potential conflicts were resolved by a third observer. Statistical analysis was performed with chi-square test, binomial logistic regression and t-test, as necessary, using the commercially available software IBM SPSS Statistics for Windows, Version 20.0 (Armonk, NY, USA). The alpha level was set to 5%.
The study protocol was approved by the Bioethics Committee of the National and Kapodistrian University of Athens, Medical School (approval reference no: 1415022715/2015).
3. Results
3.1. Study Subjects
Examined sera corresponded to 202 individuals with a male to female ratio of 0.64 (N = 202) and a mean age of 51 years (95%CI:49–54); six, 20, 67 and 109 of the participants were allocated to the age groups of 2–14, 15–29, 30–50 and >50 years, respectively (N = 201).
The majority of the examinees resided in the regional unit of Central Athens (42.6%), followed by those residing in North Athens (27.7%), Piraeus (17.3%), South Athens (6.4%), East Attica (4.5%), West Attica (1.0%) and West Athens (0.5%) (N = 202); all residencies were located in urban areas of Attica, including the Athens metropolitan area. The regional unit of Islands (less than 2% of Attica population) was not represented in this study.
Contact with companion animals was reported by 59.7% (120/201) of the participants; 24.9% (50/201) with dogs, 9.9% (20/201) with cats, 24.9% (50/201) with both dogs and cats; Among subjects with companion animal contact (N = 120), consistent administration of anti-flea treatment was implemented by 60.7% (71/117), whereas presence of fleas on the animals was perceived from 39.0% (46/118). Profession related with outdoor activities was reported by 5.1% (10/195). Gardening and/or farming was practiced by 39.0% (73/187). Insect bites, excluding mosquitoes, were subjectively perceived by 29.0% (58/200).
3.2. IgG Antibodies Prevalence Rates
IgG antibodies against B. henselae and R. typhi were identified in 17.8% (36/202) and 4.5% (9/202) of the participants, respectively. IgG solely against B. henselae were present in 14.3% (29/202), whereas solely against R. typhi in 1.0% (2/202). Co-occurring IgG against both B. henselae and R. typhi were detected in 3.5% (7/202). Titres 1/64, 1/128, 1/256, and 1/512, of anti-B. henselae IgG were identified in 6.4%, 4.5%, 4.5%, and 2.4%, whereas titres 1/40 and 1/80 of anti-R. typhi IgG were detected in 4.0%, and 0.5%, respectively (Table 1 and Supplementary Materials).

Table 1.
IgG antibodies against Bartonella henselae and Rickettsia typhi by Gender, Age Group and Contact with Companion Animals (N = 202).
3.3. Risk Factors
The residents of the regional unit of Pireaus were associated with significantly increased seropositivity for B. henselae IgG (66.7%; 14/35) (χ2(6) = 17.737, p = 0.009). The highest prevalence for R. typhi IgG (14.3%; 5/35) was also observed in Pireaus; however, it did not reach statistical significance (p > 0.05) (Figure 1 and Supplementary Materials).
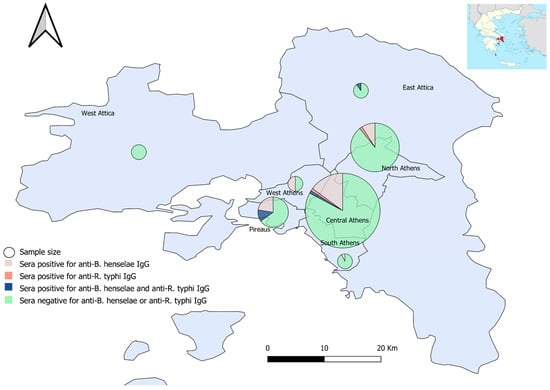
Figure 1.
Sera sampling (size proportionate circles) and seropositivity percentages for anti-B. henselae and anti-R. typhi IgG antibodies, per Regional Unit, Attica, Greece.
The B. henselae seropositive individuals had decreased mean age by 8.2 years (95%CI: −14.8 to −1.5) compared to seronegative ones (t (199) = 2.412, p = 0.017). A binomial logistic regression model revealed that for every additional year of age the likelihood for IgG antibodies detection was marginally decreased (OR = 0.976; 95%CI: 0.957 to 0.996), (χ2(1) = 5.705, p = 0.017).
A tendency for cooccurrence of R. typhi and B. henselae was observed with higher odds for R. typhi seropositivity in individuals identified with B. henselae IgG than in seronegative ones (p = 0.011; OR = 19.8).
The prevalence of R. typhi IgG and the magnitude of titres for B. henselae or R. typhi were not affected by any of the examined factors.
4. Discussion
In this serological survey we examined the prevalence and magnitude of IgG antibodies against B. henselae and R. typhi, using a commercial IFAT. This is the first study of IgG antibodies against R. typhi and B. henselae in Attica region in Greece, which includes the capital city of Athens.
The seroprevalence rates of IgG antibodies against B. henselae (17.8%) and R. typhi (4.5%) concur with previous studies conducted in the general population. Tea et al. [13] reported 19.8% seropositivity for B. henselae IgG in Greece. Variable rates were described in other countries; 15.0% in Korea, 23.0% in Poland, 9.6 to 19.6% in China, and 8.7% in Spain [14,15,16,17].
Anti-R. typhi IgG antibodies were identified at a rate of 2.0% in a previous study in Greece [18]. Rates of 3.9%, 1.3%, and 4.1% were reported in the Canary Islands of Spain, New Zealand, and China, respectively [19,20,21].
In our study, the prevalence of anti-B. henselae IgG antibodies was positively affected by younger age and a specific location of residence (Pireaus). A previous study in Greece reported increased seroprevalence at age groups of 2–14 and of 30–50 years [13] and thus concurs with our findings.
In the present study, significantly increased IgG seroreactivity for B. henselae was detected in the regional unit of Pireaus, a coastal area of Attica, featuring the biggest passenger and commercial seaport in Greece. To our knowledge, no previous studies investigated residency at seaport areas as potential risk factor for human exposure to Bartonella spp. Kosoy et al. [22] suggested that influx of imported rats by ships causes a higher Bartonella spp. infection rate in rats located in seaports compared with the ones in mainland. Xenopsylla cheopis, the rat-flea, may harbor Bartonella spp. [23] and although with a preference for feeding from rats, it may readily bite humans. Furthermore, the most common flea, Ctenocephalides felis (cat flea), has been found to parasitize rats [24]. We hypothesize that frequent introduction of new infected rats in the seaport of Pireaus, leads to higher density of infectious fleas for Bartonella spp. and increased probability of human exposure. This is further supported by the fact that the animal-related factors of our study failed to explain antibody occurrence, suggesting a universal source of infection, such as the ubiquitous fleas. As IFAT is unable to distinguish B. henseleae from species commonly identified in rodents such as B. tribocorum or B. elizabethae [22,25], exposure cannot be attributed to specific Bartonella species. Rat population size, and other ecological factors may also have an impact in the flea-borne Bartonella spp. load.
Our results did not support any modeling of anti-R. typhi IgG antibodies. However, residents of Pireaus presented the highest seroreactivity rate (p > 0.05), which is in agreement with previous reports of increased prevalence of seropositivity in human populations of seaport areas, mainly attributed to rat dynamics [26]. In a previous Greek study, higher mean age and agricultural professions were predisposing factors for anti-R. typhi IgG [18]. Bolaños-Rivero et al. reported an increased rate of R. typhi IgG to individuals older than 46 years and to those with farming activities [19]. However, our study subjects were mostly residents of urban areas with less chance of outdoor activities than the previously studied population.
The participants of our study were recruited among people visiting biopathology laboratories. However, no information regarding health status or the purpose of visit was collected. As this study focused on IgG antibodies indicating past exposure, background health-related information or the reason of presentation to the laboratory would offer limited benefit and might introduce recall bias. Similarly, seeking volunteers among people presenting in laboratories for ordering diagnostic tests, would not introduce significant bias for the scope of the particular study.
Regarding the used laboratory technique, IFAT, is considered the reference serology method for B. henselae and R. typhi [27], nevertheless, is subject to interpretation bias. We addressed this by validating the results by a second and in the case of disagreement, by a third examiner; however, no discrepancies occurred during the slide evaluation. However, IFAT is prone to false positives due to cross-reactions. Cross-reactivity of B. henselae with IgG antibodies against Coxiella burnetii, Chlamydia pneumoniae, Erlichia chaffeensis and non-henselae Bartonella [28,29], as well as between R. typhi and species of the typhus group or of the spotted fever group [30], has been well described. Discrimination among species is particularly important for R. typhi and Rickettsia felis or Rickettsia felis-like organisms, as the latter two are commonly present in C. felis fleas [30,31,32,33]. Western Blot assay and cross-absorption studies could assist in differentiating of IgG among Bartonella and Rickettsia species and could provide definite serological diagnosis [30,34]; however, due to limited resources, these techniques were not used in our study.
Our observations are based on limited data and lack validation with additional confirmatory techniques; hence, more molecular and seroepidemiologic studies focused on B. henselae and R. typhi, and particularly the role of fleas in human exposure, are required.
5. Conclusions
In summary, our results suggest exposure of the population of Attica to B. henselae and, to a lesser extent, to R. typhi. Seaports may represent high-risk areas for exposure to Bartonella spp. Physicians should consider B. henselae and R. typhi when evaluating patients with compatible symptoms.
Supplementary Materials
The spreadsheet with the relevant with the study data of all participants is available online at https://www.mdpi.com/2414-6366/5/3/145/s1, Table S1: Supplementary data.
Author Contributions
Conceptualization, G.D. and M.M.; methodology, G.D. and M.M.; software, G.D.; validation, G.D., J.P., C.B., and M.M.; formal analysis, G.D.; investigation, G.D.; resources, I.P., A.T., J.P. and C.B.; data curation, G.D.; writing—original draft preparation, G.D., M.M., J.P., and C.B.; writing—review and editing, G.D., M.M., J.P., A.T., and C.B.; visualization, G.D.; supervision, J.P., C.B., and A.T.; project administration, M.M., and J.P.; funding acquisition, A.T., and C.B. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by the University of Thessaly (Special Account for Research Grants).
Acknowledgments
We are deeply thankful to Margarita Nikolaki, Eirini Kavvadia, Georgios Kolovos, Athanassios Kolovos, Eleni Tzoumakidou, Christos Katrivesis, and Konstantinos Ladias, for their assistance in sample collection and immense contribution for the realization of this study.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Álvarez-Fernández, A.; Breitschwerdt, E.B.; Solano-Gallego, L. Bartonella infections in cats and dogs including zoonotic aspects. Parasit Vectors 2018, 11, 624. [Google Scholar] [CrossRef] [PubMed]
- Tsioutis, C.; Zafeiri, M.; Avramopoulos, A.; Prousali, E.; Miligkos, M.; Karageorgos, S.A. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: A systematic review. Acta Trop 2017, 166, 16–24. [Google Scholar] [CrossRef]
- Mouffok, N.; Parola, P.; Raoult, D. Murine typhus, Algeria. Emerg. Infect. Dis. 2008, 14, 676–678. [Google Scholar] [CrossRef]
- Eremeeva, M.E.; Warashina, W.R.; Sturgeon, M.M.; Buchholz, A.E.; Olmsted, G.K.; Park, S.Y.; Effler, P.V.; Karpathy, S.E. Rickettsia typhiand R.felis in Rat Fleas (Xenopsylla cheopis), Oahu, Hawaii. Emerg Infect. Dis 2008, 14, 1613–1615. [Google Scholar] [CrossRef]
- Civen, R.; Ngo, V. Murine Typhus: An Unrecognized Suburban Vectorborne Disease. Clin. Infect. Dis. 2008, 46, 913–918. [Google Scholar] [CrossRef]
- Cheslock, M.A.; Embers, M.E. Human Bartonellosis: An Underappreciated Public Health Problem? Trop. Med. Infect. Dis. 2019, 4, 69. [Google Scholar] [CrossRef]
- Barros, S.; Andrade, G.C.D.; Cavalcanti, C.; Nascimento, H. Cat Scratch Disease: Not a Benign Condition. Ocul. Immunol. Inflamm. 2017, 26, 1115–1122. [Google Scholar] [CrossRef]
- Mosbacher, M.E.; Klotz, S.; Klotz, J.; Pinnas, J.L. Bartonella henselae and the Potential for Arthropod Vector-Borne Transmission. Vector Borne Zoonotic Dis. 2011, 11, 471–477. [Google Scholar] [CrossRef]
- Bouhsira, E.; Ferrandez, Y.; Liu, M.; Franc, M.; Boulouis, H.J.; Biville, F. Ctenocephalides felis an in vitro potential vector for five Bartonella species. Comp. Immunol. Microbiol. Infect. Dis. 2013, 36, 105–111. [Google Scholar] [CrossRef]
- Chomel, B.B.; Boulouis, H.J.; Breitschwerdt., E.B. Cat scratch disease and other zoonotic Bartonella infections. J. Am. Vet. Med. Assoc. 2004, 224, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Chomel, B.B.; Kasten, R.W.; Stuckey, M.J.; Breitschwerdt, E.B.; Maggi, R.G.; Henn, J.B.; Koehler, J.E.; Chang, C.C. Experimental infection of cats with Afipia felis and various Bartonella species or subspecies. Vet. Microbiol. 2014, 172, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Diniz, P.P.; Maggi, R.G.; Schwartz, D.S.; Cadenas, M.B.; Bradley, J.M.; Hegarty, B.; Breitschwerdt, E.B. Canine bartonellosis: Serological and molecular prevalence in Brazil and evidence of co-infection with Bartonella henselae and Bartonella vinsonii subsp.berkhoffii. Vet. Res. 2007, 38, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Tea, A.; Arvanitidou, M.; Antoniadis, A.; Diza, E.; Alexiou-Daniel, S. Occurrence of Bartonella Henselae And Bartonella Quintana In A Healthy Greek Population. Am. J. Trop. Med. Hyg. 2003, 68, 554–556. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.Y.; Im, J.H.; Lee, S.M.; Baek, J.H.; Durey, A.; Park, S.G.; Kang, J.S.; Lee, J.S. The seroprevalence of Bartonella henselae in healthy adults in Korea. Korean J. Intern. Med. 2017, 32, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Łysakowska, M.E.; Brzezińska, O.; Szybka, M.; Konieczka, M.; Moskwa, S.; Brauncajs, M.; Makowska, J.; Pastuszak-Lewandoska, D.; Grzegorczyk, J. The seroprevalence of Bartonella spp. in the blood of patients with musculoskeletal complaints and blood donors, Poland: A pilot study. Clin. Rheumatol 2019, 38, 2691–2698. [Google Scholar]
- Liu, Q.; Eremeeva, M.E.; Li, D. Bartonella and Bartonella infections in China: From the clinic to the laboratory. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 93–102. [Google Scholar] [CrossRef]
- Pons, I.; Sanfeliu, I.; Cardeñosa, N.; Nogueras, M.M.; Font, B.; Segura, F. Serological evidence of Bartonella henselaeinfection in healthy people in Catalonia, Spain. Epidemiol. Infect. 2008, 136, 1712–1716. [Google Scholar] [CrossRef]
- Daniel, S.A.; Manika, K.; Arvanmdou, M.; Antoniadis, A. Prevalence of Rickettsia conorii and Rickettsia typhi infections in the population of northern Greece. Am. J. Trop. Med. Hyg. 2002, 66, 76–79. [Google Scholar] [CrossRef]
- Bolaños-Rivero, M.; Santana-Rodriguez, É.; Ángel-Moreno, A.; Hernández-Cabrera, M.; Limiñana-Canal, J.M.; Carranza-Rodríguez, C.; Antonio-Manuel Martín-Sánchez, A.M.; Pérez-Arellano, J.L. Seroprevalence of Rickettsia typhi and Rickettsia conorii infections in the Canary Islands. Int. J. Infect. Dis. 2011, 15, e481–e485. [Google Scholar] [CrossRef]
- Lim, M.Y.; Weinstein, P.; Bell, A.; Hambling, T.; Tompkins, D.M.; Slaney, D. Seroprevalence of antibodies to Rickettsia typhi in the Waikato region of New Zealand. Epidemiol. Infect. 2016, 144, 2283–2289. [Google Scholar] [CrossRef][Green Version]
- Zhang, L.; Shan, A.; Mathew, B.; Yin, J.; Fu, X.; Zhang, J.; Lu, J.; Xu, J.; Dumler, J.S. Rickettsial Seroepidemiology among Farm Workers, Tianjin, People’s Republic of China. Emerg. Infect. Dis. 2008, 14, 938–940. [Google Scholar] [CrossRef] [PubMed]
- Kosoy, M.; Bai, Y. Bartonella Bacteria in Urban Rats: A Movement from the Jungles of Southeast Asia to Metropoles Around the Globe. Front. Ecol. Evol. 2019, 7, 88. [Google Scholar] [CrossRef]
- Billeter, S.A.; Gundi, V.A.; Rood, M.P.; Kosoy, M.Y. Molecular Detection and Identification of Bartonella Species in Xenopsylla cheopis Fleas (Siphonaptera: Pulicidae) Collected from Rattus norvegicus Rats in Los Angeles, California. Appl. Environ. Microbiol. 2011, 77, 7850–7852. [Google Scholar] [CrossRef] [PubMed]
- Psaroulaki, A.; Papaeustathiou, A.; Loukaides, F.; Tselentis, Y.; Antoniou, M.; Toumazos, P. First Detection of Rickettsia Felis In Ctenocephalides Felis Fleas Parasitizing Rats in Cyprus. Am. J. Trop. Med. Hyg. 2006, 74, 120–122. [Google Scholar] [CrossRef]
- Gutiérrez, R.; Krasnov, B.; Morick, D.; Gottlieb, Y.; Khokhlova, I.S.; Harrus, S. Bartonella infection in rodents and their flea ectoparasites: An overview. Vector Borne Zoonotic Dis. 2015, 15, 27–39. [Google Scholar]
- Kuo, C.-C.; Wardrop, N.; Chang, C.-T.; Wang, H.-C.; Atkinson, P.M. Significance of major international seaports in the distribution of murine typhus in Taiwan. PLoS Negl. Trop. Dis. 2017, 11, e0005589. [Google Scholar]
- Mane, A.; Kamble, S.; Singh, M.K.; Ratnaparakhi, M.; Nirmalkar, A.; Gangakhedkar, R. Seroprevalence of spotted fever group and typhus group rickettsiae in individuals with acute febrile illness from Gorakhpur, India. Int. J. Infect. Dis. 2019, 79, 195–198. [Google Scholar] [CrossRef]
- Vermeulen, M.J.; Verbakel, H.; Notermans, D.W.; Reimerink, J.H.J.; Peeters, M.F. Evaluation of sensitivity, specificity and cross-reactivity in Bartonella henselae serology. J. Med. Microbiol. 2010, 59, 743–745. [Google Scholar] [CrossRef]
- Costa, P.S.G.D.; Brigatte, M.E.; Greco, D.B. Antibodies to Rickettsia rickettsii, Rickettsia typhi, Coxiella burnetii, Bartonella henselae, Bartonella quintana, and Ehrlichia chaffeensis among healthy population in Minas Gerais, Brazil. Mem. Inst. Oswaldo Cruz 2005, 100, 853–859. [Google Scholar] [CrossRef]
- Teoh, Y.T.; Hii, S.F.; Stevenson, M.A.; Graves, S.; Rees, R.; Stenos, J.; Traub, R.J. Serological evidence of exposure to Rickettsia felis and Rickettsia typhi in Australian veterinarians. Parasit Vectors 2017, 10, 129. [Google Scholar] [CrossRef]
- Maina, A.N.; Fogarty, C.; Krueger, L.; Macaluso, K.R.; Odhiambo, A.; Nguyen, K.; Farris, C.M.; Luce-Fedrow, A.; Bennett, S.; Ju, J.J.; et al. Rickettsial Infections among Ctenocephalides felis and Host Animals during a Flea-Borne Rickettsioses Outbreak in Orange County, California. PLoS ONE 2016, 11, e0160604. [Google Scholar] [CrossRef] [PubMed]
- Persichetti, M.; Solano-Gallego, L.; Serrano, L.; Altet, L.; Reale, S.; Masucci, M.; Pennisi, M. Detection of vector-borne pathogens in cats and their ectoparasites in southern Italy. Parasit. Vectors 2016, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Nogueras, M.; Pons, I.; Ortuño, A.; Lario, S.; Segura, F. Rickettsia felis in Fleas from Catalonia (Northeast Spain). Vector Borne Zoonotic Dis. 2011, 11, 479–483. [Google Scholar] [CrossRef]
- Edouard, S.; Nabet, C.; Lepidi, H.; Fournier, P.; Raoult, D. Bartonella, a Common Cause of Endocarditis: A Report on 106 Cases and Review. J. Clin. Microbiol. 2014, 53, 824–829. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).