Ensuring the Safety of Yellow Fever Vaccination in Travelers—The Experience at a Large U.S. Academic Medical Center in Colorado
Abstract
1. Introduction
2. Methods
2.1. Ethics Statement
2.2. Patients and Data Collection
2.3. Statistical Analysis
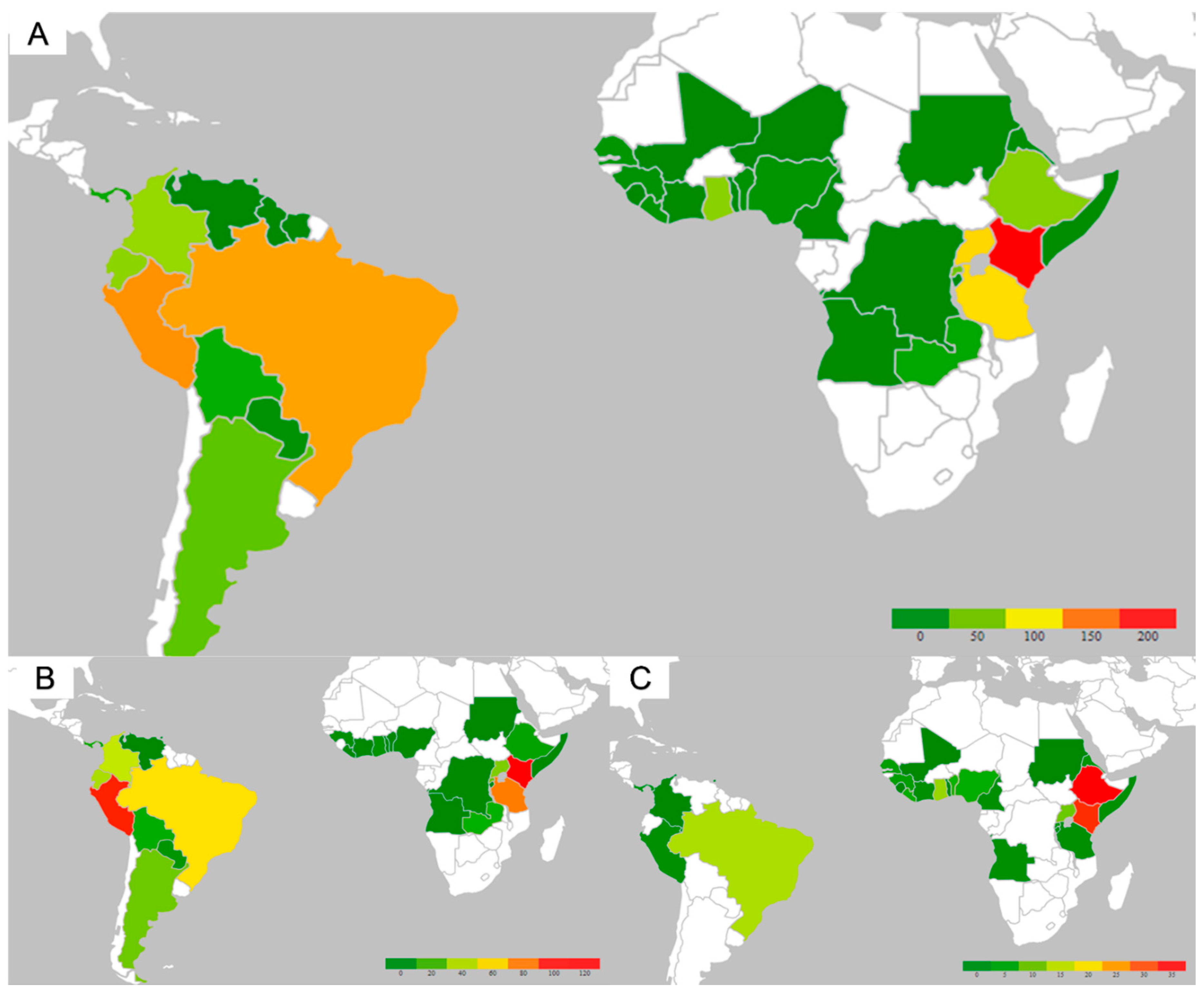
3. Results
3.1. Clinical Characteristics of Patients Receiving the Yellow Fever Vaccine:
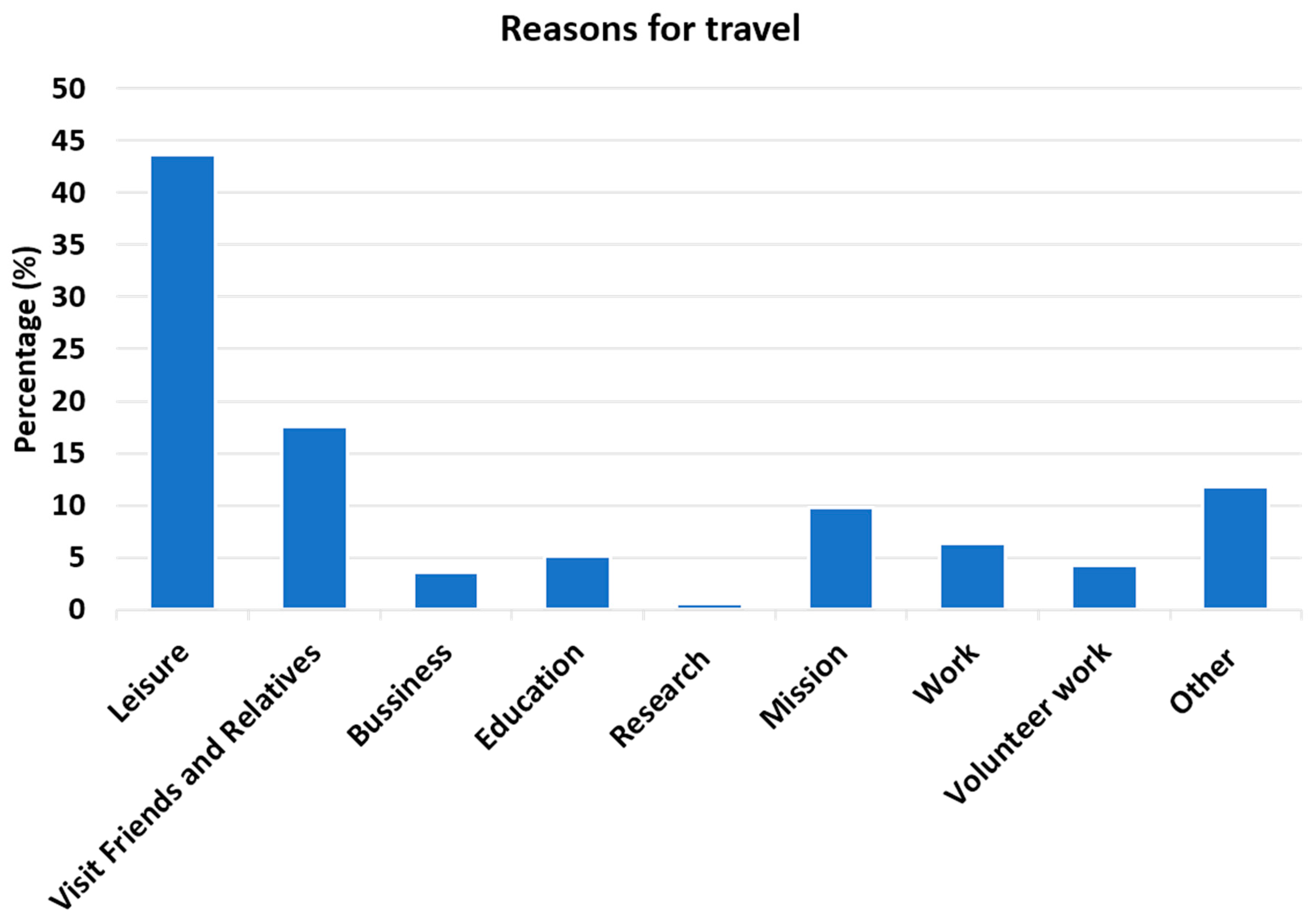
3.2. Travelers Visiting Friends and Relatives
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| ICD | ICD-Code | Diagnosis | Categories |
|---|---|---|---|
| ICD-9-CM | 250.8 | Xanthoma diabeticorum | DM |
| ICD-9-CM | 250.6 | Well controlled type 2 diabetes mellitus with peripheral neuropathy (HC code) | DM |
| ICD-9-CM | 250.7 | Well controlled type 2 diabetes mellitus with peripheral circulatory disorder (HC code) | DM |
| ICD-9-CM | 250 | Well controlled type 2 diabetes mellitus (HC code) | DM |
| ICD-9-CM | 250.61 | Well controlled type 1 diabetes mellitus with peripheral neuropathy (HC code) | DM |
| ICD-9-CM | 250.01 | Well controlled type 1 diabetes mellitus (HC code) | DM |
| ICD-9-CM | 250.5 | Visual loss due to diabetes mellitus (HC code) | DM |
| ICD-9-CM | 250.9 | Unspecified diabetes mellitus with unspecified complications | DM |
| ICD-9-CM | 250.02 | Uncontrolled type II diabetes mellitus with nephropathy | DM |
| ICD-9-CM | 250.92 | Uncontrolled type 2 diabetes mellitus with complication (HC code) | DM |
| ICD-9-CM | 249.6 | Ulnar neuropathy due to secondary DM (HC code) | DM |
| ICD-9-CM | 250.81 | Type I diabetes with complications | DM |
| ICD-10-CM | E11.9 | Type 2 diabetes mellitus without complications | DM |
| ICD-10-CM | E11.311 | Type 2 diabetes mellitus with unspecified diabetic retinopathy with macular edema | DM |
| ICD-10-CM | E11.8 | Type 2 diabetes mellitus with unspecified complications | DM |
| ICD-10-CM | E11.69 | Type 2 diabetes mellitus with other specified complication | DM |
| ICD-10-CM | E11.39 | Type 2 diabetes mellitus with other diabetic ophthalmic complication | DM |
| ICD-10-CM | E11.49 | Type 2 diabetes mellitus with other diabetic neurological complication | DM |
| ICD-10-CM | E11.59 | Type 2 diabetes mellitus with oth circulatory complications | DM |
| ICD-10-CM | E11.3393 | Type 2 diabetes mellitus with moderate nonproliferative diabetic retinopathy without macular edema, bilateral | DM |
| ICD-10-CM | E11.3293 | Type 2 diabetes mellitus with mild nonproliferative diabetic retinopathy without macular edema, bilateral | DM |
| ICD-10-CM | E11.3219 | Type 2 diabetes mellitus with mild nonproliferative diabetic retinopathy with macular edema, unspecified eye | DM |
| ICD-10-CM | E11.3213 | Type 2 diabetes mellitus with mild nonproliferative diabetic retinopathy with macular edema, bilateral | DM |
| ICD-10-CM | E11.649 | Type 2 diabetes mellitus with hypoglycemia without coma | DM |
| ICD-10-CM | E11.65 | Type 2 diabetes mellitus with hyperglycemia | DM |
| ICD-10-CM | E11.621 | Type 2 diabetes mellitus with foot ulcer | DM |
| ICD-10-CM | E11.42 | Type 2 diabetes mellitus with diabetic polyneuropathy | DM |
| ICD-10-CM | E11.40 | Type 2 diabetes mellitus with diabetic neuropathy, unsp | DM |
| ICD-10-CM | E11.610 | Type 2 diabetes mellitus with diabetic neuropathic arthropathy | DM |
| ICD-10-CM | E11.43 | Type 2 diabetes mellitus with diabetic autonomic (poly)neuropathy | DM |
| ICD-10-CM | E11.44 | Type 2 diabetes mellitus with diabetic amyotrophy | DM |
| ICD-10-CM | E11.3299 | Type 2 diab with mild nonp rtnop without macular edema, unsp | DM |
| ICD-10-CM | E10.9 | Type 1 diabetes mellitus without complications | DM |
| ICD-10-CM | E10.649 | Type 1 diabetes mellitus with hypoglycemia without coma | DM |
| ICD-10-CM | E10.65 | Type 1 diabetes mellitus with hyperglycemia | DM |
| ICD-10-CM | E13.44 | Other specified diabetes mellitus with diabetic amyotrophy | DM |
| ICD-9-CM | 359.1 | Wielander’s distal dystrophy (HC code) | Heme/Immune |
| ICD-9-CM | 287.2 | Vasculitis, hemorrhagic | Heme/Immune |
| ICD-9-CM | 287.5 | Unspecified thrombocytopenia | Heme/Immune |
| ICD-9-CM | 279.3 | Unspecified immunity deficiency | Heme/Immune |
| ICD-9-CM | 287.9 | Unspecified hemorrhagic conditions | Heme/Immune |
| ICD-9-CM | 279.9 | Unspecified disorder of immune mechanism | Heme/Immune |
| ICD-9-CM | 795.09 | Unexplained endometrial cells on cervical Pap smear | Heme/Immune |
| ICD-9-CM | 795.51 | Tuberculin test reaction | Heme/Immune |
| ICD-10-CM | D69.6 | Thrombocytopenia, unspecified | Heme/Immune |
| ICD-10-CM | D56.3 | Thalassemia minor | Heme/Immune |
| ICD-9-CM | 795.79 | Systemic lupus erythematosus inhibitor (HC code) | Heme/Immune |
| ICD-9-CM | 203 | Stage III multiple myeloma (HC code) | Neoplasms |
| ICD-10-CM | D57.3 | Sickle-cell trait | Heme/Immune |
| ICD-10-CM | D75.1 | Secondary polycythemia | Heme/Immune |
| ICD-10-CM | D86.3 | Sarcoidosis of skin | Heme/Immune |
| ICD-9-CM | 795.52 | Response to cell-mediated gamma interferon antigen without active tuberculosis | Heme/Immune |
| ICD-10-CM | D69.1 | Qualitative platelet defects | Heme/Immune |
| ICD-10-CM | D68.52 | Prothrombin gene mutation | Heme/Immune |
| ICD-9-CM | 279.49 | Progesterone dermatitis | Heme/Immune |
| ICD-9-CM | 795.05 | Positive cervical papilloma DNA test | Neoplasms |
| ICD-10-CM | Z86.73 | Personal history of transient ischemic attack (TIA), and cerebral infarction without residual deficits | Heme/Immune |
| ICD-10-CM | Z86.711 | Personal history of pulmonary embolism | Heme/Immune |
| ICD-10-CM | Z86.718 | Personal history of other venous thrombosis and embolism | Heme/Immune |
| ICD-10-CM | Z86.59 | Personal history of other mental and behavioral disorders | Heme/Immune |
| ICD-10-CM | Z92.89 | Personal history of other medical treatment | Heme/Immune |
| ICD-10-CM | Z86.19 | Personal history of other infectious and parasitic diseases | Heme/Immune |
| ICD-10-CM | Z86.69 | Personal history of other diseases of the nervous system and sense organs | Heme/Immune |
| ICD-10-CM | Z86.79 | Personal history of other diseases of the circulatory system | Heme/Immune |
| ICD-10-CM | Z86.018 | Personal history of other benign neoplasm | Neoplasms |
| ICD-10-CM | Z86.13 | Personal history of malaria | Heme/Immune |
| ICD-10-CM | Z86.008 | Personal history of in-situ neoplasm of other site | Heme/Immune |
| ICD-10-CM | Z86.39 | Personal history of endo, nutritional and metabolic disease | Heme/Immune |
| ICD-10-CM | Z86.2 | Personal history of diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism | Heme/Immune |
| ICD-10-CM | Z86.010 | Personal history of colonic polyps | Heme/Immune |
| ICD-10-CM | Z92.21 | Personal history of antineoplastic chemotherapy | Heme/Immune |
| ICD-9-CM | 795.03 | Papanicolaou smear of cervix with low grade squamous intraepithelial lesion (LGSIL) | Heme/Immune |
| ICD-9-CM | 795.01 | Papanicolaou smear of cervix with atypical squamous cells of undetermined significance (ASC-US) | Heme/Immune |
| ICD-9-CM | 795 | Papanicolaou smear - nonspecific abnormality | Heme/Immune |
| ICD-10-CM | D89.89 | Other specified disorders involving the immune mechanism, not elsewhere classified | Heme/Immune |
| ICD-10-CM | D64.89 | Other specified anemias | Heme/Immune |
| ICD-10-CM | D68.59 | Other primary thrombophilia | Heme/Immune |
| ICD-10-CM | D61.818 | Other pancytopenia | Heme/Immune |
| ICD-10-CM | D69.2 | Other nonthrombocytopenic purpura | Heme/Immune |
| ICD-10-CM | D50.8 | Other iron deficiency anemias | Heme/Immune |
| ICD-10-CM | D70.9 | Neutropenia, unspecified | Heme/Immune |
| ICD-10-CM | D70.3 | Neutropenia due to infection | Heme/Immune |
| ICD-10-CM | D50.9 | Iron deficiency anemia, unspecified | Heme/Immune |
| ICD-10-CM | D50.0 | Iron deficiency anemia secondary to blood loss (chronic) | Heme/Immune |
| ICD-10-CM | D84.9 | Immunodeficiency, unspecified | Heme/Immune |
| ICD-10-CM | D89.42 | Idiopathic mast cell activation syndrome | Heme/Immune |
| ICD-10-CM | D69.9 | Hemorrhagic condition, unspecified | Heme/Immune |
| ICD-10-CM | D72.0 | Genetic anomalies of leukocytes | Heme/Immune |
| ICD-10-CM | Z51.81 | Encounter for therapeutic drug level monitoring | Heme/Immune |
| ICD-10-CM | Z51.5 | Encounter for palliative care | Heme/Immune |
| ICD-10-CM | Z51.89 | Encounter for other specified aftercare | Heme/Immune |
| ICD-10-CM | Z51.12 | Encounter for antineoplastic immunotherapy | Heme/Immune |
| ICD-10-CM | Z51.11 | Encounter for antineoplastic chemotherapy | Heme/Immune |
| ICD-10-CM | D72.829 | Elevated white blood cell count, unspecified | Heme/Immune |
| ICD-10-CM | D72.9 | Disorder of white blood cells, unspecified | Heme/Immune |
| ICD-10-CM | D89.9 | Disorder involving the immune mechanism, unspecified | Heme/Immune |
| ICD-10-CM | D75.9 | Disease of blood and blood-forming organs, unspecified | Heme/Immune |
| ICD-10-CM | D72.819 | Decreased white blood cell count, unspecified | Heme/Immune |
| ICD-10-CM | D68.9 | Coagulation defect, unspecified | Heme/Immune |
| ICD-10-CM | D64.9 | Anemia, unspecified | Heme/Immune |
| ICD-10-CM | D68.51 | Activated protein C resistance | Heme/Immune |
| ICD-9-CM | V65.5 | Worried well | HIV |
| ICD-9-CM | V65.3 | Weight loss, intentional | HIV |
| ICD-9-CM | V65.40 | Visit for counseling | HIV |
| ICD-9-CM | V08 | Viruses, lymphadenopathy-associated | HIV |
| ICD-9-CM | V65.49 | Surgical counseling visit | HIV |
| ICD-9-CM | V65.8 | Reasons for seeking consultation | HIV |
| ICD-10-CM | B20 | Human immunodeficiency virus (HIV) disease | HIV |
| ICD-9-CM | V65.41 | Exercise counseling | HIV |
| ICD-10-CM | Z21 | Asymptomatic human immunodeficiency virus infection status | HIV |
| ICD-9-CM | 186.9 | Yolk Sac Tumour | Neoplasms |
| ICD-9-CM | 193 | Wuchernde struma langhans | Neoplasms |
| ICD-9-CM | 189 | WT (Wilms tumor) (HC code) | Neoplasms |
| ICD-9-CM | 210.2 | Warthin’s tumour | Neoplasms |
| ICD-10-CM | C88.0 | Waldenstrom macroglobulinemia | Neoplasms |
| ICD-9-CM | 184.4 | Vulvar malignant neoplasm (HC code) | Neoplasms |
| ICD-9-CM | 182 | Uterus neoplasms | Neoplasms |
| ICD-9-CM | 188.9 | Urinary bladder cancer (HC code) | Neoplasms |
| ICD-10-CM | C44.90 | Unspecified malignant neoplasm of skin, unspecified | Neoplasms |
| ICD-9-CM | 173.9 | Unspecified malignant neoplasm of skin, site unspecified | Neoplasms |
| ICD-10-CM | C44.40 | Unspecified malignant neoplasm of skin of scalp and neck | Neoplasms |
| ICD-9-CM | 173.4 | Unspecified malignant neoplasm of scalp and skin of neck | Neoplasms |
| ICD-9-CM | 201.9 | Unspecified Hodgkin’s disease, site unspecified, extranodal and solid organ sites | Neoplasms |
| ICD-9-CM | 162.9 | Undifferentiated carcinoma of lung (HC code) | Neoplasms |
| ICD-9-CM | 173.91 | Ulcers, rodent | Neoplasms |
| ICD-9-CM | 174.9 | Tubular carcinoma of breast (HC code) | Neoplasms |
| ICD-9-CM | 185 | Transitional cell carcinoma of prostate (HC code) | Neoplasms |
| ICD-9-CM | 145.9 | The mouth cancers | Neoplasms |
| ICD-9-CM | 162.3 | Syndromes, Pancoast’s | Neoplasms |
| ICD-9-CM | 173.99 | Sweat gland tumor, malignant | Neoplasms |
| ICD-9-CM | 172.9 | Superficial spreading melanoma (HC code) | Neoplasms |
| ICD-10-CM | D25.2 | Subserosal leiomyoma of uterus | Neoplasms |
| ICD-9-CM | 154.1 | Stage IV carcinoma of rectum (HC code) | Neoplasms |
| ICD-9-CM | 210.4 | Squamous papilloma of uvula | Neoplasms |
| ICD-9-CM | 173.62 | Squamous cell skin cancer, wrist | Neoplasms |
| ICD-9-CM | 173.42 | Squamous cell skin cancer, parietal | Neoplasms |
| ICD-9-CM | 173.92 | Squamous cell skin cancer | Neoplasms |
| ICD-9-CM | 173.9 | Squamous Cell Epithelioma | Neoplasms |
| ICD-10-CM | C44.92 | Squamous cell carcinoma of skin, unspecified | Neoplasms |
| ICD-10-CM | C44.42 | Squamous cell carcinoma of skin of scalp and neck | Neoplasms |
| ICD-10-CM | C44.622 | Squamous cell carcinoma of skin of right upper limb, including shoulder | Neoplasms |
| ICD-10-CM | C44.329 | Squamous cell carcinoma of skin of other parts of face | Neoplasms |
| ICD-10-CM | C44.321 | Squamous cell carcinoma of skin of nose | Neoplasms |
| ICD-9-CM | 154.3 | Squamous cell carcinoma of anus (HC code) | Neoplasms |
| ICD-9-CM | 173.41 | Skin cancer of scalp or skin of neck | Neoplasms |
| ICD-9-CM | 173.31 | Skin cancer of nose | Neoplasms |
| ICD-9-CM | 173.51 | Skin cancer of anterior chest | Neoplasms |
| ICD-9-CM | 197 | Secondary teratoma of lung (HC code) | Neoplasms |
| ICD-10-CM | C78.00 | Secondary malignant neoplasm of unspecified lung | Neoplasms |
| ICD-10-CM | C78.01 | Secondary malignant neoplasm of right lung | Neoplasms |
| ICD-10-CM | C78.02 | Secondary malignant neoplasm of left lung | Neoplasms |
| ICD-9-CM | 154 | Recurrent squamous cell carcinoma of colorectal region (HC code) | Neoplasms |
| ICD-9-CM | 173.61 | Recurrent basal cell carcinoma of shoulder | Neoplasms |
| ICD-9-CM | 174.2 | Primary malignant neoplasm of upper inner quadrant of female breast (HC code) | Neoplasms |
| ICD-10-CM | C44.99 | Other specified malignant neoplasm of skin, unspecified | Neoplasms |
| ICD-10-CM | D26.9 | Other benign neoplasm of uterus, unspecified | Neoplasms |
| ICD-10-CM | D23.9 | Other benign neoplasm of skin, unspecified | Neoplasms |
| ICD-10-CM | D23.60 | Other benign neoplasm of skin of unspecified upper limb, including shoulder | Neoplasms |
| ICD-10-CM | D23.30 | Other benign neoplasm of skin of unspecified part of face | Neoplasms |
| ICD-10-CM | D23.70 | Other benign neoplasm of skin of unspecified lower limb, including hip | Neoplasms |
| ICD-10-CM | D23.5 | Other benign neoplasm of skin of trunk | Neoplasms |
| ICD-10-CM | D23.4 | Other benign neoplasm of skin of scalp and neck | Neoplasms |
| ICD-10-CM | D23.71 | Other benign neoplasm of skin of right lower limb, including hip | Neoplasms |
| ICD-10-CM | D23.62 | Other benign neoplasm of skin of left upper limb, including shoulder | Neoplasms |
| ICD-9-CM | 210 | Neurofibroma of lip | Neoplasms |
| ICD-10-CM | D49.89 | Neoplasm of unspecified behavior of other specified sites | Neoplasms |
| ICD-10-CM | D49.7 | Neoplasm of unspecified behavior of endocrine glands and other parts of nervous system | Neoplasms |
| ICD-10-CM | D49.0 | Neoplasm of unspecified behavior of digestive system | Neoplasms |
| ICD-10-CM | D49.2 | Neoplasm of unspecified behavior of bone, soft tissue, and skin | Neoplasms |
| ICD-10-CM | D48.9 | Neoplasm of uncertain behavior, unspecified | Neoplasms |
| ICD-10-CM | D48.5 | Neoplasm of uncertain behavior of skin | Neoplasms |
| ICD-9-CM | 172.6 | Nail bed melanoma, upper extremity (HC code) | Neoplasms |
| ICD-10-CM | C90.00 | Multiple myeloma not having achieved remission | Neoplasms |
| ICD-10-CM | D47.2 | Monoclonal gammopathy | Neoplasms |
| ICD-9-CM | 172.5 | Melanoma, skin of trunk | Neoplasms |
| ICD-9-CM | 172.7 | Melanoma, skin of lower limb | Neoplasms |
| ICD-10-CM | D03.4 | Melanoma in situ of scalp and neck | Neoplasms |
| ICD-10-CM | D03.59 | Melanoma in situ of other part of trunk | Neoplasms |
| ICD-10-CM | D03.62 | Melanoma in situ of left upper limb, including shoulder | Neoplasms |
| ICD-10-CM | D22.9 | Melanocytic nevi, unspecified | Neoplasms |
| ICD-10-CM | D22.70 | Melanocytic nevi of unspecified lower limb, including hip | Neoplasms |
| ICD-10-CM | D22.60 | Melanocytic nevi of unsp upper limb, including shoulder | Neoplasms |
| ICD-10-CM | D22.5 | Melanocytic nevi of trunk | Neoplasms |
| ICD-10-CM | D22.4 | Melanocytic nevi of scalp and neck | Neoplasms |
| ICD-10-CM | D22.61 | Melanocytic nevi of right upper limb, including shoulder | Neoplasms |
| ICD-10-CM | D22.71 | Melanocytic nevi of right lower limb, including hip | Neoplasms |
| ICD-10-CM | D22.39 | Melanocytic nevi of other parts of face | Neoplasms |
| ICD-10-CM | D22.62 | Melanocytic nevi of left upper limb, including shoulder | Neoplasms |
| ICD-10-CM | D22.72 | Melanocytic nevi of left lower limb, including hip | Neoplasms |
| ICD-10-CM | C51.9 | Malignant neoplasm of vulva, unspecified | Neoplasms |
| ICD-10-CM | C55 | Malignant neoplasm of uterus, part unspecified | Neoplasms |
| ICD-9-CM | 174.4 | Malignant neoplasm of upper-outer quadrant of right female breast (HC code) | Neoplasms |
| ICD-10-CM | C50.412 | Malignant neoplasm of upper-outer quadrant of left female breast | Neoplasms |
| ICD-10-CM | C50.211 | Malignant neoplasm of upper-inner quadrant of right female breast | Neoplasms |
| ICD-10-CM | C34.12 | Malignant neoplasm of upper lobe, left bronchus or lung | Neoplasms |
| ICD-10-CM | C50.919 | Malignant neoplasm of unspecified site of unspecified female breast | Neoplasms |
| ICD-10-CM | C50.912 | Malignant neoplasm of unspecified site of left female breast | Neoplasms |
| ICD-10-CM | C34.90 | Malignant neoplasm of unspecified part of unspecified bronchus or lung | Neoplasms |
| ICD-10-CM | C34.92 | Malignant neoplasm of unspecified part of left bronchus or lung | Neoplasms |
| ICD-10-CM | C64.9 | Malignant neoplasm of unspecified kidney, except renal pelvis | Neoplasms |
| ICD-10-CM | C50.911 | Malignant neoplasm of unsp site of right female breast | Neoplasms |
| ICD-10-CM | C73 | Malignant neoplasm of thyroid gland | Neoplasms |
| ICD-10-CM | C64.1 | Malignant neoplasm of right kidney, except renal pelvis | Neoplasms |
| ICD-10-CM | C20 | Malignant neoplasm of rectum | Neoplasms |
| ICD-10-CM | C19 | Malignant neoplasm of rectosigmoid junction | Neoplasms |
| ICD-10-CM | C61 | Malignant neoplasm of prostate | Neoplasms |
| ICD-10-CM | C50.819 | Malignant neoplasm of overlapping sites of unspecified female breast | Neoplasms |
| ICD-10-CM | C50.012 | Malignant neoplasm of nipple and areola, left female breast | Neoplasms |
| ICD-10-CM | C06.9 | Malignant neoplasm of mouth, unspecified | Neoplasms |
| ICD-10-CM | C34.31 | Malignant neoplasm of lower lobe, right bronchus or lung | Neoplasms |
| ICD-10-CM | C64.2 | Malignant neoplasm of left kidney, except renal pelvis | Neoplasms |
| ICD-10-CM | C54.1 | Malignant neoplasm of endometrium | Neoplasms |
| ICD-10-CM | C62.11 | Malignant neoplasm of descended right testis | Neoplasms |
| ICD-10-CM | C50.111 | Malignant neoplasm of central portion of right female breast | Neoplasms |
| ICD-10-CM | C67.9 | Malignant neoplasm of bladder, unspecified | Neoplasms |
| ICD-10-CM | C21.0 | Malignant neoplasm of anus, unspecified | Neoplasms |
| ICD-10-CM | C43.9 | Malignant melanoma of skin, unspecified | Neoplasms |
| ICD-10-CM | C80.1 | Malignant (primary) neoplasm, unspecified | Neoplasms |
| ICD-10-CM | C62.91 | Malig neoplm of right testis, unsp descended or undescended | Neoplasms |
| ICD-10-CM | C62.90 | Malig neoplasm of unsp testis, unsp descended or undescended | Neoplasms |
| ICD-10-CM | D25.9 | Leiomyoma of uterus, unspecified | Neoplasms |
| ICD-10-CM | D25.1 | Intramural leiomyoma of uterus | Neoplasms |
| ICD-10-CM | D05.10 | Intraductal carcinoma in situ of unspecified breast | Neoplasms |
| ICD-10-CM | C81.90 | Hodgkin lymphoma, unspecified, unspecified site | Neoplasms |
| ICD-10-CM | D18.00 | Hemangioma unspecified site | Neoplasms |
| ICD-10-CM | D18.01 | Hemangioma of skin and subcutaneous tissue | Neoplasms |
| ICD-10-CM | D47.3 | Essential (hemorrhagic) thrombocythemia | Neoplasms |
| ICD-10-CM | D09.9 | Carcinoma in situ, unspecified | Neoplasms |
| ICD-10-CM | D04.39 | Carcinoma in situ of skin of other parts of face | Neoplasms |
| ICD-10-CM | D01.3 | Carcinoma in situ of anus and anal canal | Neoplasms |
| ICD-10-CM | D36.9 | Benign neoplasm, unspecified site | Neoplasms |
| ICD-10-CM | D27.9 | Benign neoplasm of unspecified ovary | Neoplasms |
| ICD-10-CM | D31.30 | Benign neoplasm of unspecified choroid | Neoplasms |
| ICD-10-CM | D12.3 | Benign neoplasm of transverse colon | Neoplasms |
| ICD-10-CM | D24.1 | Benign neoplasm of right breast | Neoplasms |
| ICD-10-CM | D36.10 | Benign neoplasm of peripheral nerves and autonomic nervous system, unspecified | Neoplasms |
| ICD-10-CM | D36.13 | Benign neoplasm of peripheral nerves and autonomic nervous system of lower limb, including hip | Neoplasms |
| ICD-10-CM | D11.0 | Benign neoplasm of parotid gland | Neoplasms |
| ICD-10-CM | D36.7 | Benign neoplasm of other specified sites | Neoplasms |
| ICD-10-CM | D10.39 | Benign neoplasm of other parts of mouth | Neoplasms |
| ICD-10-CM | D32.9 | Benign neoplasm of meninges, unspecified | Neoplasms |
| ICD-10-CM | D11.9 | Benign neoplasm of major salivary gland, unspecified | Neoplasms |
| ICD-10-CM | D24.2 | Benign neoplasm of left breast | Neoplasms |
| ICD-10-CM | D13.7 | Benign neoplasm of endocrine pancreas | Neoplasms |
| ICD-10-CM | D33.3 | Benign neoplasm of cranial nerves | Neoplasms |
| ICD-10-CM | D21.9 | Benign neoplasm of connective and other soft tissue, unspecified | Neoplasms |
| ICD-10-CM | D21.10 | Benign neoplasm of connective and other soft tissue of unspecified upper limb, including shoulder | Neoplasms |
| ICD-10-CM | D12.6 | Benign neoplasm of colon, unspecified | Neoplasms |
| ICD-10-CM | D16.4 | Benign neoplasm of bones of skull and face | Neoplasms |
| ICD-10-CM | D17.9 | Benign lipomatous neoplasm, unspecified | Neoplasms |
| ICD-10-CM | D17.20 | Benign lipomatous neoplasm of skin and subcutaneous tissue of unspecified limb | Neoplasms |
| ICD-10-CM | D17.23 | Benign lipomatous neoplasm of skin and subcutaneous tissue of right leg | Neoplasms |
| ICD-10-CM | D17.21 | Benign lipomatous neoplasm of skin and subcutaneous tissue of right arm | Neoplasms |
| ICD-10-CM | D17.24 | Benign lipomatous neoplasm of skin and subcutaneous tissue of left leg | Neoplasms |
| ICD-10-CM | D17.22 | Benign lipomatous neoplasm of skin and subcutaneous tissue of left arm | Neoplasms |
| ICD-10-CM | D17.0 | Benign lipomatous neoplasm of skin and subcutaneous tissue of head, face and neck | Neoplasms |
| ICD-10-CM | C44.611 | Basal cell carcinoma skin/ unsp upper limb, inc shoulder | Neoplasms |
| ICD-10-CM | C44.91 | Basal cell carcinoma of skin, unspecified | Neoplasms |
| ICD-10-CM | C44.41 | Basal cell carcinoma of skin of scalp and neck | Neoplasms |
| ICD-10-CM | C44.319 | Basal cell carcinoma of skin of other parts of face | Neoplasms |
| ICD-10-CM | C44.519 | Basal cell carcinoma of skin of other part of trunk | Neoplasms |
| ICD-10-CM | C44.311 | Basal cell carcinoma of skin of nose | Neoplasms |
| ICD-10-CM | Z34.81 | Encounter for suprvsn of normal pregnancy, first trimester | Pregnancy |
| ICD-10-CM | Z34.80 | Encounter for supervision of other normal pregnancy, unspecified trimester | Pregnancy |
| ICD-10-CM | Z34.83 | Encounter for supervision of other normal pregnancy, third trimester | Pregnancy |
| ICD-10-CM | Z34.82 | Encounter for supervision of other normal pregnancy, second trimester | Pregnancy |
| ICD-10-CM | Z34.90 | Encounter for supervision of normal pregnancy, unspecified, unspecified trimester | Pregnancy |
| ICD-10-CM | Z34.93 | Encounter for supervision of normal pregnancy, unspecified, third trimester | Pregnancy |
| ICD-10-CM | Z34.91 | Encounter for supervision of normal pregnancy, unspecified, first trimester | Pregnancy |
| ICD-10-CM | Z34.00 | Encounter for supervision of normal first pregnancy, unspecified trimester | Pregnancy |
| ICD-10-CM | Z34.03 | Encounter for supervision of normal first pregnancy, third trimester | Pregnancy |
| ICD-10-CM | Z34.02 | Encounter for supervision of normal first pregnancy, second trimester | Pregnancy |
| ICD-10-CM | Z34.01 | Encounter for supervision of normal first pregnancy, first trimester | Pregnancy |
| ICD-10-CM | Z32.00 | Encounter for pregnancy test, result unknown | Pregnancy |
| ICD-10-CM | Z32.01 | Encounter for pregnancy test, result positive | Pregnancy |
References
- World Health Organization. Vaccines and vaccination against yellow fever. Wkly Epidemiol. Rec. 2013, 88, 269–284. [Google Scholar]
- Garske, T.; Van Kerkhove, M.D.; Yactayo, S.; Ronveaux, O.; Lewis, R.F.; Staples, J.E.; Perea, W.; Ferguson, N.M.; Yellow Fever Expert Committee. Yellow Fever in Africa: Estimating the burden of disease and impact of mass vaccination from outbreak and serological data. PLoS Med. 2014, 11, e1001638. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P.; Vasconcelos, P.F.C. Yellow fever. J. Clin. Virol. 2015, 64, 160–173. [Google Scholar] [CrossRef] [PubMed]
- North, A.F., Jr. Comments on passive immunization procedures. J. Am. Vet. Med. Assoc. 1966, 149, 688–690. [Google Scholar] [PubMed]
- World Health Organization. New Yellow Fever Vaccination Requirements for Travellers. International Travel and Health; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Martin, M.; Weld, L.H.; Tsai, T.F.; Mootrey, G.T.; Chen, R.T.; Niu, M.; Cetron, M.S.; GeoSentinel Yellow Fever Working Group. Advanced age a risk factor for illness temporally associated with yellow fever vaccination. Emerg. Infect Dis. 2001, 7, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Mace, K.E.; Arguin, P.M. Malaria Surveillance—United States, 2014. MMWR Surveill Summ. 2017, 66, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Marasinghe, D.H.; Cheaveau, J.; Meatherall, B.; Kuhn, S.; Vaughan, S.; Zimmer, R.; Pillai, D.R. Risk of malaria associated with travel to malaria-endemic areas to visit friends and relatives: A population-based case-control study. CMAJ Open. 2020, 8, E60–E68. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Updates on Yellow fever vaccination recommendations for International Travellers related to current situation in Brazil. In International Travel and Health: WHO; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Potasman, I.; Pick, N.; Stringer, C.; Zuckerman, J.N. Inadequate protection against yellow fever of children visiting endemic areas. Am. J. Trop. Med. Hyg. 2001, 65, 954–957. [Google Scholar] [CrossRef] [PubMed]
- Akselrod, H.; Swierzbinski, M.J.; Zheng, Z.; Keiser, J.; Parenti, D.M.; Simon, G.L. Characteristics and Severity of Disease among 100 Cases of Imported Malaria Seen at a U.S. University Hospital, 2000–2017. Am. J. Trop. Med. Hyg. 2018, 99, 1511–1517. [Google Scholar] [CrossRef] [PubMed]
- Heywood, A.E.; Zwar, N. Improving access and provision of pre-travel healthcare for travellers visiting friends and relatives: A review of the evidence. J. Travel Med. 2018, 25. [Google Scholar] [CrossRef] [PubMed]
- Reno, E.; Quan, N.G.; Franco-Paredes, C.; Chastain, D.B.; Chauhan, L.; Rodriguez-Morales, A.J.; Henao-Martínez, A.F. Prevention of yellow fever in travellers: An update. Lancet Infect Dis. 2020, 20, e129–e137. [Google Scholar] [CrossRef]
- Nasidi, A.; Monath, T.P.; Vandenberg, J.; Tomori, O.; Calisher, C.H.; Hurtgen, X.; Munube, G.R.; Sorungbe, A.O.; Okafor, G.C.; Wali, S. Yellow fever vaccination and pregnancy: A four-year prospective study. Trans. R. Soc. Trop. Med. Hyg. 1993, 87, 337–339. [Google Scholar] [CrossRef]
- Tattevin, P.; Depatureaux, A.G.; Chapplain, J.M.; Dupont, M.; Souala, F.; Arvieux, C.; Poveda, J.D.; Michelet, C. Yellow fever vaccine is safe and effective in HIV-infected patients. AIDS 2004, 18, 825–827. [Google Scholar] [CrossRef] [PubMed]
- Sicre de Fontbrune, F.; Arnaud, C.; Cheminant, M.; Boulay, A.; Konopacki, J.; Lapusan, S.; Robin, C.; Bernaudin, F.; Suarez, F.; Simon, F.; et al. Immunogenicity and Safety of Yellow Fever Vaccine in Allogeneic Hematopoietic Stem Cell Transplant Recipients After Withdrawal of Immunosuppressive Therapy. J. Infect. Dis. 2017, 217, 494–497. [Google Scholar] [CrossRef]
- de Jong, W.; de Man, R.A.; Dalm, V.A.S.H.; Reusken, C.B.E.M.; Goeijenbier, M.; van Gorp, E.C.M. Yellow fever vaccination for immunocompromised travellers: Unjustified vaccination hesitancy? J. Travel Med. 2019, 26, taz015. [Google Scholar] [CrossRef] [PubMed]
- Shearer, F.M.; Moyes, C.L.; Pigott, D.M.; Brady, O.J.; Marinho, F.; Deshpande, A.; Longbottom, J.; Browne, A.J.; Kraemer, M.U.G.; O’Reilly, K.M.; et al. Global yellow fever vaccination coverage from 1970 to 2016: An adjusted retrospective analysis. Lancet Infect Dis. 2017, 17, 1209–1217. [Google Scholar] [CrossRef]
- Hamer, D.H.; Angelo, K.; Caumes, E.; van Genderen, P.J.J.; Florescu, S.A.; Popescu, C.P.; Perret, C.; McBride, A.; Checkley, A.; Ryan, J.; et al. Fatal Yellow Fever in Travelers to Brazil, 2018. MMWR Morb Mortal Wkly Rep. 2018, 67, 340–341. [Google Scholar] [CrossRef] [PubMed]
- Flasche, S.; Jit, M.; Rodríguez-Barraquer, I.; Coudeville, L.; Recker, M.; Koelle, K.; Milne, G.; Hladish, T.J.; Perkins, T.A.; Cummings, D.A.T.; et al. The Long-Term Safety, Public Health Impact, and Cost-Effectiveness of Routine Vaccination with a Recombinant, Live-Attenuated Dengue Vaccine (Dengvaxia): A Model Comparison Study. PLoS Med. 2016, 13, e1002181. [Google Scholar] [CrossRef] [PubMed]
- Goldie, S. A public health approach to cervical cancer control: Considerations of screening and vaccination strategies. Int. J. Gynecol. Obstet. 2006, 94, S95–S105. [Google Scholar] [CrossRef]
| Variables | Total (n = 964) | Visit F&R, N = 170 (18%) | Other Reason, N = 794 (82%) | p-Value |
|---|---|---|---|---|
| Age (years), mean (SD) | 39 (18) | 30 (20) | 41 (17) | <0.0001 |
| Age ≥ 60 years old | 167 (17%) | 18 (11%) | 149 (19%) | 0.01 |
| Sex, female | 502 (52%) | 74 (44%) | 428 (54%) | 0.01 |
| Race | <0.0001 | |||
| White | 614 (64%) | 36 (21%) | 578 (73%) | |
| African American | 142 (15%) | 104 (61%) | 38 (5%) | |
| Other | 208 (22%) | 30 (18%) | 178 (22%) | |
| Out of Colorado State | 41 (4%) | 5 (3%) | 36 (5%) | 0.35 |
| Pregnancy | 11 (2%) | 5 (6%) | 6 (1%) | 0.004 |
| Hematologic/Immunulogic Disease | 36 (4%) | 9 (5%) | 27 (3%) | 0.237 |
| Diabetes Mellitus | 27 (3%) | 6 (4%) | 21 (3%) | 0.526 |
| Neoplasm | 72 (7%) | 7 (4%) | 65 (8%) | 0.07 |
| HIV | 22 (2%) | 12 (7%) | 10 (1%) | <0.0001 |
| Destination | ||||
| Africa | 551 (57%) | 146 (86%) | 405 (51%) | <0.0001 |
| South America | 387 (40%) | 22 (13%) | 365 (46%) | |
| Other | 26 (3%) | 2 (1%) | 24 (3%) | |
| Time between vaccine administration and depature (days), mean (SD) | 41 (38) | 34 (39) | 43 (38) | 0.0049 |
| Vaccination < 10 days | 110 (11%) | 30 (18%) | 80 (10%) | 0.005 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bandali, M.; Schultz, J.; Than, K.; McGregor, D.; Archuleta, S.; Chadalawada, S.; Mundo, W.; Chastain, D.; Franco-Paredes, C.; Reno, E.; et al. Ensuring the Safety of Yellow Fever Vaccination in Travelers—The Experience at a Large U.S. Academic Medical Center in Colorado. Trop. Med. Infect. Dis. 2020, 5, 125. https://doi.org/10.3390/tropicalmed5030125
Bandali M, Schultz J, Than K, McGregor D, Archuleta S, Chadalawada S, Mundo W, Chastain D, Franco-Paredes C, Reno E, et al. Ensuring the Safety of Yellow Fever Vaccination in Travelers—The Experience at a Large U.S. Academic Medical Center in Colorado. Tropical Medicine and Infectious Disease. 2020; 5(3):125. https://doi.org/10.3390/tropicalmed5030125
Chicago/Turabian StyleBandali, Mehdi, Jonathan Schultz, Kimlien Than, Donna McGregor, Solana Archuleta, Sindhu Chadalawada, William Mundo, Daniel Chastain, Carlos Franco-Paredes, Elaine Reno, and et al. 2020. "Ensuring the Safety of Yellow Fever Vaccination in Travelers—The Experience at a Large U.S. Academic Medical Center in Colorado" Tropical Medicine and Infectious Disease 5, no. 3: 125. https://doi.org/10.3390/tropicalmed5030125
APA StyleBandali, M., Schultz, J., Than, K., McGregor, D., Archuleta, S., Chadalawada, S., Mundo, W., Chastain, D., Franco-Paredes, C., Reno, E., & Henao-Martínez, A. F. (2020). Ensuring the Safety of Yellow Fever Vaccination in Travelers—The Experience at a Large U.S. Academic Medical Center in Colorado. Tropical Medicine and Infectious Disease, 5(3), 125. https://doi.org/10.3390/tropicalmed5030125







