Chagas Disease Infection Reactivation after Heart Transplant
Abstract
1. Brief Historical Context
2. The Economic Burden of Chagas Disease
3. Peculiarities of Chagas Disease in the Heart Transplant Setting
3.1. Patient Selection
3.2. Immunosuppression Strategies
- Reduction in the immunosuppression (which facilitates graft rejection);
- The use of low doses of several drugs whenever feasible;
- The avoidance of excessive doses of immunosuppressive agents.
4. Allograph Rejection following Heart Transplantation
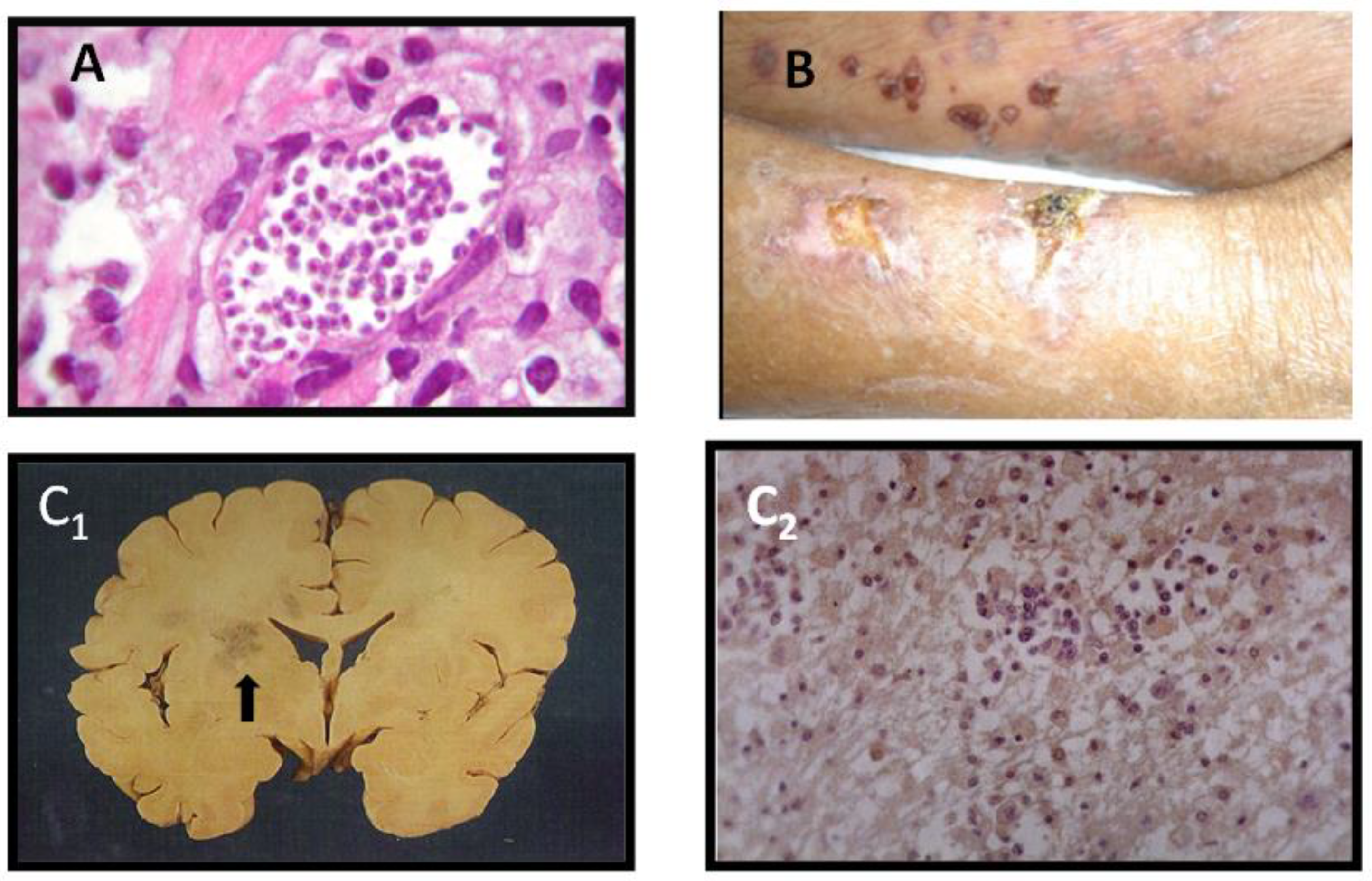
5. Reactivation
5.1. Reactivation Diagnosis
5.2. Results of Heart Transplantation in Chagasic Cardiomyopathy Concerning Reactivation
5.3. Etiological Treatment of Reactivation
6. Heart Transplantation Complications and Survival
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barnard, C.N. The operation. A human cardiac transplant: An interim report of a successful operation performed at Groote Schuur Hospital, Cape Town. S. Afr. Med. J. 1967, 41, 1271–1274. [Google Scholar] [PubMed]
- Brink, J.G.; Hassoulas, J. The first human heart transplant and further advances in cardiac transplantation at Groote Schuur Hospital and the University of Cape Town. Cardiovasc. J. Afr. 2009, 20, 31–35. [Google Scholar] [PubMed]
- Hunt, S.A.; Haddad, F. The changing face of heart transplantation. J. Am. Coll. Cardiol. 2008, 52, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Khush, K.K.; Cherikh, W.S.; Chambers, D.C.; Harhay, M.O.; Hayes, D.; Hsich, E.; Meiser, B.; Potena, L.; Robinson, A.; Rossano, J.W.; et al. The international thoracic organ transplant registry of the international society for heart and lung transplantation: Thirty-sixth adult heart transplantation report—2019; focus theme: Donor and recipient size match. J. Heart Lung Transplant. 2019, 38, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Kobashigawa, J.; Khush, K.; Colvin, M.; Acker, M.; Van Bakel, A.; Eisen, H.; Naka, Y.; Patel, J.; Baran, D.A.; Daun, T.; et al. Report from the american society of transplantation conference on donor heart selection in adult cardiac transplantation in the United States. Arab. Archaeol. Epigr. 2017, 17, 2559–2566. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-C.; Youn, J.-C.; Kobashigawa, J. The past, present and future of heart transplantation. Korean Circ. J. 2018, 48, 565–590. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Canter, C.E.; Hannan, M.M.; Semigran, M.J.; Uber, P.A.; Baran, D.A.; Danziger-Isakov, L.; Kirklin, J.K.; Kirk, R.; Kushwaha, S.S.; et al. The 2016 international society for heart lung transplantation listing criteria for heart transplantation: A 10-year update. J. Heart Lung Transplant. 2016, 35, 1–23. [Google Scholar] [CrossRef]
- Andrade, J.P.; Marin-Neto, J.A.; Paola, A.A.; Vilas-Boas, F.; Oliveira, G.M.; Bacal, F.; Bocchi, E.A.; Almeida, D.R.; Fragata-Filho, A.A.; Moreira, M.C.V.; et al. Sociedade Brasileira de Cardiologia. I Diretriz Latino Americana para o Diagnóstico e Tratamento da Cardiopatia Chagásica. Arq. Bras. Cardiol. 2011, 97, 1–48. [Google Scholar] [CrossRef]
- Fiorelli, A.; Santos, R.; Oliveira, J.L.; Lourenço-Filho, D.; Dias, R.; Oliveira, A.; Da Silva, M.; Ayoub, F.; Bacal, F.; Souza, G.; et al. Heart transplantation in 107 cases of Chagas’ disease. Transplant. Proc. 2011, 43, 220–224. [Google Scholar] [CrossRef]
- Lee, B.Y.; Bacon, K.M.; Bottazzi, M.E.; Hotez, P.J. Global economic burden of Chagas disease: A computational simulation model. Lancet Infect. Dis. 2013, 13, 342–348. [Google Scholar] [CrossRef]
- World Health Organization. Chagas Disease (American Trypanosomiaisis [Internet]. Geneva: World Health Organization 2015. Available online: http://www.who.Int/mediacentre/factsheets/fs340/en/ (accessed on 29 May 2020).
- Dias, J.C.P.; Ramos, A.N.R., Jr.; Gontijo, E.D.; Luquetti, A.; Shikanai-Yasuda, M.A.; Coura, J.R.; Torres, R.M.; Melo, J.R.D.C.; De Almeida, E.A.; Oliveira, W., Jr.; et al. 2nd Brazilian consensus on Chagas disease, 2015. Rev. Soc. Bras. Med. Trop. 2016, 49, 3–60. [Google Scholar] [CrossRef] [PubMed]
- Freitas, H.; Chizzola, P.R.; Paes, Â.; Lima, A.C.; Mansur, A.J. Risk stratification in a Brazilian hospital-based cohort of 1220 outpatients with heart failure: Role of Chagas’ heart disease. Int. J. Cardiol. 2005, 102, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Bern, C. A new epoch in antitrypanosomal treatment for Chagas disease. J. Am. Coll. Cardiol. 2017, 69, 948–950. [Google Scholar] [CrossRef] [PubMed]
- Bocchi, E.A.; Fiorelli, A. First guideline group for heart transplantation of the Brazilian Society of Cardiology: The Brazilian experience with heart transplantation: A multicenter report. J. Heart Lung Transplant. 2001, 20, 637–645. [Google Scholar] [CrossRef]
- Parra, A.V.; Rodrigues, V.; Cancella, S.; Cordeiro, J.A.; Bestetti, R.B. Impact of socioeconomic status on outcome of a Brazilian heart transplant recipients cohort. Int. J. Cardiol. 2008, 125, 142–143. [Google Scholar] [CrossRef]
- Pinazo, M.J.; Miranda, B.; Rodríguez-Villar, C.; Altclas, J.; Serra, M.B.; García-Otero, E.C.; De Almeida, E.A.; García, M.M.; Gascon, J.; Rodríguez, M.G.; et al. Recommendations for management of Chagas disease in organ and hematopoietic tissue transplantation programs in nonendemic areas. Transplant. Rev. 2011, 25, 91–101. [Google Scholar] [CrossRef]
- Pinazo, M.J.; Espinosa, G.; Cortes-Lletget, C.; Posada, E.D.J.; Aldasoro, E.; Oliveira, I.; Muñoz, J.; Gállego, M.; Gascon, J. Immunosuppression and Chagas disease: A management challenge. PLoS Negl. Trop. Dis. 2013, 7. [Google Scholar] [CrossRef]
- Costanzo, M.R.; Dipchand, A.; Starling, R.; Anderson, A.S.; Chan, M.; Desai, S.; Fedson, S.; Fisher, P.; Gonzales-Stawinski, G.; Martinelli, L.; et al. The international society of heart and lung transplantation guidelines for the care of heart transplant recipients. J. Heart Lung Transplant. 2010, 29, 914–956. [Google Scholar] [CrossRef]
- Bestetti, R.; Theodoropoulos, T.A. A systematic review of studies on heart transplantation for patients with end-stage Chagas’ heart disease. J. Card. Fail. 2009, 15, 249–255. [Google Scholar] [CrossRef]
- Bacal, F.; Silva, C.P.; Bocchi, E.A.; Pires, P.V.; Moreira, L.F.P.; Issa, V.S.; Moreira, S.A.; Cruz, F.D.D.; Strabelli, T.; Stolf, N.A.G.; et al. Mychophenolate mofetil increased Chagas disease reactivation in heart transplanted patients: Comparison between two different protocols. Arab. Archaeol. Epigr. 2005, 5, 2017–2021. [Google Scholar] [CrossRef]
- Colvin, M.M.; Cook, J.L.; Chang, P.; Francis, G.; Hsu, D.; Kiernan, M.S.; Kobashigawa, J.; Lindenfeld, J.; Masri, S.C.; Miller, D.; et al. Antibody-mediated rejection in cardiac transplantation: Emerging knowledge in diagnosis and management: A scientific statement from the American heart association. Circulation 2015, 131, 1608–1639. [Google Scholar] [CrossRef] [PubMed]
- Berry, G.J.; Angelini, A.; Burke, M.M.; Bruneval, P.; Fishbein, M.C.; Hammond, E.; Miller, D.; Neil, D.A.; Revelo, M.P.; Rodriguez, E.R.; et al. The ISHLT working formulation for pathologic diagnosis of antibody-mediated rejection in heart transplantation: Evolution and current status (2005–2011). J. Heart Lung Transplant. 2011, 30, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Berry, G.J.; Burke, M.M.; Andersen, C.; Bruneval, P.; Fedrigo, M.; Fishbein, M.C.; Goddard, M.; Hammond, E.H.; Leone, O.; Marboe, C.; et al. The 2013 international society for heart and lung transplantation working formulation for the standardization of nomenclature in the pathologic diagnosis of antibody-mediated rejection in heart transplantation. J. Heart Lung Transplant. 2013, 32, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Benatti, R.D.; Oliveira, G.H.; Bacal, F. Heart transplantation for Chagas cardiomyopathy. J. Heart Lung Transplant. 2017, 36, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, P.A.; Segatto, M.; Durso, D.F.; Moreira, W.J.D.C.; Junqueira, L.L.; De Castilho, F.M.; De Andrade, S.A.; Gelape, C.L.; Chiari, E.; Teixeira-Carvalho, A.; et al. Early polymerase chain reaction detection of Chagas disease reactivation in heart transplant patients. J. Heart Lung Transplant. 2017, 36, 797–805. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Benatti, R.D.; Al-Kindi, S.G.; Bacal, F.; Oliveira, G. Heart transplant outcomes in patients with Chagas cardiomyopathy in the United States. Clin. Transplant. 2018, 32. [Google Scholar] [CrossRef]
- Bacal, F.; Silva, C.P.; Pires, P.V.; Mangini, S.; Fiorelli, A.I.; Stolf, N.G.; Bocchi, E.A. Transplantation for Chagas’ disease: An overview of immunosuppression and reactivation in the last two decades. Clin. Transplant. 2010, 24, E29–E34. [Google Scholar] [CrossRef]
- Nogueira, S.S.; Felizardo, A.A.; Caldas, I.S.; Gonçalves, R.V.; Novaes, R.D. Challenges of immunosuppressive and anti trypanosomal drug therapy after heart transplantation in patients with chronic Chagas disease: A systematic review of clinical recommendations. Transplant. Rev. (Orlando) 2018, 33, 157–167. [Google Scholar] [CrossRef]
- Campos, S.V.; Strabelli, T.M.V.; Neto, V.A.; Silva, C.P.; Bacal, F.; Bocchi, E.A.; Stolf, N.A.G. Risk factors for Chagas’ disease reactivation after heart transplantation. J. Heart Lung Transplant. 2008, 27, 597–602. [Google Scholar] [CrossRef]
- Kransdorf, E.P.; Zakowski, P.C.; Kobashigawa, J. Chagas disease in solid organ and heart transplantation. Curr. Opin. Infect. Dis. 2014, 27, 418–424. [Google Scholar] [CrossRef]
- Camargos, S.; Moreira, M.C.V.; Portela, D.M.M.C.; Lira, J.P.I.; Modesto, F.V.S.; Menezes, G.M.M.; Moreira, D.R. CNS chagoma—Reactivation in an immunosuppressed patient. Neurology 2017, 88, 605–606. [Google Scholar] [CrossRef] [PubMed]
- From, A.M.; Maleszewski, J.J.; Rihal, C.S. Current status of endomyocardial biopsy. Mayo Clin. Proc. 2011, 86, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Diez, M.; Favaloro, L.; Bertolotti, A.; Burgos, J.M.; Vigliano, C.; Lastra, M.P.; Levin, M.J.; Arnedo, A.; Nagel, C.; Schijman, A.G.; et al. Usefulness of PCR strategies for early diagnosis of Chagas’ disease reactivation and treatment follow-up in heart transplantation. Am. J. Transp. 2007, 7, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.D.; Tiecher, F.M.; Balbinot, M.M.; Liarte, D.B.; Scholl, D.; Steindel, M.; Romanha, A.J. Efficacy of benznidazol treatment for asymptomatic chagasic patients from state of Rio Grande do Sul evaluated during a three years follow-up. Memórias Instituto Oswaldo Cruz 2009, 104, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, E.S.; Nascimento, M.S.; Kesper, N.; Coura, J.R.; Borges-Pereira, J.; Junqueira, A.C.; Camargo, M.E. Immunoblot assay using excreted-secreted antigens of Trypanosoma cruzi in serodiagnosis of congenital, acute, and chronic Chagas’ disease. J. Clin. Microbiol. 1996, 34, 2143–2147. [Google Scholar] [CrossRef]
- Jackson, Y.; Dang, T.; Schnetzler, B.; Pascual, M.; Meylan, P. Trypanosoma cruzi fatal reactivation in heart transplant recipient in Switzerland. J. Heart Lung Transplant. 2011, 30, 484–485. [Google Scholar] [CrossRef]
- Gómez-P, C.F.; Mantilla-H, J.C.; Rodriguez-Morales, A.J. Fatal Chagas disease among solid-organ transplant recipients in Colombia. Open Forum Infect. Dis. 2014, 1. [Google Scholar] [CrossRef]
- Chin-Hong, P.V.; Schwartz, B.S.; Bern, C.; Montgomery, S.P.; Kontak, S.; Kubak, B.; Morris, M.I.; Nowicki, M.; Wright, C.; Ison, M.G.; et al. Screening and treatment of Chagas disease in organ transplant recipients in the United States: Recommendations from the Chagas in transplant working group. Am. J. Transplant. 2011, 11, 672–680. [Google Scholar] [CrossRef]
- Schwartz, B.S.; Mawhorter, S.D. Parasitic Infections in Solid Organ Transplantation. Am. J. Transplant. 2013, 13, 280–303. [Google Scholar] [CrossRef]
- Bern, C.; Weller, P.F.; Baron, E.L. Chagas disease in the immunosuppressed host. Curr. Opin. Infect. Dis. 2012, 25, 450–457. [Google Scholar] [CrossRef]
- Theodoropoulos, T.A.; Silva, A.G.; Bestetti, R.B. Eosinophil blood count and anemia are associated with Trypanosoma cruzi infection reactivation in Chagas’ heart transplant recipients. Cardiovasc. Pathol. 2009, 19, 191–192. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.B.; La Hoz, R.M.; Green, J.S.; Vikram, H.R.; Benedict, T.; Rivera, H.; Montgomery, S.P. Reactivation of Chagas disease among heart transplant recipients in the United States, 2012–2016. Transpl. Infect. Dis. 2018, 20. [Google Scholar] [CrossRef] [PubMed]
- Bocchi, E.A.; Fiorelli, A. The paradox of survival results after transplantation for cardiomyopathy caused by Trypanosoma cruzi. First guidelines group for heart transplantation of the Brazilian society of cardiology. Ann. Thorac. Surg. 2001, 71, 1833–1838. [Google Scholar] [CrossRef]
- Cançado, J.R. Long term evaluation of etiological treatment of Chagas disease with benznidazole. Revista Instituto Medicina Tropical São Paulo 2002, 44, 29–37. [Google Scholar] [CrossRef]
- Olivera, M.J.; Cucunuba, Z.; Valencia-Hernandez, C.A.; Herazo, R.; Agreda-Rudenko, D.; Florez, C.; Duque, S.; Nicholls, R.S. Risk factors for treatment interruption and severe adverse effects to benznidazole in adult patients with Chagas disease. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Olivera, M.J.; Fory, J.A.; Olivera, A.J. Therapeutic drug monitoring of benznidazole and nifurtimox: A systematic review and quality assessment of published clinical practice guidelines. Rev. Soc. Bras. Med. Trop. 2017, 50, 748–755. [Google Scholar] [CrossRef]
- Bern, C.; Montgomery, S.P.; Herwaldt, B.L.; Rassi, A.; Marin-Neto, J.A.; Dantas, R.O.; Maguire, J.H.; Acquatella, H.; Morillo, C.; Kirchhoff, L.V.; et al. Evaluation and Treatment of Chagas Disease in the United States: A systematic review. JAMA 2007, 298, 2171–2181. [Google Scholar] [CrossRef]
- De Andrade, A.L.S.S.; Zicker, F.; De Oliveira, R.M.; Silva, S.A.E.; Luquetti, A.; Travassos, L.R.; Almeida, I.C.; De Andrade, S.S.; De Andrade, J.G.; Martelli, C.M.; et al. Randomised trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. Lancet 1996, 348, 1407–1413. [Google Scholar] [CrossRef]
- Sosa-Estani, S.; Porcel, B.M.; Segura, E.L.; Yampotis, C.; Ruiz, A.M.; Velazquez, E. Efficacy of chemotherapy with benznidazole in children in the indeterminate phase of Chagas’ disease. Am. J. Trop. Med. Hyg. 1998, 59, 526–529. [Google Scholar] [CrossRef]
- Morillo, C.A.; Marin-Neto, J.A.; Avezum, A.; Sosa-Estani, S.; Rosas, F.; Villena, E.; Quiroz, R.; Bonilla, R.; Britto, C.; Guhl, F.; et al. Randomized trial of benznidazole for chronic Chagas’ cardiomyopathy. N. Engl. J. Med. 2015, 373, 1295–1306. [Google Scholar] [CrossRef]
- Viotti, R.; Vigliano, C.; Armenti, H.; Segura, E. Treatment of chronic Chagas’ disease with benznidazole: Clinical and serologic evolution of patients with long-term follow-up. Am. Heart J. 1994, 127, 151–162. [Google Scholar] [CrossRef]
- Maguire, J.H. Treatment of Chagas’ disease—Time is running out. N. Engl. J. Med. 2015, 373, 1369–1370. [Google Scholar] [CrossRef] [PubMed]
- Ortega, A.E.; López, Z.R.D.A.; Pérez, R.H.; Millón, C.F.; Martín, A.D.; Palomo, Y.C.; Gallé, E.L. Kidney failure after heart transplantation. Transplant. Proc. 2010, 42, 3193–3195. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, M.d.C.V.; Renan Cunha-Melo, J. Chagas Disease Infection Reactivation after Heart Transplant. Trop. Med. Infect. Dis. 2020, 5, 106. https://doi.org/10.3390/tropicalmed5030106
Moreira MdCV, Renan Cunha-Melo J. Chagas Disease Infection Reactivation after Heart Transplant. Tropical Medicine and Infectious Disease. 2020; 5(3):106. https://doi.org/10.3390/tropicalmed5030106
Chicago/Turabian StyleMoreira, Maria da Consolação Vieira, and José Renan Cunha-Melo. 2020. "Chagas Disease Infection Reactivation after Heart Transplant" Tropical Medicine and Infectious Disease 5, no. 3: 106. https://doi.org/10.3390/tropicalmed5030106
APA StyleMoreira, M. d. C. V., & Renan Cunha-Melo, J. (2020). Chagas Disease Infection Reactivation after Heart Transplant. Tropical Medicine and Infectious Disease, 5(3), 106. https://doi.org/10.3390/tropicalmed5030106




