Levels of 25-hydroxy Vitamin D3 and Vitamin D Receptor Polymorphism in Severe Dengue Cases from New Delhi
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Inclusion Criteria for a Case of Dengue
2.3. Exclusion Criteria for a Case of Dengue
2.4. Statistical Analysis
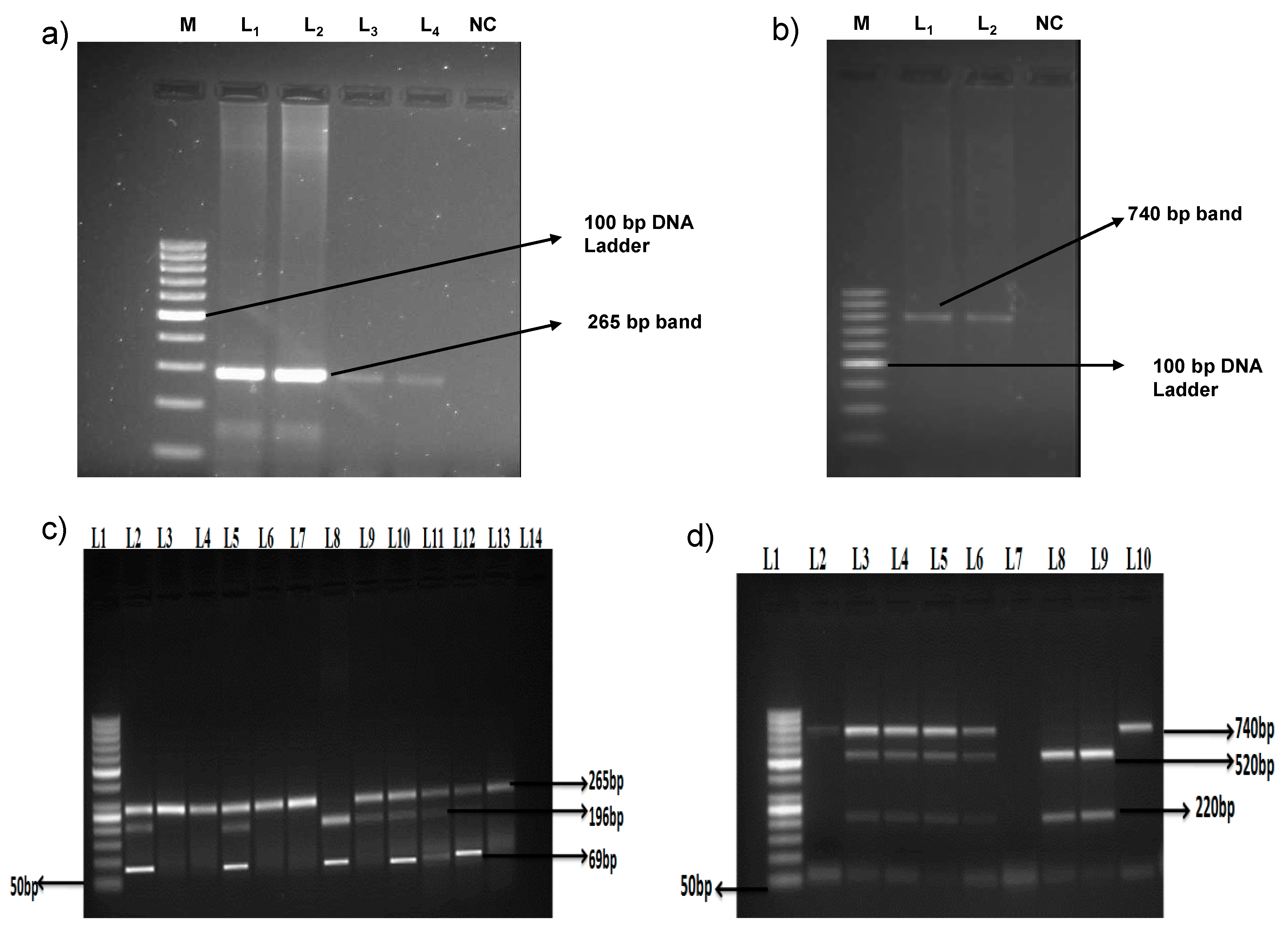
3. Results
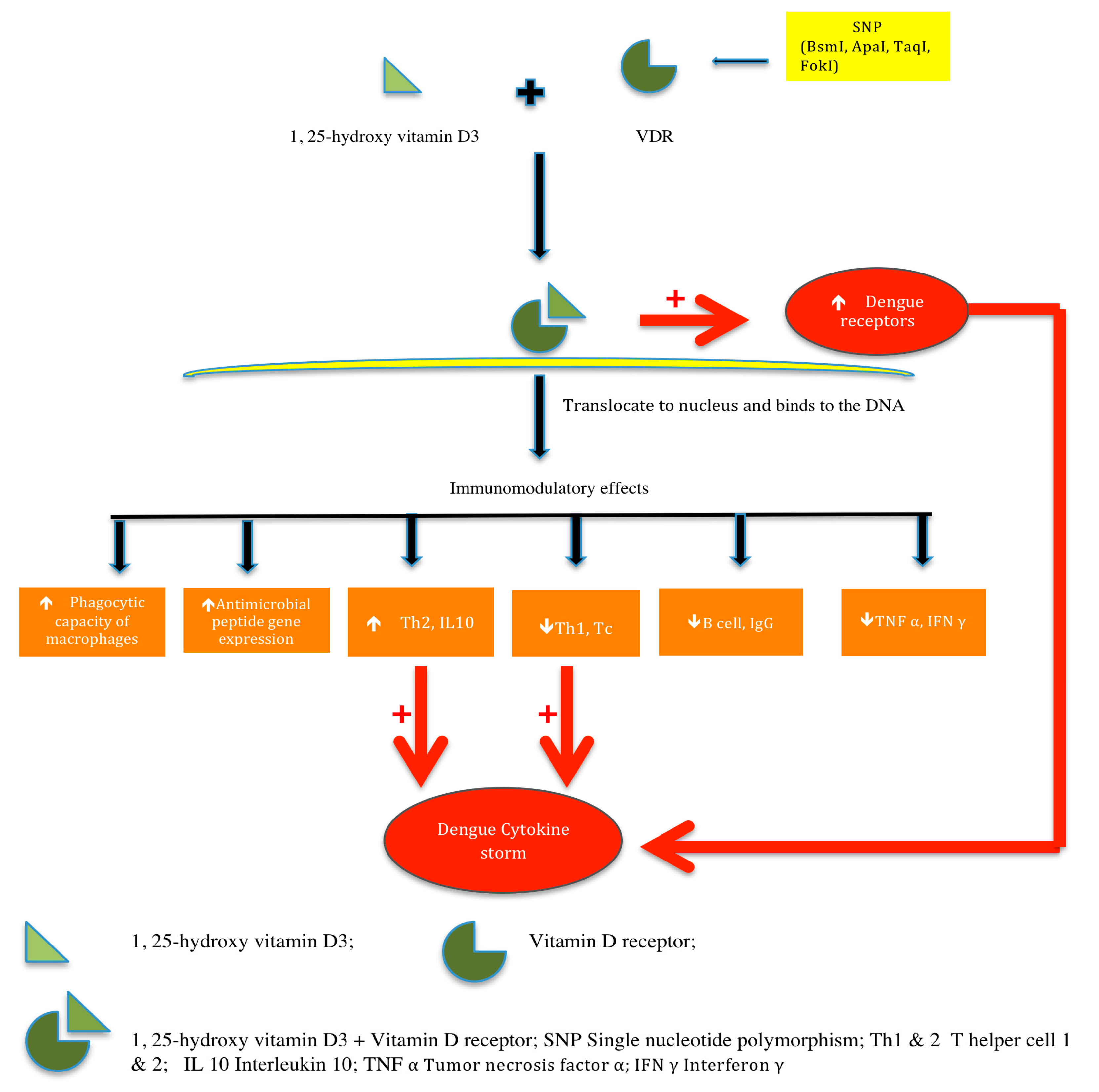
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gubler, D.J. The global pandemic of dengue/dengue haemorrhagic fever: Current status & prospects for future. Ann. Acad. Med. Singap. 1998, 27, 227–234. [Google Scholar] [PubMed]
- National Vector borne Control Program. National Guidelines for Clinical Management of Dengue Fever. 2015. Available online: http://www.searo.who.int/india/publications/national_guidelines_clinical_management_dengue1.pdf?ua=1 (accessed on 25 September 2019).
- Boonstra, A.; Barrat, F.J.; Crain, C.; Health, V.L.; Savelkoul, H.F.; O’Garra, A. 1 alpha, 25-Dihydroxyvitamin D3 has a direct effect on native CD4(+) T cells to enhance the development of Th2 cells. J. Immunol. 2001, 167, 4974–4980. [Google Scholar] [CrossRef] [PubMed]
- Bid, H.K.; Konwar, R.; Aggarwal, C.G.; Gautam, S.; Saxena, M.; Nayak, V.L.; Banerjee, M. Vitamin D receptor (FokI, BsmI and TaqI) gene polymorphisms and type 2 diabetes mellitus: A north Indian study. Indian J. Med. Sci. 2009, 63, 187–194. [Google Scholar] [PubMed]
- Zhang, J.; Li, W.; Liu, J.; Wu, W.; Ouyang, H.; Zhang, Q.; Wang, Y.; Liu, L.; Yang, R.; Liu, X.; et al. Polymorphisms in the vitamin D receptor gene and type 1 diabetes mellitus risk: An update by meta-analysis. Mol. Cell Endocrinol. 2012, 355, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Aslani, S.; Hossein-Nezhad, A.; Mirzaei, K.; Maghbooli, Z.; Afshar, A.N.; Karimi, F. VDR FokI polymorphism and its potential role in the pathogenesis of gestational diabetes mellitus and its complications. Gynecol. Endocrinol. 2011, 27, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.T.; Stengers, S.; Tang, D.H.; Modlin, R.H. Cutting edge: Vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on induction of cathelicidin. J. Immunol. 2007, 179, 2060–2063. [Google Scholar] [CrossRef]
- Mora, R.J.; Iwata, M.; Andrian, H.U. Vitamin effects on immune system: Vitamins A and D take centre stage. Nat. Rev. Immunol. 2008, 8, 685–698. [Google Scholar] [CrossRef]
- Vidyarani, M.; Selvaraj, P.; Jawahar, M.S.; Narayanan, P.R. 1,25 Dihydroxyvitamin D3 modulated cytokine response in pulmonary tuberculosis. Cytokine 2007, 40, 128–134. [Google Scholar] [CrossRef]
- Peurta-Guardo, H.; de la Cruz Hernandez, S.I.; Rosales, V.H.; Ludert, J.E.; del Angel, R.M. 1 alpha, 25-dihydroxyvitamin D3 reduces dengue virus infection in human myelomonocyte (U937) and hepatic (Huh-7) cell lines and cytokine production in the infected monocytes. Antivir. Res. 2010, 94, 57–61. [Google Scholar] [CrossRef]
- Rahmannezhad, G.; Mashayekhi, F.J.; Goodarzi, M.T.; Rezvanfar, M.R.; Sadeghi, A. Association between vitamin D receptor ApaI and TaqI gene polymorphisms and gestational diabetes mellitus in an Iranian pregnant women population. Gene 2016, 581, 43–47. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, L.; Chen, X.; He, W.; Yu, X. Associations study of vitamin D receptor gene polymorphisms with diabetic microvascular complications: A meta-analysis. Gene 2014, 546, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, R.; Ruwende, C.; Corrah, T.; McAdam, K.P.; Thursz, M.; Whittle, H.C.; Hill, A.V.S. Tuberculosis and Chronic Hepatitis B virus infection in Africans and variation in vitamin D receptor gene. J. Infect. Dis. 1999, 179, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Suneetha, P.V.; Sarin, S.K.; Goyal, A.; Kumar, G.T.; Shukla, D.K.; Hissar, S. Association between vitamin D receptor, CCR5, TNF-alpha and TNF-beta gene polymorphisms and HBV infection and severity of liver disease. J. Hepatol. 2006, 44, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Apaydın, M.; Beysel, S.; Eyerci, N.; Pinarli, F.A.; Ulubay, M.; Kizilgul, M.; Ozdemir, O.; Caliskan, M.; Cakal, E. The VDR gene FokI polymorphism is associated with gestational diabetes mellitus in Turkish women. BMC Med. Genet. 2019, 20, 1–6. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, J. Vitamin D receptor rs2228570 polymorphism and susceptibility to ovarian cancer: An updated meta-analysis. J. Obs. Gynecol. Res. 2017, 44, 556–565. [Google Scholar] [CrossRef]
- Loke, H.; Bethell, D.; Phuong, C.X.T.; Day, N.; White, N.; Farrar, J. Susceptibility to dengue hemorrhagic fever in Vietnam: Evidence of an association with variation in vitamin D receptor and FC gamma receptor IIA genes. Am. J. Trop. Med. Hyg. 2002, 67, 102–106. [Google Scholar] [CrossRef]
- Algarasu, K.; Honap, T.; Mulay, A.P.; Bachal, R.V.; Shah, P.S.; Dayaraj, C. Association of vitamin D receptor gene polymorphisms with clinical outcomes of dengue virus infection. Hum. Immunol. 2012, 73, 1194–1199. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
- Torres, C.; Sanchez de la Torre, M.; Garcia-Moroja, C.; Carrero, A.J.; Trujillo Mdel, M.; Fibla, J.; Caruz, A. Immunophenotype of vitamin D receptor pleomorphism associated to risk of HIV-1 infection and of disease progression. Curr. HIV Res. 2010, 8, 487–492. [Google Scholar] [CrossRef]
- Rigby, W.F.; Shen, L.; Ball, E.D.; Guyre, P.M.; Fanger, M.W. Differentiation of human monocytic cell line by 1,25-dihydroxyvitamin D3 (calcitriol): A morphologic phenotypic and functional analysis. Blood 1984, 64, 1110–1115. [Google Scholar] [CrossRef]
- Arboleda Alzate, J.F.; Rodenhuis-Zybert, I.A.; Hernandez, J.C.; Smit, J.M.; Urcuqui-Inchima, S. Human macrophages differentiated in the presence of vitamin D3 restrict dengue virus infection and innate responses by down regulating mannose receptor expression. PLoS Negl. Trop. Dis. 2017, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Algarasu, K.; Bachal, R.V.; Bhagat, A.B.; Shah, P.S.; Dayaraj, C. Elevated levels of vitamin D and deficiency of manose binding lectin in dengue haemorrhagic fever. Virol. J. 2012, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Pani, M.A.; Knapp, M.; Donner, H.; Braun, J.; Baur, M.P.; Usadel, K.H.; Badenhoop, K. Vitamin D receptor allele combinations influence genetic susceptibility to type I diabetes in Germans. Diabetes 2000, 49, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control—New Edition. WHO and TDR, 2009. Available online: https://www.who.int/tdr/publications/documents/dengue-diagnosis.pdf (accessed on 25 September 2019).
- Chakravarti, A.; Kumaria, R. Eco-epidemiological analysis of dengue infection during an outbreak of dengue fever, India. Virol. J. 2005, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, A.; Arora, R.; Luxemburger, C. Fifty years of dengue in India. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Abhishek, K.S.; Chakravarti, A.; Baveja, C.P.; Kumar, N.; Siddiqui, O.; Kumar, S. Association of interleukin-2, -4 and -10 with dengue severity. Ind. J. Pathol. Microbiol. 2017, 60, 66–69. [Google Scholar]
- Giraldo, D.M.; Cardona, A.; Urcuqui-inchima, S. High dose vitamin D supplement is associated with reduced susceptibility of monocyte-derived macrophages to dengue virus infection and pro-inflammatory cytokine production: An exploratory study. Clin. Chim. Acta 2018, 478, 140–151. [Google Scholar] [CrossRef]
| Clinical Symptoms | n |
| Fever | 43 |
| Headache | 33 |
| Retro orbital pain | 37 |
| Arthralgia | 29 |
| Myalgia | 27 |
| Clinical Sign | n |
| Rash | 9 |
| Malena | 10 |
| Petechiae | 27 |
| Gum bleeding | 2 |
| Epistaxis | 4 |
| Hematuria | 4 |
| Hepatomegaly | 1 |
| Splenomegaly | 1 |
| Groups (n) | Vitamin D Levels (nmol/L) Mean ± SD | 95% CI | p Value |
|---|---|---|---|
| Controls (100) | 33.8 ± 10.2 | 31.8–35.8 | - |
| Cases (43) | 42.5 ± 14.2 | 38.3–46.7 | 0.002 |
| Non-severe (28) | 36.6 ± 8.7 | 33.4–39.8 | 0.037 |
| Severe (15) | 53.5 ± 16.3 | 45.2–61.7 | <0.0001 |
| Primary (25) | 34.6 ± 8.9 | 31.1–38.1 | <0.0001 |
| Secondary (18) | 53.5 ± 13.8 | 47.1–59.9 | |
| Primary non-Severe (20) | 34.2 ± 8.8 | 30.3–38 | 0.778 |
| Primary Severe (5) | 42.6 ± 4.9 | 38.3–46.9 | |
| Secondary Non-severe (8) | 35.5 ± 5.1 | 32–39 | 0.003 |
| Secondary Severe (10) | 60.0 ± 13.6 | 59.1–60.9 |
| Genotypes | Controls (100) n (%) | Cases (43) n (%) | OR (95% CI) | p Value |
|---|---|---|---|---|
| CC | 59 (59) | 11 (25.6) | 1.0 (Ref) | |
| CT | 25 (25) | 18 (41.9) | 3.86 (1.59–9.35) | 0.002 |
| TT | 16 (16) | 14 (32.6) | 4.69 (1.79–12.3) | 0.001 |
| AA | 31 (31) | 15 (34.9) | 1.0 (Ref) | 0.032 |
| AC | 40 (40) | 24 (55.8) | 1.24 (0.55–2.75) | |
| CC | 29 (29) | 4 (9.3) | 0.28 (0.08–0.96) |
| Genotypes | Non-Severe *n (%) | Severe *n (%) | OR (95% CI) | #p Value |
|---|---|---|---|---|
| AA | 7 (58.3) | 5 (41.7) | 1.0 (Ref) | - |
| AC | 17 (70.8) | 7 (29.2) | 0.57 (0.13–2.44) | 0.453 |
| CC | 4 (57.1) | 3 (42.9) | 1.05 (0.15–6.92) | 0.959 |
| CC | 12 (57.1) | 9 (42.9) | 1.0 (Ref) | - |
| CT | 14 (82.4) | 3 (17.6) | 0.28 (0.06–1.30) | 0.09 |
| TT | 2 (65.1) | 3 (34.9) | 2.00 (0.27–14.58) | 0.48 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakravarti, A.; Bharara, T.; Kapoor, N.; Ashraf, A. Levels of 25-hydroxy Vitamin D3 and Vitamin D Receptor Polymorphism in Severe Dengue Cases from New Delhi. Trop. Med. Infect. Dis. 2020, 5, 72. https://doi.org/10.3390/tropicalmed5020072
Chakravarti A, Bharara T, Kapoor N, Ashraf A. Levels of 25-hydroxy Vitamin D3 and Vitamin D Receptor Polymorphism in Severe Dengue Cases from New Delhi. Tropical Medicine and Infectious Disease. 2020; 5(2):72. https://doi.org/10.3390/tropicalmed5020072
Chicago/Turabian StyleChakravarti, Anita, Tanisha Bharara, Neeru Kapoor, and Anzar Ashraf. 2020. "Levels of 25-hydroxy Vitamin D3 and Vitamin D Receptor Polymorphism in Severe Dengue Cases from New Delhi" Tropical Medicine and Infectious Disease 5, no. 2: 72. https://doi.org/10.3390/tropicalmed5020072
APA StyleChakravarti, A., Bharara, T., Kapoor, N., & Ashraf, A. (2020). Levels of 25-hydroxy Vitamin D3 and Vitamin D Receptor Polymorphism in Severe Dengue Cases from New Delhi. Tropical Medicine and Infectious Disease, 5(2), 72. https://doi.org/10.3390/tropicalmed5020072




