Clinical Study on the Melarsoprol-Related Encephalopathic Syndrome: Risk Factors and HLA Association
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Conduct
2.2. Study Sites
2.3. Study Population
2.4. Study Methodology
2.5. Data Management
2.6. HLA Typization
2.7. Statistical Analysis
3. Results
3.1. Characteristics of the Encephalopathic Syndrome
3.1.1. Clinical Features
3.1.2. Laboratory Evaluation of ES
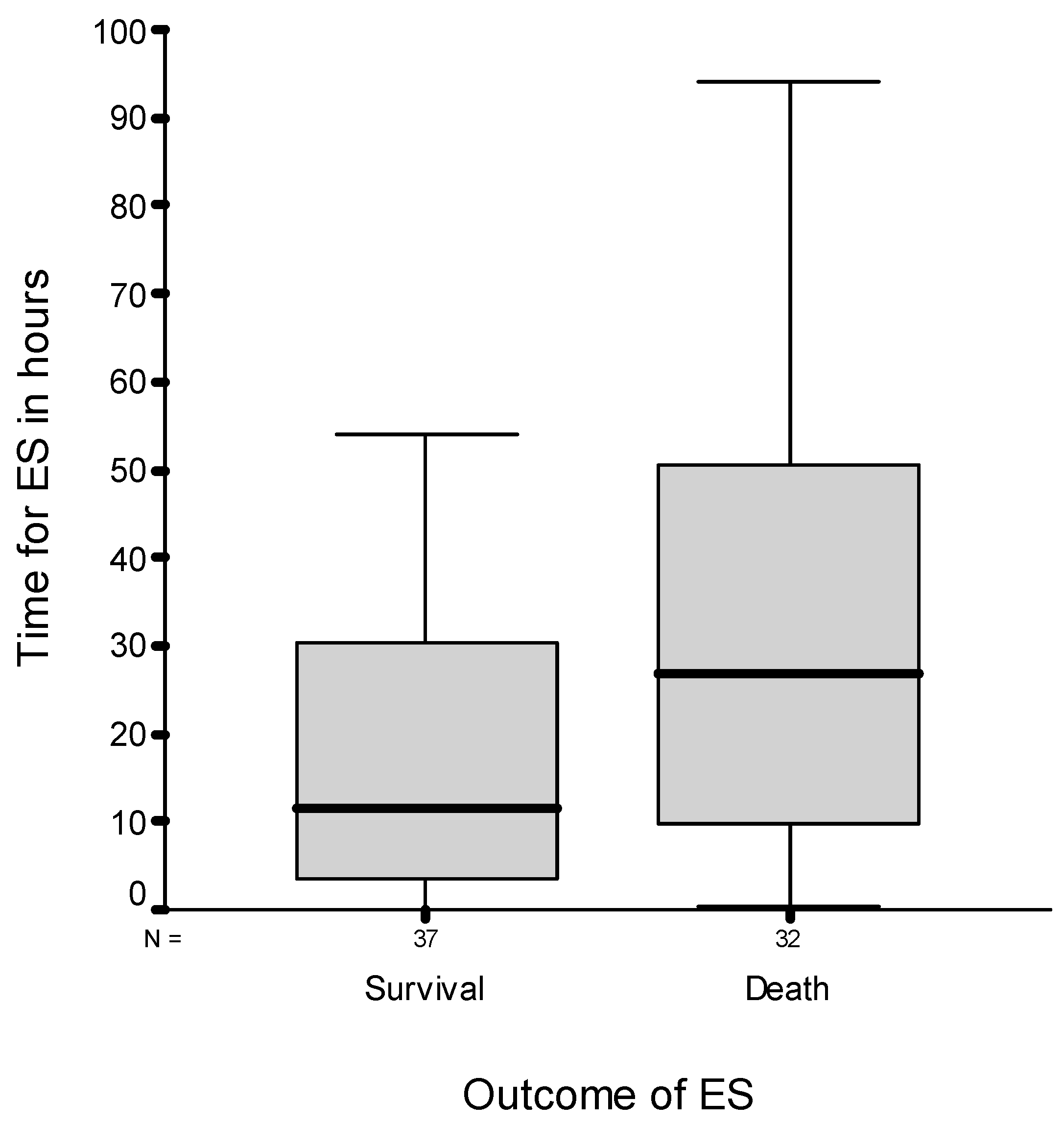
3.1.3. Time Point and Outcome for ES
3.1.4. Patient Management
3.2. Risk Factors for the Encephalopathic Syndrome
3.2.1. Clinical Factors
3.2.2. Laboratory Data
3.2.3. Concomitant Diseases
3.2.4. HLA Type
4. Discussion
4.1. Characteristics of ES
4.2. Risk Factors for ES
4.3. Patient Management
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brun, R.; Blum, J.; Chappuis, F.; Burri, C. Human African trypanosomiasis. Lancet 2010, 375, 148–159. [Google Scholar] [CrossRef]
- Franco, J.R.; Cecchi, G.; Priotto, G.; Paone, M.; Diarra, A.; Grout, L.; Simarro, P.P.; Zhao, W.; Argaw, D. Monitoring the elimination of human African trypanosomiasis: Update to 2016. PLoS Negl. Trop. Dis. 2018, 12, e0006890. [Google Scholar] [CrossRef] [PubMed]
- WHO. Accelerating Work to Overcome the Global Impact of Neglected Tropical Diseases—A Roadmap for Implementation; WHO/HTM/NTD/2012.1; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Kennedy, P.G.E.; Rodgers, J. Clinical and Neuropathogenetic Aspects of Human African Trypanosomiasis. Front. Immunol. 2019, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- WHO. Control and Surveillance of Human African Trypanosomiasis; Technical Report Series No. 984; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Pelfrene, E.; Harvey Allchurch, M.; Ntamabyaliro, N.; Nambasa, V.; Ventura, F.V.; Nagercoil, N.; Cavaleri, M. The European Medicines Agency’s. scientific opinion on oral fexinidazole for human African trypanosomiasis. PLoS Negl. Trop. Dis. 2019, 13, e0007381. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Interim Guidelines for the Treatment of Gambiense Human African Trypanosomiasis; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-4-155056-7. [Google Scholar]
- Fairlamb, A.H. Chemotherapy of human African trypanosomiasis: Current and future prospects. Trends Parasitol 2003, 19, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Blum, J.; Nkunku, S.; Burri, C. Clinical description of encephalopathic syndromes and risk factors for their occurrence and outcome during melarsoprol treatment of human African trypanosomiasis. Trop. Med. Int. Health 2001, 6, 390–400. [Google Scholar] [CrossRef]
- Pepin, J.; Milord, F.; Khonde, A.N.; Niyonsenga, T.; Loko, L.; Mpia, B.; De Wals, P. Risk factors for encephalopathy and mortality during melarsoprol treatment of Trypanosoma brucei gambiense sleeping sickness. Trans. R. Soc. Trop. Med. Hyg. 1995, 89, 92–97. [Google Scholar] [CrossRef]
- Seixas, J. Investigations on the Encephalopathic Syndrome during Melarsoprol Treatment of Human African Trypanosomiasis. Ph.D. Thesis, Instituto de Higiene e Medicina Tropical, Universidade Nova de Lisboa, Lisboa, Portugal, 2004. [Google Scholar]
- Dutertre, J.; Labusquiere, R. La thérapeutique de la trypanosomiase. Méd. Trop. (Marseille) 1966, 26, 342–356. [Google Scholar]
- Bertrand, E.; Serie, F.; Rive, J.; Compaore, P.; Sentilhes, L.; Baudin, L.; Ekra, A.; Philippe, J. La symptomatologie cardio-vasculaire dans la trypanosomiase humaine africaine à Trypanosoma gambiense. Méd. Afr. Noire 1973, 20, 327–339. [Google Scholar]
- Ginoux, P.Y.; Bissadidi, N.; Frezil, J.L. Accidents observes lors du traitement de la trypanosomiase au Congo. Méd. Trop. (Marseille) 1984, 44, 351–355. [Google Scholar]
- Scena, M.R. Melarsoprol toxicity in the treatment of human African trypanosomiasis: Ten cases treated with dimercaprol. Cent. Afr. J. Med. 1988, 34, 264–268. [Google Scholar] [PubMed]
- Haller, L.; Adams, H.; Merouze, F.; Dago, A. Clinical and pathological aspects of human African trypanosomiasis (T. b. gambiense) with particular reference to reactive arsenical encephalopathy. Am. J. Trop. Med. Hyg. 1986, 35, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Atouguia, J.L.M.; Kennedy, P.G.E. Neurological aspects of human African trypanosomiasis. In Infectious Diseases of the Nervous System, 1st ed.; Davis, L.E., Kennedy, P.G.E., Eds.; Reed Educational and Professional Publishing Ltd.: Oxford, UK, 2000. [Google Scholar]
- Pepin, J.; Milord, F. African trypanosomiasis and drug-induced encephalopathy; risk factors and pathogenesis. Trans. R. Soc. Trop. Med. Hyg. 1991, 85, 222–224. [Google Scholar] [CrossRef]
- Hunter, C.A.; Jennings, F.W.; Adams, J.H.; Murray, M.; Kennedy, P.G.E. Subcurative chemotherapy and fatal post-treatment reactive encephalopathies in African trypanosomiasis. Lancet 1992, 339, 956–958. [Google Scholar] [CrossRef]
- Bouteille, B.; Millet, P.; Enanga, B.; Mezui Me, J.; Keita, M.; Jauberteau, M.O.; Georges, A.; Dumas, M. Human African trypanosomiasis, contributions of experimental models. Bulletin de la Société de Pathologie Exotique et ses Filiales 1998, 91, 127–132. [Google Scholar]
- Keiser, J.; Ericsson, O.; Burri, C. Investigations of the metabolites of the trypanocidal drug melarsoprol. Clin. Pharmacol. Ther. 2000, 67, 478–488. [Google Scholar] [CrossRef]
- Choo, S.Y. The HLA system: Genetics, immunology, clinical testing, and clinical implications. Yonsei Med. J. 2007, 48, 11–23. [Google Scholar] [CrossRef]
- Klein, J.; Sato, A. The HLA system. First of two parts. N. Engl. J. Med. 2000, 343, 702–709. [Google Scholar] [CrossRef]
- Singh, N.; Agrawal, S.; Rastogi, A.K. Infectious diseases and immunity: Special reference to major histocompatibility complex. Emerg. Infect. Dis. 1997, 3, 41–49. [Google Scholar] [CrossRef]
- Abel, L.; Dessein, A.J. Genetic epidemiology of infectious diseases in humans: Design of population-based studies. Emerg. Infect. Dis. 1998, 4, 593–603. [Google Scholar] [CrossRef]
- Saiki, R.K.; Walsh, P.S.; Levenson, C.H.; Erlich, H.A. Genetic analysis of amplified DNA with immobilized sequence-specific oligonucleotide probes. Proc. Natl. Acad. Sci. USA 1989, 86, 6230–6234. [Google Scholar] [CrossRef] [PubMed]
- Buyse, I.; Decorte, R.; Baens, M.; Cuppens, H.; Semana, G.; Emonds, M.P.; Marynen, P.; Cassiman, J.J. Rapid DNA typing of class II HLA antigens using the polymerase chain reaction and reverse dot blot hybridization. Tissue Antigens 1993, 41, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bugawan, T.L.; Apple, R.; Erlich, H.A. A method for typing polymorphism at the HLA-A locus using PCR amplification and immobilized oligonucleotide probes. Tissue Antigens 1994, 44, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Begovich, A.B.; Erlich, H.A. HLA typing for bone marrow transplantation. New polymerase chain reaction-based methods. JAMA 1995, 273, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Bodmer, J.G.; Bodmer, W.F. Studies on African Pygmies. IV. A comparative study of the HLA polymorphism in the Babinga Pygmies and other African and Caucasian populations. Am. J. Hum. Genet. 1970, 22, 396–411. [Google Scholar] [PubMed]
- Burri, C.; Nkunku, S.; Merolle, A.; Smith, T.; Blum, J.; Brun, R. Efficacy of new, concise schedule for melarsoprol in treatment of sleeping sickness caused by Trypanosoma brucei gambiense: A randomised trial. Lancet 2000, 355, 1419–1425. [Google Scholar] [CrossRef]
- Tenembaum, S.; Chitnis, T.; Ness, J.; Hahn, J.S. Acute disseminated encephalomyelitis. Neurology 2007, 68, S23–S36. [Google Scholar] [CrossRef]
- Lann, M.A.; Lovell, M.A.; Kleinschmidt-DeMasters, B.K. Acute hemorrhagic leukoencephalitis: A critical entity for forensic pathologists to recognize. Am. J. Forensic. Med. Pathol. 2010, 1, 7–11. [Google Scholar] [CrossRef]
- Sina, G.C.; Triolo, N.; Trova, P.; Clabaut, J.M. L’encephalopathie arsenicale lors du traitement de la trypanosomiase humaine africaine a T. gambiense (à propos de 16 cas). Ann. Société Belg. Méd. Trop. 1977, 57, 67–73. [Google Scholar]
- Buyst, H. The treatment of T. rhodesiense sleeping sickness, with special reference to its physio-pathological and epidemiological basis. Ann. Socièté Belg. Méd. Trop. 1975, 55, 95–104. [Google Scholar]
- Schmid, C.; Richer, M.; Bilenge, C.M.; Josenando, T.; Chappuis, F.; Manthelot, C.R.; Nangouma, A.; Doua, F.; Asumu, P.N.; Simarro, P.P.; et al. Effectiveness of a 10-day melarsoprol schedule for the treatment of late-stage human African trypanosomiasis: Confirmation from a multinational study (IMPAMEL II). J. Infect. Dis. 2005, 191, 1922–1931. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blum, J.; Schmid, C.; Burri, C. Clinical aspects of 2541 patients with second stage human African trypanosomiasis. Acta Trop. 2006, 97, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Mohr, A.; Knauth, M.; Wildemann, B.; Storch-Hagenlocher, B. Acute disseminated encephalomyelitis: A follow-up study of 40 adult patients. Neurology 2001, 56, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Nakano, A.; Yamasaki, R.; Miyazaki, S.; Horiuchi, N.; Kunishige, M.; Mitsui, T. Beneficial effect of steroid pulse therapy on acute viral encephalitis. Eur. Neurol. 2003, 50, 225–229. [Google Scholar] [CrossRef]
- Büscher, P.; Cecchi, G.; Jamonneau, V.; Priotto, G. Human African trypanosomiasis. Lancet 2017, 390, 2397–2409. [Google Scholar] [CrossRef]
| HAT Treatment Centre | Number of ES Patients | Deaths |
|---|---|---|
| Mbuji Mayi (DRC) | 24 (35%) | 10 (41.6%) |
| Viana (Angola) | 21 (30%) | 10 (47.6%) |
| Maluku (DRC) | 8 (12%) | 4 (50%) |
| Uíge (Angola) | 7 (10%) | 4 (57.1%) |
| N’Dalatando (Angola) | 6 (9%) | 3 (50%) |
| CNPP (DRC) | 3 (4%) | 1 (33.3%) |
| Type of Manifestation of ES | Cases N/(%) | Outcome Survival n/% | Additional Signs n | Outcome Death N/% | Additional Signs n |
|---|---|---|---|---|---|
| Convulsion and coma | 38/(55) | 13/(18.8) | Fever in 3 Urticaria in 3 Fever and urticaria in 1 | 25/(36.2) | Fever in 15 Urticaria in 7 Fever and urticarial in 5 |
| Convulsion without coma | 21/(30.5) | 19/(27.5) | Fever in 6 Urticaria in 4 Fever and urticaria in 2 | 2/(2.9) | Fever in 1 |
| Coma without Convulsions | 10/(14.5) | 5/(7.2) | Fever in 2 Fever and urticaria in 1 | 5/(7.2) | Fever in 2 Urticaria in 1 |
| Totals | 69/(100) | 37/(53.6) | Fever in 11 Urticaria in 7 Fever and urticaria in 4 | 32/(46.4) | Fever in 18 Urticaria in 8 Fever and urticaria in 6 |
| Characterization of ES Symptoms and Signs | Frequency (Global) % (n) | Frequency (Degree 1) % | Frequency (Degree 2) % |
|---|---|---|---|
| Malaise | 79.7 (55) | 20.3 | 59.4 |
| Confusional state | 78.3 (51) | 26.1 | 52.2 |
| Fever | 69.6 (48) | 52.2 | 17.4 |
| Agitation | 65.2 (45) | 34.8 | 30.4 |
| Tachycardia | 60.9 (42) | 39.1 | 21.7 |
| Respiratory distress | 59.4 (41) | 36.2 | 23.2 |
| Headache | 56.5 (39) | 37.7 | 18.8 |
| Apathy | 56.5 (39) | 24.6 | 31.9 |
| Maculopapular eruption | 50.7 (35) | 10.1 | 40.6 |
| Chills | 36.2 (25) | 24.6 | 11.6 |
| Red eye syndrome | 33.3 (23) | 33.3 | 0 |
| Vertigo | 29.0 (20) | 20.3 | 8.7 |
| Oedema (facial) | 23.2 (16) | 23.2 | 0 |
| Vomiting | 23.2 (16) | 14.5 | 8.7 |
| Babinski sign | 20.3 (14) | 20.3 | 0 |
| Panic attack | 18.8 (13) | 14.5 | 4.3 |
| Nausea | 18.8 (13) | 13.0 | 5.8 |
| Hypotension | 15.9 (11) | 13.0 | 2.9 |
| Hallucinations | 10.1 (07) | 8.7 | 1.4 |
| Delirium | 10.1 (07) | 0 | 10.1 |
| Nucal rigidity | 10.1 (07) | 10.1 | 0 |
| Aggressive behaviour | 07.2 (05) | 4.3 | 2.9 |
| Variable | Encephalopathy | Death | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Anamnesis | ||||||
| Oedema | 4.0 | 1.0–15.4 | 0.03 | 1.8 | 0.2–11.5 | 0.09 |
| Bone pain | 2.3 | 1.2–4.3 | 0.10 | |||
| Paralysis | 3.0 | 0.4–22.0 | 0.20 | |||
| Hypoestesia | 2.0 | 0.3–12.2 | 0.40 | |||
| Abdominal pain | 4.5 | 1.5–14.0 | 0.006 | |||
| Conjunctivitis | 3.0 | 1.0–8.7 | 0.03 | |||
| Pruritus | 3.0 | 1.0–8.7 | 0.04 | |||
| Adenomegaly | 2.2 | 0.7–6.1 | 0.1 | |||
| Weight loss | 2 | 0.6–6.6 | 0.2 | |||
| Weakness/asthenia | 2 | 0.5–7.4 | 0.3 | |||
| Diarrhoea | 4.9 | 0.9–25.6 | 0.4 | |||
| Physical examination | ||||||
| Apathy | 1.9 | 1.0–3.8 | 0.04 | |||
| Depression | 1.9 | 0.9–4.2 | 0.07 | |||
| Facial paralysis | 3.5 | 0.6–17.8 | 0.1 | |||
| Incomprehensible Speech | 2.2 | 0.5–7.7 | 0.2 | |||
| Babinski | 2.2 | 0.6–7.7 | 0.3 | 3.6 | 0.4–36.7 | 0.2 |
| Pale mucosa | 2.4 | 0.2–27.7 | 0.4 | |||
| Neck rigidity | 2.4 | 0.2–27.7 | 0.4 | |||
| Splenomegaly | 2.8 | 0.2–33.6 | 0.4 | |||
| Agitation | 2.4 | 0.2–27.7 | 0.5 | |||
| Cases | Controls | p | OR | CI | LD Cases | |
|---|---|---|---|---|---|---|
| C*14/B*15 | 6/62 | 3/189 | 0.008 (*) | 6.64 | 1.35–41.96 | 0.038 |
| A*23/C*14 | 3/62 | 1/189 | 0.04 (*) | 9.56 | 0.74–50.4 | 0.01 |
| A*23/B*15 | 13/62 | 22/189 | 0.06 | 2.0 | 0.88–4.56 | 0.04 |
| DR*07/B*58 | 5/62 | 5/189 | 0.07 (*) | 3.23 | 0.71–14.49 | 0.017 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seixas, J.; Atouguia, J.; Josenando, T.; Vatunga, G.; Miaka Mia Bilenge, C.; Lutumba, P.; Burri, C. Clinical Study on the Melarsoprol-Related Encephalopathic Syndrome: Risk Factors and HLA Association. Trop. Med. Infect. Dis. 2020, 5, 5. https://doi.org/10.3390/tropicalmed5010005
Seixas J, Atouguia J, Josenando T, Vatunga G, Miaka Mia Bilenge C, Lutumba P, Burri C. Clinical Study on the Melarsoprol-Related Encephalopathic Syndrome: Risk Factors and HLA Association. Tropical Medicine and Infectious Disease. 2020; 5(1):5. https://doi.org/10.3390/tropicalmed5010005
Chicago/Turabian StyleSeixas, Jorge, Jorge Atouguia, Teófilo Josenando, Gedeão Vatunga, Constantin Miaka Mia Bilenge, Pascal Lutumba, and Christian Burri. 2020. "Clinical Study on the Melarsoprol-Related Encephalopathic Syndrome: Risk Factors and HLA Association" Tropical Medicine and Infectious Disease 5, no. 1: 5. https://doi.org/10.3390/tropicalmed5010005
APA StyleSeixas, J., Atouguia, J., Josenando, T., Vatunga, G., Miaka Mia Bilenge, C., Lutumba, P., & Burri, C. (2020). Clinical Study on the Melarsoprol-Related Encephalopathic Syndrome: Risk Factors and HLA Association. Tropical Medicine and Infectious Disease, 5(1), 5. https://doi.org/10.3390/tropicalmed5010005





