High Levels of Treatment Success and Zero Relapse in Multidrug-Resistant Tuberculosis Patients Receiving a Levofloxacin-Based Shorter Treatment Regimen in Vietnam
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting
2.2.1. Diagnosis and Treatment of MDR-TB
2.2.2. The Treatment Regimen
2.2.3. Follow-Up Schedule during the Treatment
2.2.4. Follow-Up Schedule Post-Treatment
2.3. Study Population
2.4. Data Variables, Sources of Data and Data Collection
2.5. Analysis and Statistics
2.6. Ethics Approval
3. Results
3.1. Baseline Sociodemographic and Clinical Characteristics
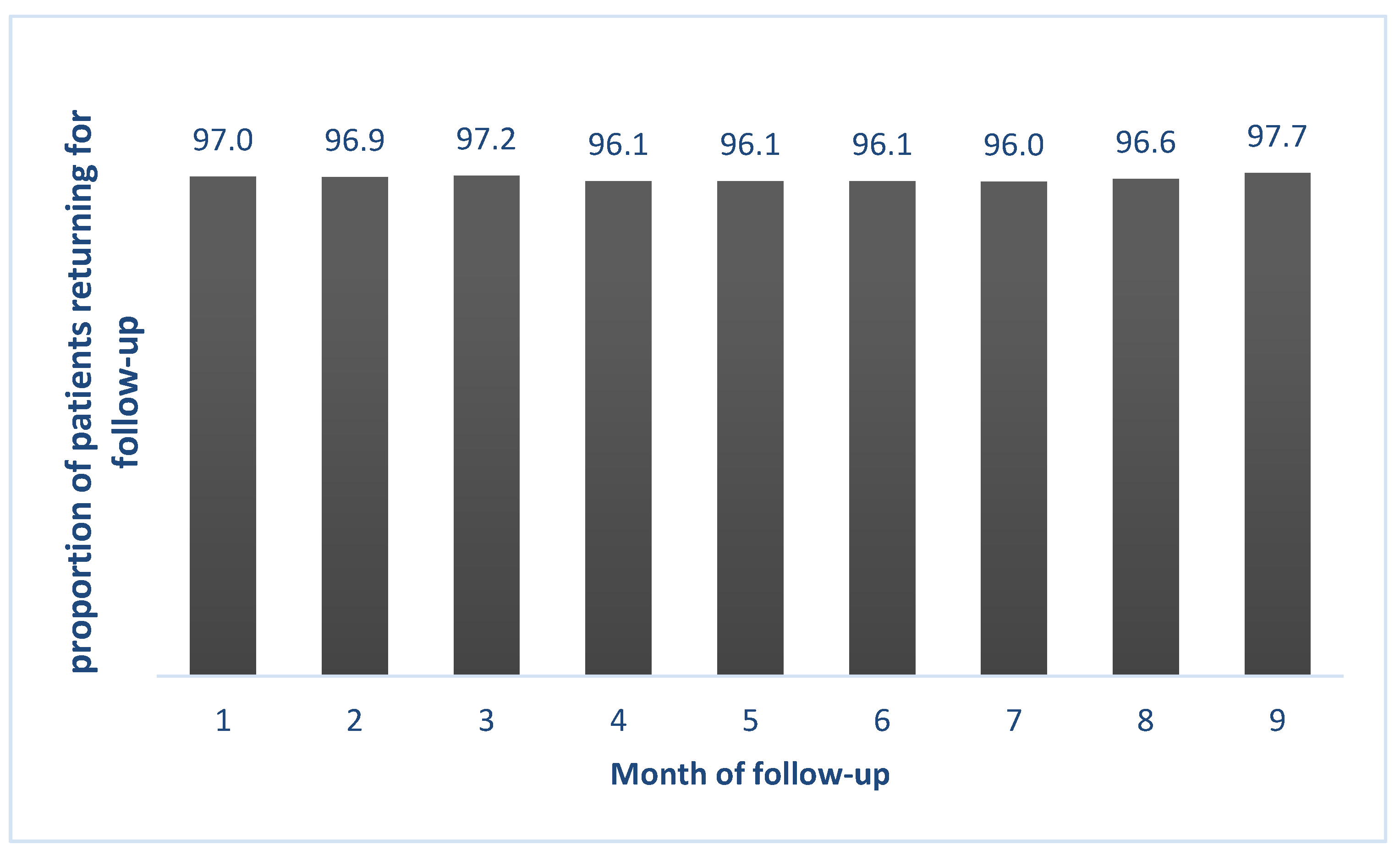
3.2. Adherence to Monthly Follow-Up Visits
3.3. Treatment Outcomes
3.4. One-Year TB Relapse
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Global Tuberculosis Report 2019; World Health Organization: Geneva, Switzerland, 2019; Volume 66. [Google Scholar]
- World Health Organization (WHO). WHO Meeting Report of a Technical Expert Consultation: Non-Inferiority Analysis of Xpert MTB/RIF Ultra Compared to Xpert MTB/RIF; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Cepheid GeneXpert Omni. Available online: http://www.cepheid.com/en/genexpert-omni/47?view=products (accessed on 7 February 2019).
- Xie, Y.L.; Chakravorty, S.; Armstrong, D.T.; Hall, S.L.; Via, L.E.; Song, T.; Yuan, X.; Mo, X.; Zhu, H.; Xu, P.; et al. Evaluation of a Rapid Molecular Drug-Susceptibility Test for Tuberculosis. N. Engl. J. Med. 2017, 377, 1043–1054. [Google Scholar]
- World Health Organization (WHO). The Use of Molecular Line Probe Assays for the Detection of Resistance to Second-Line Anti-Tuberculosis Drugs; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- World Health Organization (WHO). The Use of Bedaquiline in the Treatment of Multidrug-Resistant Tuberculosis Interim Policy Guidance; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- World Health Organization (WHO). The Use of Delamanid in the Treatment of Multidrug-Resistant Tuberculosis Interim Policy Guidance; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- World Health Organization (WHO). Rapid Communication: Key Changes to Treatment of Multidrug- and Rifampicin-Resistant Tuberculosis (MDR/RR-TB); WHO: Geneva, Switzerland, 2018. [Google Scholar]
- World Health Organization (WHO). WHO Treatment Guidelines for Drug-Resistant Tuberculosis. 2016 Updated; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Kuaban, C.; Noeske, J.; Rieder, H.L.; Aït-Khaled, N.; Abena Foe, J.L.; Trébucq, A. High effectiveness of a 12-month regimen for MDR-TB patients in Cameroon. Int. J. Tuberc. Lung Dis. 2015, 19, 517–524. [Google Scholar] [PubMed]
- Piubello, A.; Harouna, S.H.; Souleymane, M.B.; Boukary, I.; Morou, S.; Daouda, M.; Hanki, Y.; Van Deun, A. High cure rate with standardised short-course multidrugresistant tuberculosis treatment in Niger: No relapses. Int. J. Tuberc. Lung Dis. 2014, 18, 1188–1194. [Google Scholar] [PubMed]
- Aung, K.J.M.; Van Deun, A.; Declercq, E.; Sarker, M.R.; Das, P.K.; Hossain, M.A.; Rieder, H.L. Successful ‘9-month Bangladesh regimen’ for multidrugresistant tuberculosis among over 500 consecutive patients. Int. J. Tuberc. Lung Dis. 2014, 18, 1180–1187. [Google Scholar]
- Ahmad Khan, F.; Salim, M.A.H.; du Cros, P.; Casas, E.C.; Khamraev, A.; Sikhondze, W.; Benedetti, A.; Bastos, M.; Lan, Z.; Jaramillo, E.; et al. Effectiveness and safety of standardised shorter regimens for multidrug-resistant tuberculosis: Individual patient data and aggregate data meta-analyses. Eur. Respir. J. 2017, 50, 1–13. [Google Scholar]
- Trébucq, A.; Schwoebel, V.; Kashongwe, Z.; Bakayoko, A.; Kuaban, C.; Noeske, J.; Hassane, S.; Souleymane, B.; Piubello, A.; Ciza, F.; et al. Treatment outcome with a short multidrug-resistant tuberculosis regimen in nine African countries. Int. J. Tuberc. Lung Dis. 2018, 22, 17–25. [Google Scholar] [PubMed]
- Nunn, A.J.; Phillips, P.P.J.; Meredith, S.K.; Chiang, C.Y.; Conradie, F.; Dalai, D.; Van Deun, A.; Dat, P.T.O.; Lan, N.; Master, I.; et al. A trial of a shorter regimen for rifampin-resistant tuberculosis. N. Engl. J. Med. 2019, 380, 1201–1213. [Google Scholar] [PubMed]
- Nunn, A.J.; Rusen, I.D.; Van Deun, A.; Torrea, G.; Phillips, P.P.J.; Chiang, C.Y.; Squire, S.B.; Madan, J.; Meredith, S.K. Evaluation of a standardized treatment regimen of anti-tuberculosis drugs for patients with multi-drug-resistant tuberculosis (STREAM): Study protocol for a randomized controlled trial. Trials 2014, 15, 1–10. [Google Scholar]
- Park-Wyllie, L.Y.; Juurlink, D.N.; Kopp, A.; Shah, B.R.; Stukel, T.A.; Stumpo, C.; Dresser, L.; Low, D.E.; Mamdani, M.M. Outpatient Gatifloxacin Therapy and Dysglycemia in Older Adults. N. Engl. J. Med. 2006, 354, 1352–1361. [Google Scholar] [PubMed]
- Chiang, C.Y.; Van Deun, A.; Rieder, H.L. Gatifloxacin for short, effective treatment of multidrug-resistant tuberculosis. Int. J. Tuberc. Lung Dis. 2016, 20, 1143–1147. [Google Scholar] [PubMed]
- Chiang, C.Y.; Trébucq, A.; Piubello, A.; Rieder, H.L.; Van Deun, A. Should gatifloxacin be included in the model list of essential medicines? Eur. Respir. J. 2018, 51, 1702329. [Google Scholar] [PubMed]
- Chou, H.W.; Wang, J.L.; Chang, C.H.; Lee, J.J.; Shau, W.Y.; Lai, M.S. Risk of severe dysglycemia among diabetic patients receiving levofloxacin, ciprofloxacin, or moxifloxacin in Taiwan. Clin. Infect. Dis. 2013, 57, 971–980. [Google Scholar] [PubMed]
- Cox, V.; Tommasi, M.; Sa, A.; Furin, J.; Quelapio, M.; Koura, K.G.; Padanilam, X.; Dravniece, G.; Piubello, A. QTc and anti-tuberculosis drugs: A perfect storm or a tempest in a teacup? Review of evidence and a risk assessment. Int. J. Tuberc. Lung Dis. 2018, 18, 0423. [Google Scholar]
- Van Deun, A.; Decroo, T.; Piubello, A.; de Jong, B.C.; Lynen, L.; Rieder, H.L. Principles for constructing a tuberculosis treatment regimen: The role and definition of core and companion drugs. Int. J. Tuberc. Lung Dis. 2018, 22, 239–245. [Google Scholar] [PubMed]
- Makaryus, A.N.; Byrns, K.; Makaryus, M.N.; Natarajan, U.; Singer, C.; Goldner, B. Effect of ciprofloxacin and levofloxacin on the QT interval: Is this a significant “clinical” event? South. Med. J. 2006, 99, 52–56. [Google Scholar] [PubMed]
- Deshpande, D.; Pasipanodya, J.G.; Mpagama, S.G.; Bendet, P.; Srivastava, S.; Koeuth, T.; Lee, P.S.; Bhavnani, S.M.; Ambrose, P.G.; Thwaites, G.; et al. Levofloxacin Pharmacokinetics/Pharmacodynamics, Dosing, Susceptibility Breakpoints, and Artificial Intelligence in the Treatment of Multidrug-resistant Tuberculosis. Clin. Infect. Dis. 2018, 67, S293–S302. [Google Scholar] [PubMed]
- Van Deun, A.; Decroo, T.; Kuaban, C.; Noeske, J.; Piubello, A.; Aung, K.J.M.; Rieder, H.L. Gatifloxacin is superior to levofloxacin and moxifloxacin in shorter treatment regimens for multidrug-resistant TB. Int. J. Tuberc. Lung Dis. 2019, 23, 965–971. [Google Scholar] [PubMed]
- Piubello, A.; Aït-khaled, N.; Caminero, J.A.; Chiang, C.Y.; Dlodlo, R.A.; Fujiwara, P.I.; Heldal, E.; Koura, K.; Monedero, I.; Roggi, A.; et al. Field Guide for the Management of Drug-Resistant Tuberculosis, 1st ed.; International Union Against Tuberculosis and Lung Disease: Paris, France, 2018. [Google Scholar]
- Id, G.B.; Nhung, N.V.; Skrahina, A.; Ndjeka, N.; Id, D.F.; Zignol, M. Advances in clinical trial design for development of new TB treatments—Translating international tuberculosis treatment guidelines into national strategic plans: Experiences from Belarus, South Africa, and Vietnam. PLoS Med. 2019, 16, e1002896. [Google Scholar]
- Khan, F.; Ismail, M.; Khan, Q.; Ali, Z. Moxifloxacin-induced QT interval prolongation and torsades de pointes: A narrative review. Expert Opin. Drug Saf. 2018, 17, 1029–1039. [Google Scholar] [PubMed]
| Drug | Months | Drug Doses by Weight Group | |||
|---|---|---|---|---|---|
| <33 kg | 33–50 kg | >50–70 kg | >70 kg | ||
| Kanamycin *(Km) | 1–4 (6) | 0.5 g | 0.75 g | 0.75 g | 1 g |
| Levofloxacin (Lx) | 1–9 | 500 mg | 750 mg | 750 mg | 1000 mg |
| Clofazimine (Cfz) | 1–9 | 50 mg | 100 mg | 100 mg | 100 mg |
| Ethambutol (E) | 1–9 | 600 mg | 800 mg | 1000 mg | 1200 mg |
| Pyrazinamide (Z) | 1–9 | 750 mg | 1500 mg | 2000 mg | 2000 mg |
| Isoniazid (H) | 1–4 | 300 mg | 400 mg | 600 mg | 600 mg |
| Prothionamide (Pto) | 1–4 | 500 mg | 500 mg | 750 mg | 1000 mg |
| Term | Definitions |
|---|---|
| Cured | Treatment completed without evidence of failure and two consecutive negative cultures taken at least 30 days apart in the continuation phase |
| Treatment completed | Treatment completed without evidence of failure but there is no record of two consecutive negative cultures taken at least 30 days apart in the continuation phase. |
| Died | A patient who dies for any reason during the course of treatment |
| Failure | A patient who has a positive culture after ≥6 months of treatment (except for an isolated positive culture, which is a culture preceded by ≥1 and followed ≥2 negative cultures) OR |
| A patient who after an initial conversion, has a reversion after ≥6 months of treatment with two consecutive positive cultures taken at-least 30 days apart OR | |
| evidence of additional acquired resistance to fluoroquinolones or second-line injectables OR | |
| treatment terminated or need for permanent change of at least two of anti-TB drugs due to adverse drug reactions | |
| Lost to follow-up (LTFU) | A patient whose treatment was interrupted for ≥2 consecutive months |
| Not evaluated | A patient for whom no treatment outcome is assigned (this includes patients “transferred out” to another treatment unit and whose treatment outcome is unknown) |
| Treatment success | The sum of cured and treatment completed |
| Unsuccessful treatment outcomes | The sum of death, lost to follow-up, failure and not evaluated |
| Relapse | Patient after completing a course of STR and declared “cured” or “treatment completed”, is diagnosed with another episode of confirmed RR-TB (based on Xpert MTB/RIF assay or culture) during a follow-up period of one year post-treatment |
| Adherence to follow-up | Number who had a follow-up smear or culture divided by number eligible for follow-up for a given month. Number eligible will be calculated by subtracting the number dead and lost to follow-up before the scheduled follow-up time. |
| Bacteriological effectiveness | This is calculated by dividing the number successfully treated by the number of patients who had a bacteriological outcome (excluding death, LTFU and not evaluated) |
| Characteristics | Number | (%) | |
|---|---|---|---|
| Total | 302 | (100.0) | |
| Age categories in years | |||
| 15–24 | 35 | (11.6) | |
| 25–34 | 76 | (25.2) | |
| 35–44 | 77 | (25.5) | |
| 45–54 | 57 | (18.9) | |
| 55–64 | 38 | (12.6) | |
| ≥65 | 19 | (6.2) | |
| Gender | |||
| Male | 224 | (74.2) | |
| Female | 78 | (25.8) | |
| Weight categories | |||
| <33 kg | 3 | (1.0) | |
| 33–50 kg | 133 | (44.0) | |
| 51–70 kg | 114 | (37.8) | |
| 71–80 kg | 3 | (1.0) | |
| Missing | 49 | (18.2) | |
| Body Mass Index (kg/m2) | |||
| Underweight (<18.5) | 113 | (37.4) | |
| Normal (18.5–22.9) | 99 | (32.8) | |
| Overweight/obese (≥23.0) | 17 | (5.6) | |
| Missing | 73 | (24.2) | |
| HIV | |||
| Negative | 253 | (84.0) | |
| Positive | 3 | (1.0) | |
| Missing | 46 | (15.0) | |
| TB categories | |||
| New | 117 | (38.7) | |
| Relapse | 112 | (37.1) | |
| Treatment after LTFU | 2 | (0.7) | |
| Treatment after failure | 60 | (19.9) | |
| Others | 5 | (1.6) | |
| Missing | 6 | (2.0) | |
| Sputum smear microscopy | |||
| Negative | 82 | (27.1) | |
| Scanty positive | 35 | (11.6) | |
| 1+ positive | 77 | (25.5) | |
| 2+ positive | 45 | (14.9) | |
| 3+ positive | 53 | (17.6) | |
| Unknown/missing | 10 | (3.3) | |
| Culture positive | |||
| Negative | 61 | (20.2) | |
| Positive | 199 | (65.9) | |
| Unknown | 42 | (13.9) | |
| Year of enrolment | |||
| 2016 | 99 | (32.8) | |
| 2017 | 72 | (23.8) | |
| 2018 | 131 | (43.4) | |
| Treatment Outcomes | Culture Positive | Culture Negative | Culture Unknown | Total | ||||
|---|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | N | (%) | |
| Total | 199 | (100) | 61 | (100) | 42 | (100) | 302 | (100) |
| Successful Outcomes | 166 | (83.4) | 55 | (90.2) | 38 | (90.5) | 259 | (85.8) |
| Cured | 158 | (79.4) | 52 | (85.3) | 36 | (85.7) | 246 | (81.5) |
| Completed | 8 | (4.0) | 3 | (4.9) | 2 | (4.8) | 13 | (4.3) |
| Unsuccessful Outcomes | 33 | (16.6) | 6 | (9.8) | 4 | (9.5) | 43 | (14.2) |
| Failure | 13 | (6.6) | 1 | (1.6) | 2 | (4.8) | 16 | (5.3) |
| LTFU | 10 | (5.0) | 2 | (3.3) | 2 | (4.8) | 14 | (4.6) |
| Died | 10 | (5.0) | 3 | (4.9) | 0 | (0) | 13 | (4.3) |
| Factors | Total | Unsuccessful Outcome | RR | (95%CI) | aRR | (95%CI) | ||
|---|---|---|---|---|---|---|---|---|
| N | n | (%)# | ||||||
| Total | 302 | 43 | (14.2) | |||||
| Age in years | ||||||||
| 15–44 | 188 | 22 | (11.7) | ref | ref | |||
| 45–64 | 95 | 17 | (17.9) | 1.53 | (0.85–2.74) | 1.57 | (0.84–2.93) | |
| ≥ 65 | 19 | 4 | (21.0) | 1.80 | (0.69–4.68) | 2.97 | (1.22–7.22) * | |
| Gender | ||||||||
| Male | 224 | 35 | (15.6) | 1.52 | (0.74–3.14) | 1.31 | (0.71–2.44) | |
| Female | 78 | 8 | (10.3) | ref | ref | |||
| BMI | ||||||||
| Under weight (<18.5) | 113 | 15 | (13.3) | 1.10 | (0.54–2.23) | 0.98 | (0.46–2.06) | |
| Normal (18.5–22.9) | 99 | 12 | (12.1) | ref | Ref | |||
| Overweight/Obese (≥23.0) | 17 | 3 | (17.6) | 1.46 | (0.46–4.62) | 1.52 | (0.48–4.81) | |
| Missing | 73 | 13 | (17.8) | 1.47 | (0.71–3.03) | 1.82 | (0.93–3.57) | |
| TB category | ||||||||
| New | 113 | 13 | (11.5) | ref | ref | |||
| Previously treated | 175 | 26 | (14.9) | 1.29 | (0.69–2.41) | 1.22 | (0.65–2.28) | |
| Missing | 6 | 2 | (33.3) | 2.9 | (0.84–10.03) | 3.21 | (1.04–9.92) * | |
| HIV | ||||||||
| Positive | 3 | 2 | (66.6) | 4.82 | (2.04–11.34) | 7.14 | (3.51–15.65) * | |
| Negative | 253 | 35 | (13.8) | ref | ref | |||
| Unknown | 46 | 6 | (13.0) | 0.94 | (0.42–2.11) | 1.14 | (0.52–2.51) | |
| Year of enrolment | ||||||||
| 2016 | 99 | 20 | (20.2) | 1.76 | (0.95–3.27) | 1.79 | (0.99–3.25) | |
| 2017 | 72 | 8 | (11.1) | 0.97 | (0.43–2.18) | 0.90 | (0.40–1.99) | |
| 2018 | 131 | 15 | (11.4) | ref | ||||
| Culture | ||||||||
| Negative | 61 | 6 | (9.8) | ref | ||||
| Positive | 199 | 33 | (16.6) | 1.69 | (0.74–3.83) | 2.39 | (1.09–5.24) * | |
| Unknown | 42 | 4 | (9.5) | 0.97 | (0.29–3.22) | 1.44 | (0.41–4.99) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anh, L.T.N.; M. V. Kumar, A.; Ramaswamy, G.; Htun, T.; Thanh Hoang Thi, T.; Hoai Nguyen, G.; Quelapio, M.; Gebhard, A.; Nguyen, H.B.; Nguyen, N.V. High Levels of Treatment Success and Zero Relapse in Multidrug-Resistant Tuberculosis Patients Receiving a Levofloxacin-Based Shorter Treatment Regimen in Vietnam. Trop. Med. Infect. Dis. 2020, 5, 43. https://doi.org/10.3390/tropicalmed5010043
Anh LTN, M. V. Kumar A, Ramaswamy G, Htun T, Thanh Hoang Thi T, Hoai Nguyen G, Quelapio M, Gebhard A, Nguyen HB, Nguyen NV. High Levels of Treatment Success and Zero Relapse in Multidrug-Resistant Tuberculosis Patients Receiving a Levofloxacin-Based Shorter Treatment Regimen in Vietnam. Tropical Medicine and Infectious Disease. 2020; 5(1):43. https://doi.org/10.3390/tropicalmed5010043
Chicago/Turabian StyleAnh, Le T. N., Ajay M. V. Kumar, Gomathi Ramaswamy, Thurain Htun, Thuy Thanh Hoang Thi, Giang Hoai Nguyen, Mamel Quelapio, Agnes Gebhard, Hoa Binh Nguyen, and Nhung Viet Nguyen. 2020. "High Levels of Treatment Success and Zero Relapse in Multidrug-Resistant Tuberculosis Patients Receiving a Levofloxacin-Based Shorter Treatment Regimen in Vietnam" Tropical Medicine and Infectious Disease 5, no. 1: 43. https://doi.org/10.3390/tropicalmed5010043
APA StyleAnh, L. T. N., M. V. Kumar, A., Ramaswamy, G., Htun, T., Thanh Hoang Thi, T., Hoai Nguyen, G., Quelapio, M., Gebhard, A., Nguyen, H. B., & Nguyen, N. V. (2020). High Levels of Treatment Success and Zero Relapse in Multidrug-Resistant Tuberculosis Patients Receiving a Levofloxacin-Based Shorter Treatment Regimen in Vietnam. Tropical Medicine and Infectious Disease, 5(1), 43. https://doi.org/10.3390/tropicalmed5010043





