Yield of Systematic Longitudinal Screening of Household Contacts of Pre-Extensively Drug Resistant (PreXDR) and Extensively Drug Resistant (XDR) Tuberculosis Patients in Mumbai, India
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting
2.2.1. Household Contact (HHC) Investigation
2.2.2. Follow-Up of HHCs
2.3. Study Population and Participants
2.4. Data Variables and Sources, Data Analysis
2.5. Operational Definitions
2.6. Ethics
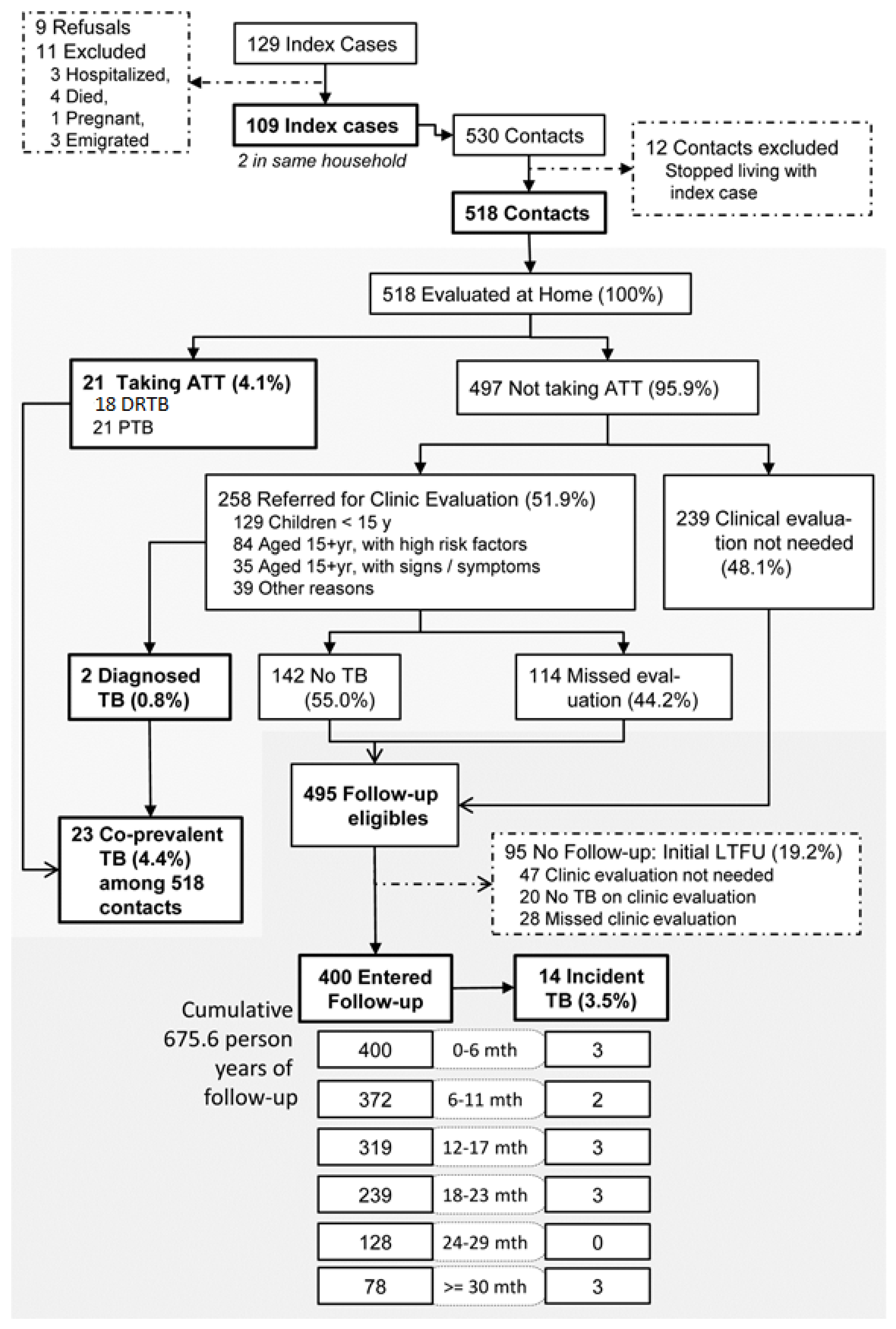
3. Results
3.1. TB Diagnosis among HHCs
3.2. Overall Yield of TB in HHCs
3.3. Drug Resistance Patterns
4. Discussion
4.1. Limitations
4.2. Implications for Policy and Practice
4.3. Implications for Future Research
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2017; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Central TB Division. India TB Report 2019: Revised National Tuberculosis Control Program—Annual Status Report; Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India: New Delhi, India, 2019.
- Report of the First National Anti-Tuberculosis Drug Resistance Survey India 2014–2016 [Internet]; Ministry of Health and Family Welfare, Government of India: New Delhi, India, 2018.
- Shah, I.; Shah, F. Changing prevalence and resistance patterns in children with drug-resistant tuberculosis in Mumbai. Paediatr. Int. Child Health 2017, 37, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Goyal, V.; Kadam, V.; Narang, P.; Singh, V. Prevalence of drug-resistant pulmonary tuberculosis in India: Systematic review and meta-analysis. BMC Public Health 2017, 17, 817. [Google Scholar] [CrossRef] [PubMed]
- Dalal, A.; Pawaskar, A.; Das, M.; Desai, R.; Prabhudesai, P.; Chhajed, P.; Rajan, S.; Reddy, D.; Babu, S.; Jayalakshmi, T.K.; et al. Resistance patterns among multidrug-resistant tuberculosis patients in greater metropolitan Mumbai: Trends over time. PLoS ONE 2015, 10, e0116798. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.; Pai, M.; Hopewell, P.C. Review Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: A systematic review and meta-analysis. Lancet Infect. Dis. 2008, 8, 359–368. [Google Scholar] [CrossRef]
- Shah, N.S.; Yuen, C.M.; Heo, M.; Tolman, A.W.; Becerra, M.C. Yield of Contact Investigations in Households of Patients with Drug-Resistant Tuberculosis: Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2014, 58, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Fox, G.J.; Barry, S.E.; Britton, W.J.; Marks, G.B. Contact investigation for tuberculosis: A systematic review and meta-analysis. Eur. Respir. J. 2013, 41, 140–156. [Google Scholar] [CrossRef]
- Javaid, A.; Khan, M.A.M.A.; Khan, M.A.M.A.; Mehreen, S.; Basit, A.; Khan, R.A.; Ihtesham, M.; Ullah, I.; Khan, A.; Ullah, U. Screening outcomes of household contacts of multidrug-resistant tuberculosis patients in Peshawar, Pakistan. Asian Pac. J. Trop. Med. 2016, 9, 909–912. [Google Scholar] [CrossRef] [PubMed]
- Fox, G.J.; Nhung, N.V.; Sy, D.N.; Hoa, N.L.P.; Anh, L.T.N.; Anh, N.T.; Hoa, N.B.; Dung, N.H.; Buu, T.N.; Loi, N.T.; et al. Household-Contact Investigation for Detection of Tuberculosis in Vietnam. N. Engl. J. Med. 2018, 378, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Lu, L.; Wu, J.; Yang, C.; Prakash, R.; Zuo, T.; Liu, Q.; Hong, J.; Guo, X.; Gao, Q. Assessment of tuberculosis contact investigation in Shanghai, China: An 8-year cohort study. Tuberculosis 2018, 108, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Mumbai (Greater Mumbai) City Population Census 2011 | Maharashtra [Internet]. Available online: https://www.census2011.co.in/census/city/365-mumbai.html (accessed on 28 November 2019).
- Registrar General and Census Commissioner-India. Census of India 2011: Provisional Population Totals-India Data Sheet; Office of the Registrar General and Census Commissioner: Delhi, India, 2011.
- Isaakidis, P.; Cox, H.S.; Varghese, B.; Montaldo, C.; Silva, E.D.; Mansoor, H.; Ladomirska, J.; Sotgiu, G.; Migliori, G.B.; Pontali, E.; et al. Ambulatory multi-drug resistant tuberculosis treatment outcomes in a cohort of HIV-infected patients in a slum setting in Mumbai, India. PLoS ONE 2011, 6, e28066. [Google Scholar] [CrossRef] [PubMed]
- Leung, E.C.C.; Leung, C.C.; Kam, K.M.; Yew, W.W.; Chang, K.C.; Leung, W.M.; Tam, C.M. Transmission of multidrug-resistant and extensively drug-resistant tuberculosis in a metropolitan city. Eur. Respir. J. 2013, 41, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yang, M.; Wu, Z.; Shen, X.; Wang, Y.; Zhao, G. High incidence and low case detection rate among contacts of tuberculosis cases in Shanghai, China. BMC Infect. Dis. 2019, 19, 320. [Google Scholar] [CrossRef] [PubMed]
- Hill, P.C.; Jackson-Sillah, D.J.; Fox, A.; Brookes, R.H.; de Jong, B.C.; Lugos, M.D.; Adetifa, I.M.; Donkor, S.A.; Aiken, A.M.; Howie, S.R.; et al. Incidence of Tuberculosis and the Predictive Value of ELISPOT and Mantoux Tests in Gambian Case Contacts. PLoS ONE 2008, 3, e1379. [Google Scholar] [CrossRef] [PubMed]
- Saunders, M.J.; Tovar, M.A.; Collier, D.; Baldwin, M.R.; Montoya, R.; Valencia, T.R.; Gilman, R.H.; Evans, C.A. Active and passive case-finding in tuberculosis-affected households in Peru: A 10-year prospective cohort study. Lancet Infect. Dis. 2019, 19, 519–528. [Google Scholar] [CrossRef]
- Cudahy, P.G.T.; Andrews, J.R.; Bilinski, A.; Dowdy, D.W.; Mathema, B.; Menzies, N.A.; Salomon, J.A.; Shrestha, S.; Cohen, T. Spatially targeted screening to reduce tuberculosis transmission in high-incidence settings. Lancet Infect. Dis. 2019, 19, e89–e95. [Google Scholar] [CrossRef]
- National Strategic Plan for Tuberculosis Elimination 2017–2025 [Internet]; Central TB Division, Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India: New Delhi, India, 2017. Available online: https://tbcindia.gov.in/WriteReadData/NSP%20Draft%2020.02.2017%201.pdf (accessed on 25 June 2019).
- Lung, T.; Marks, G.B.; Nhung, N.V.; Anh, N.T.; Hoa, N.L.P.; Anh, L.T.N.; Hoa, N.B.; Britton, W.J.; Bestrashniy, J.; Jan, S.; et al. Household contact investigation for the detection of tuberculosis in Vietnam: Economic evaluation of a cluster-randomised trial. Lancet Glob. Health 2019, 7, E376–E384. [Google Scholar] [CrossRef]
| Characteristics | Groups | Index Cases | |
|---|---|---|---|
| N | % | ||
| Type of TB drug resistance | Pre-Extensive Drug Resistance (pre-XDR) | 51 | (47) |
| Extensive Drug Resistance (XDR) | 58 | (53) | |
| Age at enrolment (years) | 0–14 | 9 | (8) |
| 15 and above | 100 | (92) | |
| Sex | Male | 49 | (45) |
| Female | 60 | (55) | |
| Previous TB treatment | No treatment | 6 | (6) |
| History of previous TB treatment | 103 | (94) | |
| Site of TB in Index Case | Extra-pulmonary (EPTB) | 15 | (14) |
| Pulmonary (PTB) | 94 | (86) | |
| X-ray results (n = 105) | Abnormal | 92 | (88) |
| Normal | 13 | (12) | |
| Sputum conversion (in months) [Median (IQR)] (n = 76) | 2 (1–4) | ||
| Human Immunodeficiency Virus (HIV) co-infection | 5 | (5) | |
| Diabetes mellitus co-morbidity | 10 | (9) | |
| Overcrowding (area per person <= 50 square feet) | 60 | (55) | |
| Staying in rented house | 33 | (30) | |
| Variable | Household Contacts (n = 400) * | Household Contacts with Incident TB(n = 14) ** | p-Value | |
|---|---|---|---|---|
| Index Case Characteristics | n (%) | n (%) | ||
| Type of index case | Pre-XDR | 166 (41) | 7 (4) | 0.51 |
| XDR | 234 (59) | 7 (3) | ||
| Age (Median(IQR), years) † | 24 (19–30) | 24 (18–26) | 0.43 | |
| Sex | Male | 156 (39) | 6 (4) | 0.76 |
| Female | 244 (61) | 8 (3) | ||
| Prior TB treatment | No treatment | 23 (6) | 2 (9) | 0.43 |
| TB treatment | 377 (94) | 12 (3) | ||
| HIV in index case | No | 384 (96) | 12 (3) | 0.10 |
| Yes | 16 (4) | 2 (13) | ||
| AFB result | Negative | 183 (47) | 8 (4) | 0.59 |
| Scanty | 42 (11) | 2 (5) | ||
| Positive | 162 (42) | 4 (3) | ||
| Site of TB | EPTB | 51 (13) | 0 (0) | |
| PTB | 349 (87) | 14 (4) | ||
| X-ray results | Abnormal | 344 (88) | 14 (4) | |
| Normal | 47 (12) | 0 (0) | ||
| Number of household contacts † | 6 (4–8) | 6 (5–10) | 0.21 | |
| Household Contact Characteristics | ||||
| Age group | 0–14 years | 94 (23) | 4 (4) | 0.75 |
| 15 years or more | 306 (97) | 10 (3) | ||
| Sex | Male | 205 (51) | 6 (3) | 0.52 |
| Female | 195 (49) | 8 (4) | ||
| Relationship with index case | Child | 68 (17) | 3 (4) | 0.37 |
| Parent | 120 (30) | 4 (3) | ||
| Sibling | 97 (24) | 6 (6) | ||
| Spouse | 22 (6) | 0 (0) | ||
| Other | 93 (23) | 1 (1) | ||
| Area < 50 sq/feet/capita | No | 226 (59) | 10 (4) | 0.32 |
| Yes | 159 (41) | 4 (3) | ||
| Past history of TB | No | 358 (90) | 11 (3) | 0.17 |
| Yes | 42 (10) | 3 (7) | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paryani, R.H.; Gupta, V.; Singh, P.; Verma, M.; Sheikh, S.; Yadav, R.; Mansoor, H.; Kalon, S.; Selvaraju, S.; Das, M.; et al. Yield of Systematic Longitudinal Screening of Household Contacts of Pre-Extensively Drug Resistant (PreXDR) and Extensively Drug Resistant (XDR) Tuberculosis Patients in Mumbai, India. Trop. Med. Infect. Dis. 2020, 5, 83. https://doi.org/10.3390/tropicalmed5020083
Paryani RH, Gupta V, Singh P, Verma M, Sheikh S, Yadav R, Mansoor H, Kalon S, Selvaraju S, Das M, et al. Yield of Systematic Longitudinal Screening of Household Contacts of Pre-Extensively Drug Resistant (PreXDR) and Extensively Drug Resistant (XDR) Tuberculosis Patients in Mumbai, India. Tropical Medicine and Infectious Disease. 2020; 5(2):83. https://doi.org/10.3390/tropicalmed5020083
Chicago/Turabian StyleParyani, Roma Haresh, Vivek Gupta, Pramila Singh, Madhur Verma, Sabira Sheikh, Reeta Yadav, Homa Mansoor, Stobdan Kalon, Sriram Selvaraju, Mrinalini Das, and et al. 2020. "Yield of Systematic Longitudinal Screening of Household Contacts of Pre-Extensively Drug Resistant (PreXDR) and Extensively Drug Resistant (XDR) Tuberculosis Patients in Mumbai, India" Tropical Medicine and Infectious Disease 5, no. 2: 83. https://doi.org/10.3390/tropicalmed5020083
APA StyleParyani, R. H., Gupta, V., Singh, P., Verma, M., Sheikh, S., Yadav, R., Mansoor, H., Kalon, S., Selvaraju, S., Das, M., Laxmeshwar, C., Ferlazzo, G., & Isaakidis, P. (2020). Yield of Systematic Longitudinal Screening of Household Contacts of Pre-Extensively Drug Resistant (PreXDR) and Extensively Drug Resistant (XDR) Tuberculosis Patients in Mumbai, India. Tropical Medicine and Infectious Disease, 5(2), 83. https://doi.org/10.3390/tropicalmed5020083





